Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 15
Association Between Serum Albumin Level and Microvascular Complications of Type 2 Diabetes Mellitus
Authors Zhang J , Deng Y , Wan Y, He S, Cai W, Xu J
Received 4 May 2022
Accepted for publication 13 July 2022
Published 23 July 2022 Volume 2022:15 Pages 2173—2182
DOI https://doi.org/10.2147/DMSO.S373160
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Juei-Tang Cheng
Jie Zhang,1 Yuanyuan Deng,1 Yang Wan,1 Shasha He,1 Wei Cai,2 Jixiong Xu1,3,4
1Department of Endocrinology and Metabolism, First Affiliated Hospital of Nanchang University, Nanchang, Jiangxi, 330006, People’s Republic of China; 2Department of Medical Genetics and Cell Biology, Medical College of Nanchang University, Nanchang, 330006, People’s Republic of China; 3Jiangxi Clinical Research Center for Endocrine and Metabolic Disease, Nanchang, Jiangxi, 330006, People’s Republic of China; 4Jiangxi Branch of National Clinical Research Center for Metabolic Disease, Nanchang, Jiangxi, 330006, People’s Republic of China
Correspondence: Jixiong Xu, Email [email protected]
Objective: To analyze the associations between serum albumin (sALB) level and diabetic microvascular complications, including diabetic retinopathy (DR) and diabetic kidney disease (DKD), in patients with type 2 diabetes mellitus (T2DM).
Methods: This retrospective study included 951 hospitalized patients with T2DM who had completed screening for DR and DKD during hospitalization. Patients were divided into three groups according to sALB tertiles. Multivariate logistic regression analysis was used to assess the association of sALB with microvascular complications.
Results: The prevalence of DR, DKD and macroalbuminuria increased with decreasing sALB levels. Multivariate logistic regression analysis showed that lower levels of sALB (Q1) were associated with higher risk of DR (odds ratio [OR]: 1.59, 95% confidence interval [CI]: 1.12– 2.26), DKD (OR: 3.00, 95% CI: 2.04– 4.41) and macroalbuminuria (OR: 9.76, 95% CI: 4.62– 20.63) compared with higher levels of sALB (Q3) after adjustment for other risk factors. After stratification by sex and age, the effect of lower levels of sALB (Q1) on DR incidence was more obvious in patients with male (OR: 1.60, 95% CI: 1.00– 2.56), and aged< 65 years (OR: 1.74, 95% CI: 1.14– 2.65) (P < 0.05 for all); the effect of lower levels of sALB (Q1) on the incidence of DKD was significant in both males (OR: 3.78, 95% CI: 2.26– 6.32) and females (OR: 2.35, 95% CI: 1.26– 4.35) (P < 0.05 for all), while only the age < 65 years (OR: 3.46, 95% CI: 2.16– 5.53) was significant in the age subgroup (P < 0.001).
Conclusion: Decreased sALB levels may be an independent risk indicator of DR and DKD in patients with T2DM, and significantly associated with DKD progression. For DR screening, special attention should be paid to men aged < 65 years, while screening for DKD should pay attention to people < 65 years old.
Keywords: serum albumin, diabetic kidney disease, diabetic retinopathy, type 2 diabetes mellitus
Introduction
Type 2 diabetes mellitus (T2DM) is a common chronic disease worldwide, and its prevalence is increasing at an alarming rate. According to the International Diabetes Federation, the global prevalence of diabetes was estimated at 9.3% (463 million people) in 2019, and it is predicted to rise to 10.2% (578 million people) by 2030 and 10.9% (700 million people) by 2045.1 The threat of T2DM to human health is mainly due to various complications. Diabetic kidney disease (DKD) and diabetic retinopathy (DR) are the main microvascular complications of T2DM. Research has found that approximately half of the people with T2DM will develop chronic kidney disease, which causes individual frailty, reduced quality of life, and even develop end-stage renal disease.2 DR is the leading cause of acquired vision loss in middle-aged and economically active people worldwide.3 In 2002, the World Health Organization estimated that DR accounted for 4.8% of the global blindness cases through the global visual impairment data.4 However, in clinical work, we found that good blood sugar control cannot prevent the occurrence of chronic microvascular complications; therefore, we speculate on the presence of other risk factors that affect DR and DKD occurrence.
