Back to Journals » International Journal of General Medicine » Volume 16
Association Between SPARC Polymorphisms and Ankylosing Spondylitis and Its mRNA and Protein Expression in a Chinese Han Population: A Case–Control Study
Authors Liu D, Wang L, Hu Z , Ma Z, Yang Q
Received 27 April 2023
Accepted for publication 5 August 2023
Published 16 August 2023 Volume 2023:16 Pages 3533—3542
DOI https://doi.org/10.2147/IJGM.S419094
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Luca Testarelli
Dongxia Liu,1 Liya Wang,1,2 Zhongdan Hu,3 Zhenzhen Ma,1 Qingrui Yang1
1Department of Rheumatology and Immunology, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan, 250021, People’s Republic of China; 2Department of Rheumatology and Immunology, Heze Medical College, Heze, 274000, People’s Republic of China; 3Tianjin Zhonghe Clinic Co. Ltd. of Heping District, Tianjin, 300450, People’s Republic of China
Correspondence: Liya Wang; Zhongdan Hu, Email [email protected]; [email protected]
Objective: We explore the association of polymorphisms in Secreted protein acidic and rich in cysteine (SPARC) with ankylosing spondylitis (AS) and detect SPARC mRNA and protein expression in a Chinese Han population.
Methods: Nine single-nucleotide polymorphisms (SNPs) of SPARC were genotyped in 768 AS patients and 768 controls by TaqMan genotyping assay. mRNA expression of SPARC was detected by real-time polymerase chain reaction (RT-PCR), and serum level of SPARC protein was detected by ELISA.
Results: The frequency of A allele of rs171121187 was significantly higher in AS patients than in controls (Pc=0.003, odds ratio [OR]=1.45, 95% confidence interval [95% CI] = 1.18– 1.77), the AA and AC genotypes increased the risk of AS when compared with CC genotype (Pc=0.003, OR=3.96, 95% CI=1.80– 8.75, and Pc=0.003, OR=1.27, 95% CI=1.01– 1.61, respectively). The frequency of G allele of rs4958487 was significantly lower in AS than in controls (Pc=0.001, OR=0.60, 95% CI=0.47– 0.68), the GG and GA genotypes reduced the risk of AS when compared with AA genotype (Pc=0.005, OR=0.46, 95% CI 0.18– 1.14, and Pc=0.005, OR=0.60, 95% CI=0.45– 0.79, respectively). The haplotype AA of rs17112187/rs4958487 significantly increased the risk of AS (P=2.31E-5, OR=1.60, 95% CI=1.28– 1.98), while haplotype CG decreased the risk of AS (P=5.42E-5, OR=0.55, 95% CI=0.41– 0.74). Expression levels of SPARC mRNA were significantly lower in both Peripheral blood mononuclear cells (PBMC) and granulocytes in AS patients than in controls (P=0.008 and P=0.005, respectively). SPARC protein levels were also reduced in AS patients versus the controls (P= 0.002).
Conclusion: This study indicates that polymorphisms in SPARC are associated with AS susceptibility, and both mRNA and protein levels of SPARC are decreased in AS patients in a Chinese Han population.
Keywords: secreted protein acidic and rich in cysteine, SPARC, ankylosing spondylitis, single nucleotide polymorphism, SNP, case–control study
Introduction
Ankylosing spondylitis (AS) is a chronic inflammatory disease and the main subtype of spondyloarthritis. Clinical features of AS include sacroiliitis, spinal inflammation, asymmetrical peripheral oligoarthritis, and enthesitis.1 The exact pathogenesis and etiology of AS are not fully elaborated till now, but it is well established that genetic factors play a key role in determining a patient’s susceptibility to AS.2 The gene encoding human leukocyte antigen B-27 (HLA-B27) is strongly associated with AS development.3 However, only 1–5% of all HLA-B27 positive individuals will develop AS.4,5 Previously, in addition to this well-known HLA-B27 gene, more than 14 susceptibility genes outside of the MHC region, such as ERAP1, IL23R, ANTXR2, IL12B, CARD9, PTGER4, KIF21B, and STAT3, have been identified as the risk factors for AS.6–11
Osteoporosis (OP) or osteopenia is common even in the early stages of AS.12–14 Cortical bone loss starts early and is evident within the first 2 years of the disease.15 It is reported that the prevalence of OP and osteopenia in AS patients ranged from 2.0% to 47.0% and 5.0%–78.3%, respectively.16 Compared with patients with mechanical back pain, patients with AS showed significant bone loss assessed by Dual-energy x-ray absorptiometry (DXA) at the lumbar spine.17 Osteoporosis of spine is much more common than that of femur, and presence of syndesmophytes did not affect estimation of osteoporosis of spine.18 Inflammation is associated with trabecular bone loss leading to osteoporosis but also with cordal new bone formation.17,19–21 The bone mineral density (BMD) at the femoral neck was reduced in AS patients and was correlated with increased risk of vertebral fractures, suggesting that bone mass loss is still the basic pathological change of this disease.12
