Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 16
Association Between Physical Activity and Arterial Stiffness in Patients with Type 2 Diabetes in Ningbo, China: A Cross-Sectional Study
Authors Li X , Chattopadhyay K , Chen X , Li J , Xu M , Chen X, Li L
Received 5 October 2023
Accepted for publication 12 December 2023
Published 18 December 2023 Volume 2023:16 Pages 4133—4141
DOI https://doi.org/10.2147/DMSO.S438344
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Juei-Tang Cheng
Xueyu Li,1,* Kaushik Chattopadhyay,2,* Xiaoting Chen,3 Jialin Li,1 Miao Xu,1 Xueqin Chen,4 Li Li1
1Department of Endocrinology and Metabolism, The First Affiliated Hospital of Ningbo University, Ningbo, People’s Republic of China; 2Lifespan and Population Health, School of Medicine, University of Nottingham, Nottingham, UK; 3School of Medicine, Ningbo University, Ningbo, People’s Republic of China; 4Department of Traditional Chinese Medicine, The First Affiliated Hospital of Ningbo University, Ningbo, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Li Li, Email [email protected]
Aim: The study aimed to investigate the association between physical activity and arterial stiffness in patients with type 2 diabetes mellitus (T2DM) in Ningbo, China.
Methods: A cross-sectional study was conducted using the Metabolic Management Center (MMC) dataset of The First Affiliated Hospital of Ningbo University from 1st March 2018 to 28th February 2023. 4444 adults with T2DM were included in the study. Physical activity was assessed using the International Physical Activity Questionnaire (IPAQ)-Short and was categorized into high, moderate, and low. Arterial stiffness was defined as brachial-ankle pulse wave velocity (baPWV) ≥ 1800cm/s or common carotid artery intima-media thickness (CCA IMT) ≥ 1mm. Multiple logistic regression analyses were performed to identify the association between physical activity and arterial stiffness.
Results: 6.5%, 47.0%, and 46.5% of patients with T2DM had high, moderate, and low physical activity, respectively. 18.8% and 17.5% of patients had arterial stiffness based on baPWV and CCA IMT, respectively. The odds of arterial stiffness (based on baPWV) were lower in patients having moderate to high physical activity (OR 0.82, 95% CI 0.68 to 0.98 and OR 0.58, 95% CI 0.39 to 0.87, respectively). The odds of arterial stiffness (based on CCA IMT) were found to be lower in patients having high physical activity (OR 0.49, 95% CI 0.33 to 0.74).
Conclusion: Higher physical activity was found to be associated with lower arterial stiffness in patients with T2DM in Ningbo, China. This was a cross-sectional study, and there is a need to conduct longitudinal studies on this topic.
Keywords: type 2 diabetes mellitus, physical activity, arterial stiffness, China
Introduction
Around 140 million people in China have diabetes, and the type 2 diabetes mellitus (T2DM) population in China is the largest in the world.1 T2DM is a chronic condition, and comorbidities like hypertension and hyperlipidemia are common.2 All these are risk factors for cardiovascular diseases (CVD).3 Compared to non-T2DM individuals, CVD risk is two-to-four-fold higher in patients with T2DM.1,4,5 CVD is one of the primary causes of death in patients with T2DM, accounting for approximately half of all deaths.6
Physical activity is an important lifestyle advice given to patients with T2DM.7 Regular physical activity can significantly improve insulin sensitivity, glucose metabolism, and body composition.8 It can also reduce the risk of developing CVD.9,10 However, physical activity levels are lower in China.11,12
Atherosclerosis is a well-known and main pathological basis of CVD.13 Arterial stiffness is one of the earliest stages of atherosclerosis14 and is an independent predictor of CVD even after adjusting for cardiovascular risk factors.15 Compared to healthy individuals, patients with T2DM have a higher risk of arterial stiffness.16 The increasing arterial stiffness with accumulating comorbidities like diabetes and hypertension is associated with heart failure with preserved ejection fraction.17 Brachial-ankle pulse wave velocity (baPWV) is a non-invasive method for detecting atherosclerosis and arterial stiffness in large and medium arteries.18 Common carotid artery intima-media thickness (CCA IMT) is another safe, simple, inexpensive, and widely used method for detecting atherosclerosis and arterial stiffness.19 High pulse wave velocity in China is a grave issue.20
Studies have been conducted to determine the relationship between physical activity and arterial stiffness in patients with T2DM, mostly focusing on carotid-femoral pulse wave velocity (cfPWV) or carotid artery thickness with inconsistent results.21–30 A study conducted in patients with T2DM in China reported that higher sedentary time was associated with higher carotid plaque.27 However, no study has examined the relationship between physical activity and multiple measures of arterial stiffness in patients with T2DM in China. Therefore, the study aimed to determine the association between physical activity and arterial stiffness, based on baPWV and CCA IMT, in patients with T2DM in Ningbo, China.