Human serum albumin (sALB) is an aglycosylated protein synthesized and secreted by hepatocytes.5 Due to the free thiol group of Cys34, sALB can bind to a variety of small molecules of biological and clinical importance as a trap for reactive oxygen and nitrogen species, thereby participating in redox processes, and is the primary antioxidant exposed to continuous oxidative stress in the blood stream.6 Oxidative stress, as an “important promoter” of occurrence and development of diabetic microvascular complications,7 so in recent years, the relationship between sALB and diabetic microvascular complications has attracted attention. Studies have found that hypoalbuminemia is associated with poorer renal prognosis in patients with diabetic nephropathy;8 and sALB in non-proliferative DR and vision-threatening DR was significantly lower than that in patients without DR, and the prognostic nutritional index calculated based on sALB was inversely and independently correlated with the severity and prevalence of DR.9 However, both DKD and DR are microvascular complications of diabetes, and to our knowledge, their association with sALB has not been comprehensively simultaneously analyzed in one study.
Therefore, this study aimed to analyze the associations between sALB and T2DM complicated by DR and DKD, and investigate the association of sALB with the associated risk factors for microvascular disease, while considering the potential confounders, and subgroup analyze the based on patient characteristics.
Materials and Methods
Patients and Study Design
This retrospective analysis was conducted on inpatients with T2DM (aged 19–92 years) who underwent treatment from August 2020 to March 2022 at the Endocrinology Department of the First Affiliated Hospital of Nanchang University. Inclusion criteria were as follows: 1) participants aged 18 years and above and 2) with T2DM. Exclusion criteria were as follows: 1) type 1 diabetes; 2) diabetes secondary to other diseases; 3) retinopathy or nephropathy diagnosed before diabetes was diagnosed; 4) severe chronic liver disease; 5) suffering from cancer, acute diabetic complications, infection, and other serious circulatory, respiratory or digestive disorders; 6) pregnant or breastfeeding. The study protocol was approved by the Ethics Committee of the First Affiliated Hospital of Nanchang University, and the study was conducted in accordance with the declaration of Helsinki (Ethics number:2022-3-031).
Data Collection
Clinical data of study participants were collected from electronic medical records. Gender, age, diabetes duration, and the presence of complications were recorded for each patient. Participants’ height, weight, body mass index (BMI), systolic blood pressure (SBP), and diastolic blood pressure (DBP) were measured. BMI is calculated by dividing the weight (in kilograms) by the square of the height (in meters).
All patients underwent blood drawing after at least 8 h of fasting. Fasting plasma glucose (FPG), total cholesterol (TC), triglycerides (TG), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), alanine transaminase (ALT), aspartate aminotransferase (AST), serum creatinine (SCr), serum uric acid (SUA), and sALB were all measured by an automatic biochemistry analyzer (AU5421, Olympus, Shizuoka, Japan). Glycated hemoglobin (HbA1c) was measured using a glycated hemoglobin analyzer (D-10, Bio-Rad, Hercules, CA, USA).
The first urine sample in the morning was collected from each subject. The urinary albumin/creatinine ratio (UACR) was measured by automatic biochemical analyzer (Biosystems A25, Spain) or multifunctional urine analyzer (AUTION ELEVEN AE-4020). The estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation.10
In our study, DR was diagnosed by an ophthalmologist based on typical retinopathy changes on funduscopic examination due to diabetes, including background, pre-proliferative, proliferative, or maculopathy. DKD was defined as eGFR of <60 mL/min/1.73 m2 and/or UACR of ≥30 mg/g.11
Statistical Analyses
Non-normally distributed continuous data are expressed as median (25th–75th percentiles), and comparisons of continuous variables between groups were performed using Kruskal—Wallis test. Categorical variables are presented as numbers and percentages, and between-group differences are assessed using the chi-square test. Associations between sALB and other variables were examined using the Spearman correlation analysis and partial correlation analysis controlling for age, sex, BMI, and diabetes duration.