Human SPARC (Secreted protein acidic and rich in cysteine) is a single-copy gene localized on chromosome 5q31-33 with a high degree of evolutionary conservation. Termine et al primarily identified that SPARC was a pivotal noncollagenous constituent of bovine bone in 1981.22 SPARC is also called osteonectin or BM-40, and it is a matricellular protein secreted by many cell types found in the extracellular (ECM) during a variety of processes such as bone formation, wound healing, adipogenesis, and angiogenesis.23–25 It has been shown that matricellular SPARC regulates ECM tightness, dendritic cells migration, and T-cell priming with antigen-specific immune response.26 When tissues undergo remodeling during normal development or in response to injury, the expression of SPARC mRNA and protein is markedly elevated.27 While in some osteopenic states, such as osteogenesis imperfecta, osteonectin expression decreases,28 as Delany et al reported that osteonectin-null mice had decreased bone formation, resulting in decreased bone remodeling with a negative bone balance and causing profound osteopenia. Mansergh et al have established a Sparc-deficient mouse model for human osteoporosis.29 These data suggest that SPARC/osteonectin participated in bone remodeling and the maintenance of bone mass. Sharma et al first implicated SPARC as a component of the innate immune system in the pathogenesis of spondyloarthropathy (SpA).30 In order to evaluate whether SPARC contributes to the susceptibility of AS, we studied SPARC polymorphisms and its mRNA and protein expression in a Chinese Han population.
Materials and Methods
Subjects
A total of 768 AS patients were recruited from the Department of Rheumatology and Immunology of Shandong Provincial Hospital affiliated to Shandong First Medical University from 2014 to 2016, 768 healthy controls were recruited from those who underwent physical examination in the Health Center of the same hospital, who had no previous medical history or family history diseases. All the participants underwent genotyping. For the following mRNA test, we recruited 68 patients from the AS group who were first diagnosed without any treatment, and collected 76 individuals randomly from the control group. For further protein analysis, we randomly chose 39 from 68 patients and 45 from 76 controls, respectively. All AS patients were diagnosed according to the modified 1984 New York criteria.31 The exclusion criteria of this study included: those who were not of Chinese Han nationality, those with a family history of AS, those aged under 18 years; those with psoriasis, psoriatic arthritis, inflammatory bowel disease (IBD), diabetes, viral hepatitis, cirrhosis, severe hepatic or renal function insufficiency, thyroid disease, those who were diagnosed with cardiovascular or cerebrovascular diseases, those who underwent organ transplants, and patients with active tuberculosis or other infections. Blood samples were obtained after their informed consent. The study was approved by the Human Ethics Committee of Provincial Hospital affiliated with Shandong First Medical University (No. 2014–030). The main demographic and clinical characteristics of AS patients and controls are shown in Table 1. Questionnaires (Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) and Bath Ankylosing Spondylitis Functional Index (BASFI)) were obtained before the initiation of treatments.
 |
Table 1 The Main Demographic and Clinical Characteristics of the AS Patients and Controls |
Tag SNP Selection and Genotyping
Tag SNPs were identified using International HapMap data with the Chinese Beijing Han population (CHB) of Phase I+II release, 24. The region of SNPs data was downloaded covering the SPARC gene and 2 kb upstream of the gene. We used Haploview software to figure out the linkage disequilibrium structure between SNPs, with minor allele frequency (MAF) >0.05 and r2 >0.8. Finally, in order to contain the maximum information of the haplotype blocks, we chose the SNPs rs3776959, rs4958279, rs7733793, rs2881558, rs2347128, rs4958485, rs17112187, rs4958487and rs1545030 as tag SNPs. The basic information of the nine SNPs is shown in Table 2.
 |
Table 2 The Basic Information of the Nine SNPs |
DNA samples from the 768 AS patients and 768 controls were extracted from peripheral blood leukocytes using TIANGEN Genomic DNA Purification Kit according to the manufacturer’s instructions and stored at −80°C before genotyping. The nine SNPs were genotyped using TaqMan chemistry of the Applied Biosystems real-time Prism 7900HT Sequence Detection System (ABI Inc. CA, USA). Primers and the TaqMan fluorogenic probes were designed using the Primer Express software V2.0 (ABI Inc., Foster City, CA, USA) and procured from Applied Biosystems (ABI, Warrington, UK). One probe (for allele 1) was labeled with VIC dye and the other (for allele 2) with FAM dye at the 5’-end, and serial dilutions were run to determine the optimal working concentrations. For each reaction, a 25 μL mixture was prepared by mixing 5 μL containing 50 ng DNA, 12.5 μL of 2x Universal mix (Eurogentec, Liege Science Park, Seraing Belgium), 1.25 μL of 20x probe assay mix, and 6.25 μL DNase-free distilled water. Three no-template controls were included in each plate for normalization of emission signal. The thermal profile for amplification for the first cycle occurred at 50°C for 2 min and 95°C for 10 min, followed by 50 cycles of 94°C for 15 sec and 60°C for 30 sec. The plates were then scanned for FRET signal using the 7900HT sequence detection system and data analyzed using SDS 2.0 software (ABI, Foster City, CA, USA).