Materials and Methods
Study Design, Site, Population, Data Source, and Period
A cross-sectional study was conducted using the Metabolic Management Center (MMC) dataset of The First Affiliated Hospital of Ningbo University from 1st March 2018 to 28th February 2023. MMC, led by Ruijin Hospital, Shanghai, is a multi-hospital program on metabolic diseases that is running across mainland China.31 In the dataset, there were 5892 patients with metabolic diseases, and 4444 adults (18 to 75 years) with T2DM were included in this study. The rest were excluded due to the following reasons: <18 or >75 years of age (60), not T2DM (606), missing data on T2DM (130), missing or inaccurate physical activity data (49), and missing arterial stiffness data (603).
Data Collection and Study Variables
A standardized questionnaire developed and piloted by MMC is used for data collection, and parameters such as physiological, anthropometric, and biochemical are assessed by trained hospital staff using the MMC standardized protocol.31 In this study, the following routinely collected data from the first MMC visit were extracted and used:
- Self-reported sociodemographic factors:
- Age (18 to 39 years, 40 to 59 years, or ≥60 years)
- Sex (male or female)
- Self-reported lifestyle factors:
- Health-related conditions:
- Overweight/obesity (ie, body mass index (BMI) ≥24kg/m2,33 body weight and height were measured with light clothes and without shoes in standing position using a calibrated automatic digital body weight and height scale (HNH-318, Omron, Japan), body weight was measured to the nearest 0.1 kg, height was measured to the nearest 0.5 cm, and BMI was calculated as weight in kg divided by height in m2)
- High blood pressure (ie, systolic blood pressure ≥130 mmHg and/or diastolic blood pressure ≥80 mmHg on the day of visit using an automated blood pressure monitor (HBP-1100U, Omron, Japan) in a seated position, self-reported history of hypertension, or use of antihypertensive medicines)
- Hyperlipidemia (ie, low-density lipoprotein cholesterol ≥2.6 mmol/L or non-high-density lipoprotein cholesterol ≥2.6 mmol/L,34 determined by the enzymatic assays (AU5400, Beckman Coulter, USA), self-reported history of hyperlipidemia, or use of antihyperlipidemic medicines)
- Self-reported duration of T2DM (<5 years, ≥5 to <10 years, or ≥10 years)
- Glycosylated hemoglobin (HbA1c, <7% or ≥7%,35 measured using the high-performance liquid chromatographic method (D-10 Hemoglobin Analyzer, Bio-Rad, USA))
- Arterial stiffness (ie, baPWV ≥1800cm/s36 or CCA IMT ≥1mm37). BaPWV was measured by a specially trained nurse, using the plethysmography apparatus (BP-203RPE III, Omron, Japan). The assessment was conducted in a well-controlled room (with temperature around 22–25°C) and after resting for at least 5 minutes. Patients were requested not to smoke for at least 2–4 hours before the test. They were requested to wear light clothes, lie down on the examination bed in the supine position, and remain quiet during the test. Blood pressure cuffs were wrapped on both the arms and ankles. The lower edge of the arm cuff was positioned 2–3 cm above the cubital fossa transverse striation, and the lower edge of the ankle cuff was positioned 1–2 cm above the medial malleolus. The heartbeat monitor was placed on the left edge of the sternum, and electrocardiogram electrodes were placed on the wrists. BaPWV measurements on the left and right sides were taken, and the average of these was recorded.38 CCA IMT was assessed using the B-mode ultrasound (Resona