Unconditional logistic regression analysis was used to estimate odds ratios (OR) and 95% confidence intervals (CI) for the association between sALB and microvascular complications. Model 1 has no covariate adjustment, whereas model 2 was adjusted for age and sex, model 3 was adjusted for age, sex, diabetes duration, BMI, FPG, HbA1c, TC, TG and treatment of diabetes.
All data were analyzed by SPSS software (Version 26.0, SPSS Chicago, IL, USA), and P < 0.05 was considered statistically significant.
Results
Patient Characteristics
This study included 951 T2DM patients, 566 males (59.50%) and 385 females (40.50%), with a mean age of 56.25 ± 12.48 years. DR and DKD prevalence were 46.48% and 35.12%, respectively. The sALB concentrations of participants ranged from 20.5 to 53.2 g/L. Patients were divided into the following three groups according to sALB tertiles: Q1 (≤39.3 g/L), Q2 (39.4–42.4 g/L), Q3 (>42.4 g/L). Significant differences were found among the three groups in terms of age, gender, diabetes duration, BMI, SBP, DBP, HbA1c, TC, TG, ALT, AST, UACR, microalbuminuria, macroalbuminuria, eGFR, treatment of diabetes and the prevalence of DR and DKD (all P < 0.05). While no significant differences were found among the groups in terms of FPG, LDL-C, HDL-C, and SUA (all P > 0.05) (Table 1).
 |
Table 1 Baseline Patient Characteristics Based on sALB Tertiles |
DR, DKD and Macroalbuminuria Prevalence According to sALB Tertiles
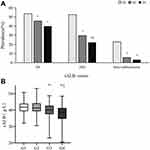
We investigated the association of the sALB levels with DR, DKD and macroalbuminuria prevalence. Decreasing prevalences of DR, DKD and macroalbuminuria were observed with increasing sALB tertiles (P = 0.003, P < 0.001 and P < 0.001 respectively). Further investigation revealed that the prevalence of DR and macroalbuminuria was significantly lower in Q2 and Q3 than in Q1 (all P < 0.05), while there was no significant difference between Q2 and Q3 (all P > 0.05); the prevalence of DKD was significantly lower in Q2 and Q3 than in Q1 (P < 0.001, P < 0.001, respectively), and significantly lower in Q3 than Q2 (P = 0.033) (Figure 1A).
All patients were subsequently divided into four groups based on the presence of DR and/or DKD, resulting in the following patient numbers: Group 1, 366 patients without DR or DKD; Group 2, 251 patients with DR only; Group 3, 143 patients with DKD only; and Group 4, 191 patients with DR and DKD. The medians of sALB in these four groups were 41.8g/L, 41.3g/L, 40.1g/L and 38.6g/L, respectively. The sALB levels in groups 1 and 2 were significantly higher than that in groups 3 and 4 (all P < 0.05), and the sALB level in group 3 was significantly higher than that in group 4 (P < 0.05). However, the difference in sALB levels between groups 1 and 2 was not statistically significant (P > 0.05) (Figure 1B).
Association Between sALB and Risk Factors Related to Microvascular Disease in Study Subjects
Spearman correlation analysis showed that sALB levels were positively correlated with BMI, DBP, TC, TG, ALT, AST and eGFR and negatively correlated with age, female, diabetes duration, SBP, HbA1c, UACR, and DR and DKD prevalence (all P < 0.05) (Table 2). After adjusting for age, gender, diabetic duration and BMI, the associations among sALB and metabolic parameters (SBP, TG, ALT, and HbA1c), DKD-related parameters (UACR and eGFR), and DR and DKD prevalence remained statistically significant (all P < 0.05) (Table 2).