Isolation of PBMC and Granulocytes
Blood samples from 68 patients and 76 healthy controls were deposited in tubes with sodium citrate. Peripheral blood mononuclear cells (PBMC) were isolated from heparinized blood samples by Ficoll gradient centrifugation (Lymphoprep, Nycomed Pharma AS). Cells from the interphase were collected and washed twice with HBSS (HyClone). Granulocytes were collected from heparinized blood samples after being processed by lymphocyte separation liquid and red cell lysing reagent, washed twice with Hanks liquid. The PBMC and granulocytes are stored in 1mL of Trizol (Invitrogen) at –80°C, respectively, until total RNA extraction.
Total RNA Extraction and cDNA Synthesis
Total RNA from PBMC was extracted using the TRIzol Reagent (Invitrogen, Carlsbad, CA, USA), following the manufacturer’s instructions. RNA quantitation and quality were determined using an Agilent 2100 Bioanalyzer (Agilent) and the RNA 6000 Nano Kit (Agilent), according to the manufacturer’s manuals. The isolated RNA samples were quantified by a spectrophotometer (Titertek-Berthold Colibri, Germany). cDNA was synthesized using the UEIris reverse transcription real-time polymerase chain reaction system. Total RNA (500 ng/mL in a final volume of 2 μL) was added to a mixture (10 μL) containing RNase-free water, 10 × DNase I buffer, and DNase I. This mixture was placed into a thermal cycler (Bioer Life Express, China) at 37°C for 10 min, before 1 μL of 50 mM EDTA was added to the mix. This mixture was heated to 65°C for 10 min to deactivate DNase I; then, UEIris RT MasterMix (5×) RNase-free water was added to the mix (20μL). This mix was placed into a thermal cycler, and a reverse transcription reaction was facilitated to produce cDNA at 55°C for 30 min, with a final step conducted for 10 sec at 85°C.
Quantitative Real-Time PCR (qRT-PCR)
Expression levels of SPARC mRNA were detected by qRT-PCR using QuantiFast SYBR Green PCR Kit (Qiagen) following the manufacturer’s instructions. Primers for SPARC gene were designed and obtained from the GenBank Database, which were as follows: forward 5’-AGAAGGTGTGCAGCAATGAC-3’, reverse: 5’-TGTAGTCCAGGTGGAGCTTG-3’. PCR amplification conditions were as follows: one cycle at 95°C for 2 min, 40 cycles at 95°C for 5 sec, one cycle at 60°C for 15 sec, and elongation at 72°C for 25 sec. Each cDNA sample was analyzed in triplicate, and β-actin was used as the endogenous control. The relative expression of SPARC mRNA was calculated using the 2−ΔΔCt comparative method, where −ΔCt refers to the difference between the numbers of cycles (Ct) of the target gene and the endogenous control.
Enzyme-Linked Immunosorbent Assay (ELISA)
The blood samples of 39 AS patients and 45 healthy controls were centrifuged, and the serum specimens were stored at −80°C. Serum SPARC protein levels were detected by Human SPARC ELISA kit from R&D systems (Minneapolis, MN55413, USA) according to the manufacturer’s instructions. In this ELISA system, human SPARC ELISA kit was used to generate the standard curve to quantify the SPARC protein based on absorbance data. The intra- and inter-assay CVs were 5.3% and 9.2%, respectively. All samples were tested in duplicates in a blinded manner.
Statistical Analysis
The data were analyzed using GraphPad Prism software (version 5.0). Mann–Whitney U-tests were used to compare mRNA expression and serum protein levels of SPARC in patients with AS and healthy controls. P value of less than 0.05 was considered significant. The Hardy–Weinberg equilibrium (HWE) of the nine SNPs was tested by chi-square test. Genotypic and allelic frequencies between patients and controls, the odds ratio (OR) with 95% confidence interval (CI), P value were calculated with respect to the minor allele compared with the major allele, and haplotype frequencies analyzed by SHEsis and SNPStats program. The linkage disequilibrium (LD) was calculated using Haploview 4.2. Pc values were corrected by the Bonferroni method, and the statistical significance was defined as Pc< 0.05.