R9T, Mindray, China), by an ultrasound doctor. CCA IMT was observed as a double line pattern visualized between the intimal-luminal and the medial-adventitial interfaces of the carotid wall in a longitudinal view. CCA IMT was measured near the carotid bifurcation in a region that was free of plaque (usually found in a segment of the distal CCA). The arterial wall was assessed in a lateral probe position with an insonification angle of 90° to acquire high-quality images. CCA IMT was measured along a 10 mm length, preferably on the posterior wall of the CCA at least 5 mm below its termination to avoid inter-individual variability. Manual measurements on the left and right sides were taken three times, and the average of these was recorded.39
Statistics Analyses
IBM SPSS Statistics version 28.0 for Windows was used for data analyses. Numbers and percentages are reported for categorical data and mean (standard deviation (SD)) and median (interquartile range (IQR)) are reported for normally distributed and skewed continuous data, respectively. Chi-square test was used to assess the correlations between physical activity and arterial stiffness (based on baPWV and CCA IMT) in patients with T2DM. To identify any association between physical activity and arterial stiffness, the following models were created:
- Unadjusted: Simple logistic regression analyses were performed.
- Adjusted: Multiple logistic regression analyses were performed, and the adjustment was done for age, sex, current smoker, current alcohol drinker, sedentary time, overweight/obesity, high blood pressure, hyperlipidemia, duration of T2DM, and HbA1c. Data missing (unknown) on the adjusted variables were included in the models.
Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated. A P value of ≤0.05 was considered statistically significant.
Ethics
Ethics approval was obtained from the Research Ethics Committees of Ruijin Hospital (2017-42) and The First Affiliated Hospital of Ningbo University (2019-R057). The study was conducted in accordance with the Declaration of Helsinki. All the patients gave written informed consent to use the routinely collected data on them for research purposes.
Results
Table 1 shows the characteristics of patients with T2DM in the study. Their mean age was 51.0 years (SD ±11.9), and 63.4% were men. 6.5%, 47.0%, and 46.5% of patients with T2DM had high, moderate, and low physical activity, respectively. 18.8% and 17.5% of patients had arterial stiffness based on baPWV and CCA IMT, respectively.
 |
Table 1 Characteristics of Patients with T2DM in the Study |
Table S1 shows the correlations between physical activity and arterial stiffness (based on baPWV and CCA IMT) in patients with T2DM. The proportion of arterial stiffness was found to be lower in patients having high physical activity, employing a p-value threshold of 0.05.
Table 2 reports the association between physical activity and arterial stiffness (based on baPWV) in patients with T2DM. In the adjusted model, the odds of arterial stiffness were lower in patients having moderate to high physical activity (OR 0.82, 95% CI 0.68 to 0.98 and OR 0.58, 95% CI 0.39 to 0.87, respectively).
 |
Table 2 Association Between Physical Activity and Arterial Stiffness (Based on baPWV) in Patients with T2DM |
Table 3 shows the association between physical activity and arterial stiffness (based on CCA IMT) in patients with T2DM. In the adjusted model, the odds of arterial stiffness were found to be lower in patients having high physical activity (OR 0.49, 95% CI 0.33 to 0.74).