 |
Table 2 Association Between sALB and Risk Factors Related to Microvascular Disease in Study Subjects |
Associations Between sALB Tertiles and Microvascular Disease in the Participants
In the multivariable-adjusted model, age, sex, diabetes duration, BMI, FPG, HbA1c, TC, TG, and treatment of diabetes were adjusted. The results of the fully adjusted covariate model using Q3 (>42.4 g/L) as reference revealed a significantly higher risk of DR [OR (95% CI) = 1.59 (1.12–2.26), P = 0.010], DKD [OR (95% CI) = 3.00 (2.04–4.41), P < 0.001] and macroalbuminuria [OR (95% CI) = 9.76 (4.62–20.63), P < 0.001] in Q1 (≤39.3 g/L) (Table 3). After stratification by sex and age, the effect of sALB of ≤39.3 g/L on DR incidence was more obvious in patients with male (OR: 1.60, 95% CI: 1.00–2.56), and aged<65 years (OR: 1.74, 95% CI: 1.14–2.65) (P < 0.05 for all); the effect of sALB≤39.3 g/L on the incidence of DKD was significant in both males (OR: 3.78, 95% CI: 2.26–6.32) and females (OR: 2.35, 95% CI: 1.26–4.35) (P < 0.05 for all), while only the age <65 years (OR: 3.46, 95% CI: 2.16–5.53) was significant in the age subgroup (P < 0.001) (Table 4).
 |
Table 3 Associations Between sALB Tertiles and Microvascular Disease in Study Subject |
 |
Table 4 Subgroup Analysis of Association Between sALB Tertiles and Microvascular Complication Stratified by Sex and Age |
Discussion
The present study revealed that the prevalence of DR, DKD and macroalbuminuria significantly increased with decreasing sALB levels, and patients with T2DM with DKD or DKD combined with DR had significantly lower sALB levels compared with patients without DR or DKD. The multivariate analysis showed that patients with lower sALB levels were consistently at a significantly higher risk of DR and DKD compared with patients with higher sALB levels and were strongly associated with DKD progression, despite adjustment for potential confounders. Further subgroup analysis showed that the effect of lower levels of sALB on the incidence of DR was more obvious in male T2DM patients aged <65 years, while the effect on the incidence of DKD was more obvious in T2DM patients aged <65 years. The clinical significance of the study lies in the comprehensive analysis of the association of sALB with DR and DKD in patients with T2DM and the first identification of the key population, for which this indicator is applicable.
sALB plays an important role in maintaining homeostasis and is the main carrier of endogenous and exogenous ligands, thereby contributing to the transport of various biochemical molecules in the body.12–15 Hypoalbuminemia may be caused by decreased energy or amino acid supply, impaired hepatic synthesis, increased losses, increased tissue catabolism or distribution problems, and it is a predictor of poor prognosis in many different conditions, not only in the disease setting but also in healthy populations.16 Patients with lower levels of sALB in our study were associated with the older age and lower BMI, possibly related to the decreased protein intake in frail elderly patients. Evidence suggests a high prevalence of diabetes in elderly and frail patients, and that frailty is a predictor of poor diabetes outcomes.17 Our study also showed that low sALB was a predictor of concurrent DR or DKD in patients with T2DM.
The higher risk of microvascular complications in the low sALB group may be due to the following reasons. First, sALB is an important extracellular antioxidant with multiple enzymatic activities, including glucuronidase activity, lipid peroxidase activity, and antioxidant activity.14 sALB reversibly or irreversibly binds glucuronide conjugates, thus reducing their plasma levels.18 Studies have shown that glucuronides are significantly associated with the long-term progression of microvascular complications in patients with type 1 diabetes,19 and the amount of p-cresol glucuronide is significantly increased in patients with chronic kidney disease, and the ultrafiltrate of patients with uremia contains oligosaccharides composed of glucuronide conjugates.20–22 Additionally, sALB inhibits erythrocyte membrane lipid peroxidation, whereas persistent hypoalbuminemia decreases serum antioxidant activity and increases oxidative cell damage.23 Moreover, sALB can inhibit oxidative stress by reducing reactive oxygen and nitrogen species (ROS and RNS, respectively) production and accumulation depending on glutathione-linked thiol peroxidase activity.14 Second, sALB has anti-inflammatory effects. Recent animal studies have shown that serum levels of proinflammatory cytokines, such as tumor necrosis factor alpha (TNF-α), interleukin-1 (IL-1), and C-reactive protein, in rats receiving albumin infusion and a high casein diet are significantly lower than those in hypoalbuminemia control.24 Studies have found that sALB may exert anti-inflammatory effects by specifically regulating cellular glutathione (GSH) levels and reducing TNF-α-mediated activation of nuclear factor kappa B (NF-κB).25 Additionally, sALB inhibits NF-κB activation in a GSH-independent manner, thereby inhibiting TNF-α-induced vascular cell adhesion molecule-1 (VCAM-1) expression and monocyte adhesion, exerting anti-inflammatory effects on vascular endothelial cells.26 Oxidative stress and inflammation are key factors in patients with T2DM with concurrent DR and DKD.27 The increased inflammation and oxidative stress caused by sALB reduction may be the main mechanism, by which sALB is associated with DR and DKD.