Results
Overall, 768 AS patients (474 men, mean age 35.7±9.8 years) and 768 controls (444 men, mean age 37.1±8.7 years) were enrolled in our study. The age of all participants ranged from 18 to 60. There were no statistical differences in gender and age between AS patients and controls (for gender, P=0.119; for age, P=0.537). In the AS group, positivity of HLA-B27 was 95%, mean BASDAI was 4.67±1.62, and mean BASFI was 3.86±2.14.
Genetic Association Between SPARC SNPs and AS
Nine SNPs of SPARC were genotyped in all subjects. The distribution of the nine SPARC SNPs was in accordance with the Hardy-Weinberg equilibrium (HWE) (P>0.05, Supplementary Table S1). In this study, Minor Allele Frequency (MAF) was consistent with the International HapMap project data of CHB.
The allele and genotype frequencies of the tested SPARC SNPs are illustrated in Table 3. The results revealed that rs17112187 and rs4958487 of the tested SNPs correlated with the development of AS. For rs17112187, the major A allele was associated with the increased risk of AS (Pc=0.003, OR=1.45), the AA and AC genotypes appeared to increase the risk of AS when compared with CC genotype (Pc=0.003, OR=0.25 and Pc=0.003, OR=0.79, respectively). For rs4958487, the minor G allele frequency in AS patients was significantly lower than that in controls (Pc=0.001, OR=1.67), the GG and GA genotypes appeared to reduce the risk of AS when compared with AA genotype (Pc=0.005, OR=2.18 and Pc=0.005, OR=1.68, respectively). The haplotype AA of rs17112187/rs4958487 was strongly associated with AS and significantly increased the risk of AS (P=2.31E-5, OR=1.60), while the haplotype CG reduced the risk (P=5.42E-5, OR=0.55), we further got a global P=1.90E-7, which suggests the haplotypes of both SNPs were related to AS susceptibility (Table 4). The linkage analysis was performed by Haploview software 4.2, all the nine SNPs haplotypes were compared between AS patients and healthy controls, but all of them were in weak linkage disequilibrium (Supplementary Figure S1).
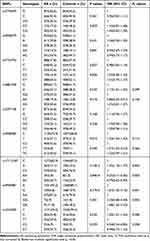 |
Table 3 Frequencies of Alleles and Genotypes of SPARC Polymorphisms in AS Patients and Controls |
 |
Table 4 The Haplotypes Between rs17112187 and rs4958487 Related to AS |
Expression Levels of SPARC mRNA and Protein
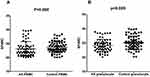
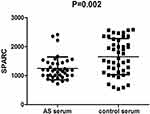
The subjects included 68 AS patients (59 male and 9 female, mean age 33.2±9.1 years) and 76 healthy controls (64 males and 12 females, mean age 36.7±8.3 years). There were no statistical differences in gender and age between the AS patients and healthy controls (for gender, P=0.814; for age, P=0.726). The SPARC mRNA expression levels were examined using qRT-PCR. The results showed that SPARC mRNA expression levels in PBMC of the 68 AS patients were significantly lower than that of the 76 healthy controls (P=0.008, Figure 1A). Further, the granulocyte mRNA expression levels were also significantly lower in AS patients than in healthy controls (P=0.005, Figure 1B). Next, SPARC protein levels in the serum were investigated by ELISA, the result indicated that SPARC protein levels in 39 AS patients were significantly reduced compared with 45 healthy controls (P=0.002, Figure 2). All the results showed that the mRNA and protein levels of SPARC were significantly decreased in patients with AS.
Discussion
In this case–control study based on a Chinese Han population, we evaluated the association between SPARC genetic polymorphisms and AS susceptibility, we then detected mRNA and protein expression levels of SPARC in AS patients and healthy controls. We found the allele frequencies and genotype of rs17112187 and rs4958487 correlated with the development of AS. Both mRNA and protein levels of SPARC were significantly decreased in AS patients compared with controls.