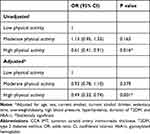 |
Table 3 Association Between Physical Activity and Arterial Stiffness (Based on CCA IMT) in Patients with T2DM |
Discussion
In the present study, higher physical activity was found to be associated with lower arterial stiffness in patients with T2DM in Ningbo, China. The finding is consistent with existing observational epidemiological studies conducted in Denmark, Canada, and Finland.23–25 A large cross-sectional study in China reported an association between sedentary time and arterial stiffness.27 Some randomized controlled trials (RCTs) conducted in Portugal and Greece reported a beneficial effect of physical activity on arterial stiffness,28,30 whereas no effect of the intervention was found in other RCTs conducted in the USA, Canada, Finland, and Norway.21,22,26,29 It is difficult to generalize the RCT findings due to several factors, including trial-related issues (eg, small sample sizes, shorter trial durations) and intervention-related issues (eg, heterogeneous content, structure, and delivery characteristics of interventions).
The underlying mechanism of how physical activity delays arteriosclerosis is still unclear and the following pathways are proposed: (i) physical activity improves blood glucose, blood pressure, and blood lipids, which are well-known risk factors of arterial stiffness,40 (ii) physical activity improves body composition, leading to fat mass reduction, which in turn inhibits the secretion of various inflammatory mediators (eg, interleukin-1β (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α)) that are known to cause atherosclerosis,41,42 and (iii) physical activity improves blood circulation and releases vasodilators (eg, nitric oxide) to improve blood and oxygen supply to the tissues and protects vascular endothelial cells, which in turn delays arteriosclerosis.43–45
To the best of the authors’ knowledge, this was the first study to investigate the association between physical activity and multiple measures of arterial stiffness in patients with T2DM in China. The study included a relatively large number of patients with T2DM, and the routinely collected data quality was high. Missing data on the adjusted variables were low in the study and were included in the adjusted models. However, the physical activity assessment was subjective, and IPAQ-Short was used for this purpose. Although IPAQ-Short is a well-tested tool,32 in the future, there is a need to assess physical activity objectively. Similarly, some other variables were self-reported (eg, other lifestyle factors, some health-related conditions), and social desirability bias and recall bias could have been an issue. Since it was a cross-sectional study, the causal relationship between physical activity and arterial stiffness could not be determined, and there is a need for longitudinal studies.
In conclusion, higher physical activity was found to be associated with lower arterial stiffness in patients with T2DM in Ningbo, China. This was a cross-sectional study, and there is a need to conduct cohort or case-control studies on this topic.
Data Sharing Statement
The dataset will be available upon request unless there are legal or ethical reasons for not doing so.
Acknowledgment
The authors thank Yanshu Chen and Miao Chen for managing the database. Xueyu Li and Kaushik Chattopadhyay have contributed equally to this work and share the first authorship.
Funding
This work was supported by the Major Science and Technology Projects for Health of Zhejiang Province [Grant No. WKJ-ZJ-2216] and Key Project of Scientific and Technological Innovation 2025 of Ningbo Science and Technology Bureau [Grant No. 2011ZDYF020003].
Disclosure
The authors report no conflicts of interest in this work.
References
1. Sun H, Saeedi P, Karuranga S, et al. IDF diabetes atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022;183:109–119. doi:10.1016/j.diabres.2021.109119