It is well known that a large amount of sALB is lost from the urine in DKD patients due to impaired renal filtration barrier,28 which is an important cause of hypoalbuminemia in DKD patients. This was also reflected in our study, with a higher proportion of macroalbuminuria in patients with low sALB, suggesting more severe renal disease. We also found that patients with low sALB were associated with higher HbA1c and longer duration of diabetes, and poor glycemic control and longer duration have been identified as risk factors for DKD and DR in previous studies.29,30 In addition, patients with low sALB are also accompanied by high SBP and low levels of ALT, which may also be the reason for the higher incidence of DKD and DR in patients with low sALB. Studies have shown that hypertension and ALT are related to the incidence of DKD and DR.31–33 Therefore, patients with T2DM with lower sALB levels must be screened for DR or DKD. Combined with our subgroup analysis results, DR screening should pay special attention to men aged <65 years, whereas DKD screening should pay attention to people <65 years old.
Additionally, sALB differences in DR or DKD alone and their comorbidities were also explored, which, to our knowledge, have not been found in previous studies. No significant difference was found in the sALB of patients with DR only compared with those without DR or DKD. None of the previous studies on sALB and DR excluded patients with co-morbidity of DR and DKD.9,34 The occurrence of this situation in our study may be related to the insufficient sample size of patients in the DR only group because some patients with DR are divided into the DR + DKD group, and the sALB level in the DR + DKD group is significantly lower than that in the DKD only, DR only, and control group. However, patients with DR and DKD comorbidities may have different pathophysiological mechanisms from those with DR or DKD alone. After all, studies have suggested that patients with DR and DKD comorbidities have different blood glucose phenotypes and uric acid levels compared with patients with DR or DKD alone.35,36 This should be further investigated in future studies.
This study has some limitations. First, retrospective studies cannot infer causality. Therefore, prospective studies are needed to confirm our findings. Second, all the study participants were inpatients, which may have selection bias. Third, due to the lack of clear grading of DR outcomes reported by ophthalmologists, and the lack of data on the severity of DR, the association between sALB and DR progression was not further analyzed, and future research is needed.
In general, we devoted little attention to low sALB as a predictor of DKD progression or DR screening, although sALB concentration can be measured easily in the clinical laboratory. Therefore, it is of great significance to clarify the clinical significance of sALB in DKD and DR and to identify the high-risk groups for application. More importantly, our findings revealed the potential of sALB as an interventional target for microvascular complications of diabetes.
Conclusions
In conclusion, our study shows that decreased sALB levels may be an independent risk indicator of DR and DKD in patients with T2DM, and significantly associated with DKD progression. DR screening should pay special attention to men aged <65 years, whereas DKD screening should pay attention to people <65 years old. Therefore, sALB levels in T2DM patients should be carefully monitored. Future studies are needed to verify whether modulating sALB levels could be beneficial to patients with DKD or DR.
Data Sharing Statement
All data in this study are available from the corresponding authors upon reasonable request.
Ethics Approval and Consent to Participate
Our study protocol had been approved by the research committee of the First Affiliated Hospital of Nanchang University (no. 2022-3-031). Local ethical committee approved the use of anonymized historic data for the study and waived informed consent from patients.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data, took part in drafting the article or revising it critically for important intellectual content, agreed to submit to the current journal, gave final approval to the version to be published, and agree to be accountable for all aspects of the work.
Funding
This study was supported by grants from the National Natural Science Funds of China (grant no. 82160140) and Key research and development projects of the Social Development Department of Jiangxi Provincial Department of Science and Technology (grant no. 20201BBG71006).
Disclosure
The authors declare that they have no competing interests.