SPARC is a 34,632 Dalton extracellular matrix-associated glycoprotein in the extracellular matrix (ECM), also called osteonectin or BM-40.32 Termine et al first described that SPARC was a bone-specific protein enriched in mineralized bone trabeculae, connecting hydroxyapatite and collagen to compose a bone matrix.22,33 SPARC could influence pathways involved in extracellular matrix assembly such as procollagen processing and collagen fibril formation.34 In many studies of animal models, SPARC was found to participate in the regulation mechanism of growth factors and matrix protease in the microenvironment. SPARC could link with TGFβ/TGFβRII complex combination to enhance its downstream signals.35 In HMVEC and BAEC cells, SPARC inhibits the β-FGF receptor phosphorylation, combines with VEGF and its receptor against angiogenesis.36 In fibroblasts, SPARC inhibits the binding of PDGF-B to its cell-surface receptors.37 SPARC mediates peripheral blood mononuclear cells to express MMP-1 and MMP-9, and mediates fibroblasts to express MMP-1, MMP-3, and MMP-9.38 Moreover, SPARC participates in a variety of signal transduction pathways, such as PI3K/AKT, MAPK, and Wnt signal transduction pathway,39,40 relative to the occurrence of glioma, colorectal cancer, and ovarian cancer.41,42 SPARC interacts with actin in skeletal muscle in vitro and in vivo, and appears to be an important modulator of the actin cytoskeleton, implicating maintenance of muscular function, which suggests a new role of SPARC during tissue remodeling.43 A recent animal study demonstrated that SPARC played a role in maintaining the stiffness of skeletal muscle by regulating collagen accumulation, showing that accumulation of fibrillar collagen was significantly reduced in SPARC-null mice compared to WT mice after 5 months of age.44 SPARC can also adjust the osteoblasts and osteoclasts to promote the growth of bone.34 In osteoblasts of patients with osteogenesis imperfecta, expression of SPARC was significantly lower than in that of healthy controls. In SPARC-null mice, the numbers of osteoclasts and osteoblasts were significantly decreased.45–47 It indicated that SPARC was one of the necessary factors in the phases of osteoblast formation, maturity, and survival. A newly published paper demonstrated that SPARC regulated the extracellular matrix mineralization of osteoblasts through P38 signaling pathway.48 Jundt et al reported that in bone cells, SPARC was a marker of osteoblastic functional differentiation.49
In recent years, the complex interactions between the skeletal and immune systems have become more and more clear, and some investigators have verified that SPARC is one of the critical factors in both systems.50 Several studies indicated that SPARC was associated with some autoimmune diseases such as rheumatoid arthritis (RA), primary Sjogren’s syndrome (PSS), and scleroderma. SPARC synthesis was increased in the synovial cells of RA and OA joints, and the levels of SPARC in synovial fluids of patients with RA were significantly elevated.51 In addition, another study conducted by our team suggested that two SNPs (rs3210714 and rs1950384) of SPARC gene were associated with the pathogenesis of RA.52 Strong expression of SPARC promoted calcinosis cutis in PSS and scleroderma.53,54 Zhou et al first reported that polymorphisms of the SPARC gene were associated with susceptibility to systemic sclerosis (SSc) and its clinical manifestations.55 Evidence showed that serum SPARC expression levels in AS patients accompanied with OP were significantly lower than that in healthy controls, and the correlation analysis showed that serum SPARC levels were negatively correlated with TNF-α, while positively correlated with L2-4BMD, TGF-β1 and BSAP.56 In the present study, we first investigated the association between SPARC and the susceptibility to AS in a Chinese Han population by functional and genetic association studies.
Our study showed that two SNPs of SPARC gene were associated with susceptibility to AS. The major A allele, AA and AC genotype of rs17112187 was associated with an increased risk of AS. The minor G allele, GG and GA genotype of rs4958487 appeared to reduce the risk of AS. The haplotypes for both rs17112187 and rs4958487 were related to AS susceptibility. We also found that the expression of SPARC mRNA levels in PBMC and granulocytes of AS patients was significantly lower than in those of the healthy controls. Moreover, the serum level of SPARC protein was also significantly reduced in AS patients. Thus, we suppose that SPARC might be a protective factor in the pathogenesis of AS. To our knowledge, our study is the first to demonstrate that both SPARC gene and its protein are significantly associated with AS. Bone metabolism disorder is one of the pathological changes in AS, SPARC may be related to the bone metabolic disorder of AS.
There are some limitations in our study, the first is that our study just enrolled a small size of AS patients of Han population in a single center of local hospital, the result could not represent the whole Chinese population, more RCTs studies with larger sample size are needed to elaborate the association between SPARC and AS. Secondly, the present conclusion is just based on the initial SNP study of SPARC in AS patients, we need to do further research to determine the effect of SPACR on disease manifestations such as X-ray progression. Thirdly, when recruiting AS patients, we set the inclusion criteria as that who were first diagnosed without any treatment, we did not account for effect of treatments on our measurements. Therefore, whether the treatment of AS would affect the expression level of SPARC protein and the relationship between SPARC level and inflammatory markers still needs to be further elaborated.
Conclusions
Genetic polymorphisms and mRNA and protein expression of SPARC are associated with AS in a Chinese Han population. How the specific physiological process of SPARC influences AS is still not fully elucidated. Further studies are needed to figure out the specific pathway of SPARC in the pathogenesis of AS, so as to provide a better therapeutic strategy for AS.
Data Sharing Statement
The data used to support the findings of this study are available from the corresponding author upon request.
Ethics Statement
The studies titled “Association between SPARC Polymorphisms and Expression and Ankylosing Spondylitis in a Chinese Han Population: A Case-Control Study” involving human participants were reviewed and approved by Shandong Provincial Hospital Affiliated to Shandong First Medical University. Patients/participants provided their written informed consent to participate in this study. Participants were assured of confidentiality and anonymity of information relating to the survey. This study was conducted according to the guidelines of the Declaration of Helsinki.