2. Li X, Chattopadhyay K, Xu S, et al. Prevalence of comorbidities and their associated factors in patients with type 2 diabetes at a tertiary care department in Ningbo, China: a cross-sectional study. BMJ Open. 2021;11(1):e040532. doi:10.1136/bmjopen-2020-040532
3. Teo K, Rafiq T. Cardiovascular risk factors and prevention: a perspective from developing countries. Can J Cardiol. 2021;37(5):733–743. doi:10.1016/j.cjca.2021.02.009
4. Taylor K, Heneghan C, Farmer A, et al. All-cause and cardiovascular mortality in middle-aged people with type 2 diabetes compared with people without diabetes in a large U.K. primary care database. Diabetes Care. 2013;36(8):2366–2371. doi:10.2337/dc12-1513
5. Harding J, Pavkov M, Magliano D, et al. Global trends in diabetes complications: a review of current evidence. Diabetologia. 2019;62(1):3–16. doi:10.1007/s00125-018-4711-2
6. Einarson T, Acs A, Ludwig C, et al. Prevalence of cardiovascular disease in type 2 diabetes: a systematic literature review of scientific evidence from across the world in 2007–2017. Cardiovasc Diabetol. 2018;17(1):83. doi:10.1186/s12933-018-0728-6
7. Kanaley J, Colberg S, Corcoran M, et al. Exercise/physical activity in individuals with type 2 diabetes: a consensus statement from the American college of sports medicine. Med Sci Sports Exerc. 2022;54(2):353–368. doi:10.1249/MSS.0000000000002800
8. Langleite T, Jensen J, Norheim F, et al. Insulin sensitivity, body composition and adipose depots following 12 w combined endurance and strength training in dysglycemic and normoglycemic sedentary men. Arch Physiol Biochem. 2016;122(4):167–179. doi:10.1080/13813455.2016.1202985
9. Jeong S, Kim S, Kang S, et al. Mortality reduction with physical activity in patients with and without cardiovascular disease. Eur Heart J. 2019;40(43):3547–3555. doi:10.1093/eurheartj/ehz564
10. Martinez-Gomez D, Cabanas-Sanchez V, Yu T, et al. Long-term leisure-time physical activity and risk of all-cause and cardiovascular mortality: dose-response associations in a prospective cohort study of 210 327 Taiwanese adults. Br J Sports Med. 2022;56(16):919–926. doi:10.1136/bjsports-2021-104961
11. Zhou Y, Wu J, Zhang S, et al. Prevalence and risk factors of physical inactivity among middle-aged and older Chinese in Shenzhen: a cross-sectional study. BMJ Open. 2018;8(10):e019775. doi:10.1136/bmjopen-2017-019775
12. Chen S, Liu Y, Hong J, et al. Co-existence of physical activity and sedentary behavior among children and adolescents in Shanghai, China: do gender and age matter? BMC Public Health. 2018;18(1):1287. doi:10.1186/s12889-018-6167-1
13. Sedding D, Boyle E, Demandt J, et al. Vasa vasorum angiogenesis: key player in the initiation and progression of atherosclerosis and potential target for the treatment of cardiovascular disease. Front Immunol. 2018;9:706. doi:10.3389/fimmu.2018.00706
14. Palombo C, Kozakova M. Arterial stiffness, atherosclerosis and cardiovascular risk: pathophysiologic mechanisms and emerging clinical indications. Vascul Pharmacol. 2016;77:1–7. doi:10.1016/j.vph.2015.11.083
15. Stanek A, Grygiel-Górniak B, Brożyna-Tkaczyk K, et al. The influence of dietary interventions on arterial stiffness in overweight and obese subjects. Nutrients. 2023;15(6):1440. doi:10.3390/nu15061440
16. Mohammedi K, Compaoré A, Potier L, et al. Outpatient measurement of arterial stiffness in patients with type 2 diabetes and obesity. J Diabetes. 2017;9(3):237–242. doi:10.1111/1753-0407.12405
17. Ali D, Tran P, Ennis S, et al. Rising arterial stiffness with accumulating comorbidities associates with heart failure with preserved ejection fraction. ESC Heart Fail. 2023;10(4):2487–2498. doi:10.1002/ehf2.14422
18. Wang A, Tao J, Guo X, et al. The product of resting heart rate times blood pressure is associated with high brachial-ankle pulse wave velocity. PLoS One. 2014;9(9):e107852. doi:10.1371/journal.pone.0107852
19. Stein J, Korcarz C, Hurst R, et al. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: a consensus statement from the American society of echocardiography carotid intima-media thickness task force. Endorsed by the society for vascular medicine. J Am Soc Echocardiogr. 2008;21(2):