References
1. Saeedi P, Petersohn I, Salpea P, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res Clin Pract. 2019;157:107843. doi:10.1016/j.diabres.2019.107843
2. Thomas MC, Brownlee M, Susztak K, et al. Diabetic kidney disease. Nat Rev Dis Prim. 2015;1:15018. doi:10.1038/nrdp.2015.18
3. Ting DS, Cheung GC, Wong TY. Diabetic retinopathy: global prevalence, major risk factors, screening practices and public health challenges: a review. Clin Experiment Ophthalmol. 2016;44:260–277. doi:10.1111/ceo.12696
4. Resnikoff S, Pascolini D, Etya’Ale D, et al. Global data on visual impairment in the year 2002. Bull World Health Organ. 2004;82:844–851.
5. Don BR, Kaysen G. Serum albumin: relationship to inflammation and nutrition. Semin Dial. 2004;17:432–437. doi:10.1111/j.0894-0959.2004.17603.x
6. Belinskaia DA, Voronina PA, Shmurak VI, Jenkins RO, Goncharov NV. Serum albumin in health and disease: esterase, antioxidant, transporting and signaling properties. Int J Mol Sci. 2021;22(19):10318. doi:10.3390/ijms221910318
7. Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010;107:1058–1070. doi:10.1161/CIRCRESAHA.110.223545
8. Zhang J, Zhang R, Wang Y, et al. The level of serum albumin is associated with renal prognosis in patients with diabetic nephropathy. J Diabetes Res. 2019;2019:7825804. doi:10.1155/2019/7825804
9. Yang L, Yu W, Pan W, et al. A clinical epidemiological analysis of prognostic nutritional index associated with diabetic retinopathy. Diabetes Metab Syndr Obes. 2021;14:839–846. doi:10.2147/DMSO.S295757
10. Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi:10.7326/0003-4819-150-9-200905050-00006
11. American Diabetes Association. 11. Microvascular complications and foot care: standards of medical care in diabetes-2021. Diabetes Care. 2021;44:S151–S167. doi:10.2337/dc21-S011
12. Gatta A, Verardo A, Bolognesi M. Hypoalbuminemia. Intern Emerg Med. 2012;7(Suppl 3):S193–S199. doi:10.1007/s11739-012-0802-0
13. Fanali G, Di Masi A, Trezza V, et al. Human serum albumin: from bench to bedside. Mol Aspects Med. 2012;33:209–290.
14. De Simone G, Di Masi A, Ascenzi P. Serum albumin: a multifaced enzyme. Int J Mol Sci. 2021;22(18):10086. doi:10.3390/ijms221810086
15. Belinskaia DA, Voronina PA, Shmurak VI, et al. The universal soldier: enzymatic and non-enzymatic antioxidant functions of serum albumin. Antioxidants. 2020;9. doi:10.3390/antiox9100966
16. Franch-Arcas G. The meaning of hypoalbuminaemia in clinical practice. Clin Nutr. 2001;20:265–269. doi:10.1054/clnu.2001.0438
17. Assar ME, Laosa O, Rodriguez ML. Diabetes and frailty. Curr Opin Clin Nutr Metab Care. 2019;22:52–57. doi:10.1097/MCO.0000000000000535
18. Mizuma T, Benet LZ, Lin ET. Interaction of human serum albumin with furosemide glucuronide: a role of albumin in isomerization, hydrolysis, reversible binding and irreversible binding of a 1-O-acyl glucuronide metabolite. Biopharm Drug Dispos. 1999;20:131–136. doi:10.1002/(SICI)1099-081X(199904)20:3<131::AID-BDD166>3.0.CO;2-X
19. Sell DR, Sun W, Gao X, et al. Skin collagen fluorophore LW-1 versus skin fluorescence as markers for the long-term progression of subclinical macrovascular disease in type 1 diabetes. Cardiovasc Diabetol. 2016;15:30. doi:10.1186/s12933-016-0343-3
20. Sell DR, Nemet I, Liang Z, Monnier VM. Evidence of glucuronidation of the glycation product LW-1: tentative structure and implications for the long-term complications of diabetes. Glycoconj J. 2018;35:177–190. doi:10.1007/s10719-017-9810-7
21. Le Moel G, Troupel S, Rottembourg J, et al. Glucuronoconjugates in chronic renal failure. Comparative determination with values in healthy adult. Biomater Artif Cells Artif Organs. 1987;15:191–197. doi:10.3109/10731198709118519
22. Liabeuf S, Glorieux G, Lenglet A, et al. Does p-cresylglucuronide have the same impact on mortality as other protein-bound uremic toxins? PLoS One. 2013;8:e67168. doi:10.1371/journal.pone.0067168
23. Soejima A, Matsuzawa N, Miyake N, et al. Hypoalbuminemia accelerates erythrocyte membrane lipid peroxidation in chronic hemodialysis patients. Clin Nephrol. 1999;51:92–97.