Acknowledgments
We thank all AS patients and control individuals who participated in this study.
Funding
This work was supported by Natural Science Foundation of China Grant [No. 81172861/H1008 and No. 81671605/H1008], Foundation of Scientific and Technological Project of Shandong Province [No. 2011GG21829], Shandong Province Excellent Middle-aged and Young Scientists Encouragement Research Foundation [No. BS2009YY025], and the National Natural Science Foundation of China (Youth fund project, Grant No. 82201994).
Disclosure
The authors declare that there is no conflict of interest for this work.
References
1. Braun J, Sieper J. Ankylosing spondylitis. Lancet. 2007;369:1379–1390. doi:10.1016/S0140-6736(07)60635-7
2. Chatzikyriakidou A, Georgiou I, Voulgari PV, Drosos AA. The role of tumor necrosis factor (TNF)-alpha and TNF receptor polymorphisms in susceptibility to ankylosing spondylitis. Clin Exp Rheumatol. 2009;27:645–648.
3. Ebrahimiadib N, Berijani S, Ghahari M, Pahlaviani FG. Ankylosing Spondylitis. J Ophthalmic Vis Res. 2021;16:462–469.
4. Brown MA, Wordsworth BP, Reveille JD. Genetics of ankylosing spondylitis. Clin Exp Rheumatol. 2002;20:S43–49.
5. Reveille JD. Genetics of spondyloarthritis--beyond the MHC. Nat Rev Rheumatol. 2012;8:296–304. doi:10.1038/nrrheum.2012.41
6. Maksymowych WP, Brown MA. Genetics of ankylosing spondylitis and rheumatoid arthritis: where are we at currently, and how do they compare?. Clin Exp Rheumatol. 2009;27:S20–25.
7. Burton PR, Clayton DG, Cardon LR, et al. Association scan of 14,500 nonsynonymous SNPs in four diseases identifies autoimmunity variants. Nat.Genet. 2007;39:1329–1337.
8. Danoy P, Pryce K, Hadler J, et al. Association of variants at 1q32 and STAT3 with ankylosing spondylitis suggests genetic overlap with Crohn’s disease. PLoS Genet. 2010;6:e1001195. doi:10.1371/journal.pgen.1001195
9. Gagliardi MC, Teloni R, Mariotti S, et al. Endogenous PGE2 promotes the induction of human Th17 responses by fungal ss-glucan. J Leukoc Biol. 2010;88:947–954. doi:10.1189/jlb.0310139
10. Evans DM, Spencer CC, Pointon JJ, et al. Interaction between ERAP1 and HLA-B27 in ankylosing spondylitis implicates peptide handling in the mechanism for HLA-B27 in disease susceptibility. Nat Genet. 2011;43:761–767. doi:10.1038/ng.873
11. Pimentel-Santos FM, Ligeiro D, Matos M, et al. Association of IL23R and ERAP1 genes with ankylosing spondylitis in a Portuguese population. Clin Exp Rheumatol. 2009;27:800–806.
12. Magrey M, Khan MA. Osteoporosis in ankylosing spondylitis. Curr Rheumatol Rep. 2010;12:332–336. doi:10.1007/s11926-010-0122-1
13. Davey-Ranasinghe N, Deodhar A. Osteoporosis and vertebral fractures in ankylosing spondylitis. Curr Opin Rheumatol. 2013;25:509–516. doi:10.1097/BOR.0b013e3283620777
14. Klingberg E, Lorentzon M, Mellstrom D, et al. Osteoporosis in ankylosing spondylitis - prevalence, risk factors and methods of assessment. Arthritis Res Ther. 2012;14:R108. doi:10.1186/ar3833
15. Neumann A, Haschka J, Kleyer A, et al. Cortical bone loss is an early feature of nonradiographic axial spondyloarthritis. Arthritis Res Ther. 2018;20:202. doi:10.1186/s13075-018-1620-1
16. Kilic E, Ozgocmen S. Bone mass in axial spondyloarthritis: a literature review. World J Orthop. 2015;6:298–310. doi:10.5312/wjo.v6.i2.298
17. Akgol G, Kamanli A, Ozgocmen S. Evidence for inflammation-induced bone loss in non-radiographic axial spondyloarthritis. Rheumatology. 2014;53:497–501. doi:10.1093/rheumatology/ket385
18. Singh HJ, Nimarpreet K, Das S, Kumar A, Prakash S. Study of bone mineral density in patients with ankylosing spondylitis. J Clin Diagn Res. 2013;7:2832–2835. doi:10.7860/JCDR/2013/6779.3770
19. Carter S, Lories RJ. Osteoporosis: a paradox in ankylosing spondylitis. Curr Osteoporos Rep. 2011;9:112–115. doi:10.1007/s11914-011-0058-z
20. Lim MJ, Kang KY. A contemporary view of the diagnosis of osteoporosis in patients with axial spondyloarthritis. Front Med. 2020;7:569449. doi:10.3389/fmed.2020.569449