24. Utariani A, Rahardjo E, Perdanakusuma DS. Effects of albumin infusion on serum levels of albumin, proinflammatory cytokines (TNF-alpha, IL-1, and IL-6), CRP, and MMP-8; tissue expression of EGRF, ERK1, ERK2, TGF-beta, collagen, and MMP-8; and wound healing in sprague dawley rats. Int J Inflam. 2020;2020:3254017. doi:10.1155/2020/3254017
25. Cantin AM, Paquette B, Richter M, Larivee P. Albumin-mediated regulation of cellular glutathione and nuclear factor kappa B activation. Am J Respir Crit Care Med. 2000;162:1539–1546. doi:10.1164/ajrccm.162.4.9910106
26. Zhang WJ, Frei B. Albumin selectively inhibits TNF alpha-induced expression of vascular cell adhesion molecule-1 in human aortic endothelial cells. Cardiovasc Res. 2002;55:820–829. doi:10.1016/S0008-6363(02)00492-3
27. Barrett EJ, Liu Z, Khamaisi M, et al. Diabetic microvascular disease: an endocrine society scientific statement. J Clin Endocrinol Metab. 2017;102:4343–4410. doi:10.1210/jc.2017-01922
28. Qi C, Mao X, Zhang Z, Wu H. Classification and differential diagnosis of diabetic nephropathy. J Diabetes Res. 2017;2017:8637138. doi:10.1155/2017/8637138
29. Seid MA, Akalu Y, Gela YY, et al. Microvascular complications and its predictors among type 2 diabetes mellitus patients at Dessie town hospitals, Ethiopia. Diabetol Metab Syndr. 2021;13:86. doi:10.1186/s13098-021-00704-w
30. Cheema S, Maisonneuve P, Zirie M, et al. Risk factors for microvascular complications of diabetes in a high-risk middle east population. J Diabetes Res. 2018;2018:8964027. doi:10.1155/2018/8964027
31. Shi R, Niu Z, Wu B, et al. Nomogram for the risk of diabetic nephropathy or diabetic retinopathy among patients with Type 2 diabetes mellitus based on questionnaire and biochemical indicators: a cross-sectional study. Diabetes Metab Syndr Obes. 2020;13:1215–1229. doi:10.2147/DMSO.S244061
32. Yanagawa T, Koyano K, Azuma K. Retrospective study of factors associated with progression and remission/regression of diabetic kidney disease-hypomagnesemia was associated with progression and elevated serum alanine aminotransferase levels were associated with remission or regression. Diabetol Int. 2021;12:268–276. doi:10.1007/s13340-020-00483-1
33. Xu J, Shi X, Pan Y. The association of aspartate aminotransferase/alanine aminotransferase ratio with diabetic nephropathy in patients with Type 2 diabetes. Diabetes Metab Syndr Obes. 2021;14:3831–3837. doi:10.2147/DMSO.S330741
34. Zhu Y, Cai X, Liu Y, et al. Serum albumin, but not bilirubin, is associated with diabetic chronic vascular complications in a Chinese Type 2 diabetic population. Sci Rep. 2019;9:12086. doi:10.1038/s41598-019-48486-6
35. Liu G, Dou J, Zheng D, et al. Association between abnormal glycemic phenotypes and microvascular complications of Type 2 diabetes mellitus outpatients in China. Diabetes Metab Syndr Obes. 2020;13:4651–4659. doi:10.2147/DMSO.S242148
36. Hou L, Shi Y, Wang S, et al. Associations of serum uric acid level with diabetic retinopathy and albuminuria in patients with type 2 diabetes mellitus. J Int Med Res. 2020;48:1220763532. doi:10.1177/0300060520963980
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.