21. Thudium CS, Nielsen SH, Sardar S, et al. Bone phenotypes in rheumatology - there is more to bone than just bone. BMC Musculoskelet Disord. 2020;21:789. doi:10.1186/s12891-020-03804-2
22. Termine JD, Kleinman HK, Whitson SW, Conn KM, McGarvey ML, Martin GR. Osteonectin, a bone-specific protein linking mineral to collagen. Cell. 1981;26:99–105. doi:10.1016/0092-8674(81)90037-4
23. Bradshaw AD. Diverse biological functions of the SPARC family of proteins. Int J Biochem Cell Biol. 2012;44:480–488. doi:10.1016/j.biocel.2011.12.021
24. Peixoto E, Atorrasagasti C, Aquino JB, et al. SPARC (secreted protein acidic and rich in cysteine) knockdown protects mice from acute liver injury by reducing vascular endothelial cell damage. Gene Ther. 2015;22:9–19. doi:10.1038/gt.2014.102
25. Melouane A, Yoshioka M, Kanzaki M, St-Amand J. Sparc, an EPS-induced gene, modulates the extracellular matrix and mitochondrial function via ILK/AMPK pathways in C2C12 cells. Life Sci. 2019;229:277–287. doi:10.1016/j.lfs.2019.05.070
26. Sangaletti S, Gioiosa L, Guiducci C, et al. Accelerated dendritic-cell migration and T-cell priming in SPARC-deficient mice. J Cell Sci. 2005;118:3685–3694. doi:10.1242/jcs.02474
27. Motamed K. SPARC (osteonectin/BM-40). Int J Biochem Cell Biol. 1999;31:1363–1366. doi:10.1016/S1357-2725(99)00090-4
28. Delany AM, Amling M, Priemel M, Howe C, Baron R, Canalis E. Osteopenia and decreased bone formation in osteonectin-deficient mice. J Clin Invest. 2000;105(7):915–923. doi:10.1172/JCI7039
29. Mansergh FC, Wells T, Elford C, et al. Osteopenia in Sparc (osteonectin)-deficient mice: characterization of phenotypic determinants of femoral strength and changes in gene expression. Physiol Genomics. 2007;32(1):64–73. doi:10.1152/physiolgenomics.00151.2007
30. Sharma SM, Choi D, Planck SR, et al. Insights in to the pathogenesis of axial spondyloarthropathy based on gene expression profiles. Arthritis Res Ther. 2009;11:R168. doi:10.1186/ar2855
31. van der Linden CJ, Breslau PJ, de Jong PC, Soeters PB, Timmermans F, Greep JM. Prospective study on the incidence of complications of right heart catheterization. Neth J Surg. 1984;36:127–129.
32. Murphy-Ullrich JE, Sage EH. Revisiting the matricellular concept. Matrix Biol. 2014;37:1–14. doi:10.1016/j.matbio.2014.07.005
33. Brekken RA, Sage EH. SPARC, a matricellular protein: at the crossroads of cell-matrix. Matrix Biol. 2000;19:569–580. doi:10.1016/S0945-053X(00)00105-0
34. Rosset EM, Bradshaw AD. SPARC/osteonectin in mineralized tissue. Matrix Biol. 2016;52–54:78–87. doi:10.1016/j.matbio.2016.02.001
35. Socha MJ, Manhiani M, Said N, Imig JD, Motamed K. Secreted protein acidic and rich in cysteine deficiency ameliorates renal inflammation and fibrosis in angiotensin hypertension. Am J Pathol. 2007;171:1104–1112. doi:10.2353/ajpath.2007.061273
36. Motamed K, Blake DJ, Angello JC, et al. Fibroblast growth factor receptor-1 mediates the inhibition of endothelial cell proliferation and the promotion of skeletal myoblast differentiation by SPARC: a role for protein kinase A. J Cell Biochem. 2003;90:408–423. doi:10.1002/jcb.10645
37. Raines EW, Lane TF, Iruela-Arispe ML, Ross R, Sage EH. The extracellular glycoprotein SPARC interacts with platelet-derived growth factor (PDGF)-AB and -BB and inhibits the binding of PDGF to its receptors. Proc Natl Acad Sci USA. 1992;89:1281–1285. doi:10.1073/pnas.89.4.1281
38. Arnold S, Mira E, Muneer S, et al. Forced expression of MMP9 rescues the loss of angiogenesis and abrogates metastasis of pancreatic tumors triggered by the absence of host SPARC. Exp Biol Med. 2008;233:860–873. doi:10.3181/0801-RM-12
39. Nie J, Sage EH. SPARC inhibits adipogenesis by its enhancement of beta-catenin signaling. J Biol Chem. 2009;284:1279–1290. doi:10.1074/jbc.M808285200
40. Wang Q, Yang Q, Zhang A, Kang Z, Wang Y, Zhang Z. Silencing of SPARC represses heterotopic ossification via inhibition of the MAPK signaling pathway. Biosci Rep. 2019;2019:39.
41. Nie J, Chang B, Traktuev DO, et al. IFATS collection: combinatorial peptides identify alpha5beta1 integrin as a receptor for the matricellular protein SPARC on adipose stromal cells. Stem Cells. 2008;26:2735–2745. doi:10.1634/stemcells.2008-0212
42. Tang MJ, Tai IT. A novel interaction between procaspase 8 and SPARC enhances apoptosis and potentiates chemotherapy sensitivity in colorectal cancers. J Biol Chem. 2007;282:34457–34467. doi:10.1074/jbc.M704459200
43. Jorgensen LH, Jepsen PL, Boysen A, et al. SPARC interacts with actin in skeletal muscle in vitro and in vivo. Am J Pathol. 2017;187:457–474. doi:10.1016/j.ajpath.2016.10.013
44. Omi S, Yamanouchi K, Nakamura K, Matsuwaki T, Nishihara M. Reduced fibrillar collagen accumulation in skeletal muscle of secreted protein acidic and rich in cysteine (SPARC)-null mice. J Vet Med Sci. 2019;81:1649–1654. doi:10.1292/jvms.19-0485
45. Delany AM, Hankenson KD. Thrombospondin-2 and SPARC/osteonectin are critical regulators of bone remodeling. J Cell Commun Signal. 2009;3:227–238. doi:10.1007/s12079-009-0076-0
46. Delany AM, McMahon DJ, Powell JS, Greenberg DA, Kurland ES. Osteonectin/SPARC polymorphisms in Caucasian men with idiopathic osteoporosis. Osteoporos Int. 2008;19:969–978. doi:10.1007/s00198-007-0523-9
47. Boskey AL, Moore DJ, Amling M, Canalis E, Delany AM. Infrared analysis of the mineral and matrix in bones of osteonectin-null mice and their wildtype controls. J Bone Miner Res. 2003;18:1005–1011. doi:10.1359/jbmr.2003.18.6.1005
48. Zhu YS, Gu Y, Jiang C, Chen L. Osteonectin regulates the extracellular matrix mineralization of osteoblasts through P38 signaling pathway. J Cell Physiol. 2020;235:2220–2231. doi:10.1002/jcp.29131
49. Jundt G, Berghauser KH, Termine JD, Schulz A. Osteonectin--a differentiation marker of bone cells. Cell Tissue Res. 1987;248:409–415. doi:10.1007/BF00218209
50. Takayanagi H. Osteoimmunology: shared mechanisms and crosstalk between the immune and bone systems. Nat Rev Immunol. 2007;7:292–304. doi:10.1038/nri2062
51. Nakamura S, Kamihagi K, Satakeda H, et al. Enhancement of SPARC (osteonectin) synthesis in arthritic cartilage. Increased levels in synovial fluids from patients with rheumatoid arthritis and regulation by growth factors and cytokines in chondrocyte cultures. Arthritis Rheum. 1996;39:539–551. doi:10.1002/art.1780390402
52. Yang XL, Hu ZD, Wu Q, et al. Association of polymorphisms in SPARC and NLRP2 genes with rheumatoid arthritis in a Chinese Han population. Mod Rheumatol. 2015;25:67–71. doi:10.3109/14397595.2014.903595
53. Fueki H, Hino R, Yoshioka M, Nakamura M, Tokura Y. Calcinosis cutis associated with primary Sjogren’s syndrome: strong expression of osteonectin and matrix Gla protein. Rheumatology. 2011;50:2318–2320. doi:10.1093/rheumatology/ker304
54. Davies CA, Jeziorska M, Freemont AJ, Herrick AL. Expression of osteonectin and matrix Gla protein in scleroderma patients with and without calcinosis. Rheumatology. 2006;45(11):1349–1355. doi:10.1093/rheumatology/kei277
55. Zhou X, Tan FK, Reveille JD, et al. Association of novel polymorphisms with the expression of SPARC in normal fibroblasts and with susceptibility to scleroderma. Arthritis Rheum. 2002;46:2990–2999. doi:10.1002/art.10601
56. Xu JR, Lin Y, Zhang CY, Li WM, Guo CJ, Ye L. Effects of comprehensive therapy on serum SPARC levels in ankylosing spondylitis patients accompanied with osteoporosis. Chin J Integrat Trad West Med. 2013;33:466–470.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.