20. Lu Y, Kiechl SJ, Wang J, et al. Global distributions of age- and sex-related arterial stiffness: systematic review and meta-analysis of 167 studies with 509,743 participants. EBioMedicine. 2023;92:104619. doi:10.1016/j.ebiom.2023.104619
21. Dobrosielski D, Gibbs B, Ouyang P, et al. Effect of exercise on blood pressure in type 2 diabetes: a randomized controlled trial. J Gen Intern Med. 2012;27(11):1453–1459. doi:10.1007/s11606-012-2103-8
22. Dasgupta K, Rosenberg E, Joseph L, et al. Physician step prescription and monitoring to improve ARTERial health (SMARTER): a randomized controlled trial in patients with type 2 diabetes and hypertension. Diabetes Obes Metab. 2017;19(5):695–704. doi:10.1111/dom.12874
23. Funck K, Laugesen E, Høyem P, et al. Low physical activity is associated with increased arterial stiffness in patients recently diagnosed with type 2 diabetes. Am J Hypertens. 2016;29(7):882–888. doi:10.1093/ajh/hpv197
24. Dasgupta K, Rosenberg E, Joseph L, et al. Carotid femoral pulse wave velocity in type 2 diabetes and hypertension: capturing arterial health effects of step counts. J Hypertens. 2017;35(5):1061–1069. doi:10.1097/HJH.0000000000001277
25. Metsämarttila E, Rodilla E, Jokelainen J, et al. Effect of physical activity on pulse wave velocity in elderly subjects with normal glucose, prediabetes or Type 2 diabetes. Sci Rep. 2018;8(1):8045. doi:10.1038/s41598-018-25755-4
26. Loimaala A, Groundstroem K, Rinne M, et al. Effect of long-term endurance and strength training on metabolic control and arterial elasticity in patients with type 2 diabetes mellitus. Am J Cardiol. 2009;103(7):972–977. doi:10.1016/j.amjcard.2008.12.026
27. Ke J, Li K, Ke T, et al. Association of sedentary time and carotid atherosclerotic plaques in patients with type 2 diabetes. J Diabetes. 2022;14(1):64–72. doi:10.1111/1753-0407.13242
28. Magalhães J, Melo X, Correia I, et al. Effects of combined training with different intensities on vascular health in patients with type 2 diabetes: a 1-year randomized controlled trial. Cardiovasc Diabetol. 2019;18(1):34. doi:10.1186/s12933-019-0840-2
29. Byrkjeland R, Stensæth K, Anderssen S, et al. Effects of exercise training on carotid intima-media thickness in patients with type 2 diabetes and coronary artery disease. Influence of carotid plaques. Cardiovasc Diabetol. 2016;15:13. doi:10.1186/s12933-016-0336-2
30. Kadoglou N, Fotiadis G, Kapelouzou A, et al. The differential anti-inflammatory effects of exercise modalities and their association with early carotid atherosclerosis progression in patients with type 2 diabetes. Diabet Med. 2013;30(2):e41–50. doi:10.1111/dme.12055
31. Zhang Y, Wang W, Ning G. Metabolic management center: an innovation project for the management of metabolic diseases and complications in China. J Diabetes. 2019;11(1):11–13. doi:10.1111/1753-0407.12847
32. Craig C, Marshall A, Sjöström M, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35(8):1381–1395. doi:10.1249/01.MSS.0000078924.61453.FB
33. Hu L, Huang X, You C, et al. Prevalence of overweight, obesity, abdominal obesity and obesity-related risk factors in southern China. PLoS One. 2017;12(9):e0183934. doi:10.1371/journal.pone.0183934
34. Grundy S, Stone N, Bailey A, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139(25):e1082–e1143. doi:10.1161/CIR.0000000000000625
35. American Diabetes Association. Standards of medical care in diabetes: 2018 abridged for primary care providers. Clin Diabetes. 2018;36(1):14–37. doi:10.2337/cd17-0119
36. Munakata M. Brachial-ankle pulse wave velocity in the measurement of arterial stiffness: recent evidence and clinical applications. Curr Hypertens Rev. 2014;10(1):49–57. doi:10.2174/157340211001141111160957
37. Jae S, Heffernan K, Fernhall B, et al. Cardiorespiratory fitness and carotid artery intima media thickness in men with type 2 diabetes. J Phys Act Health. 2012;9(4):549–553. doi:10.1123/jpah.9.4.549
38. Townsend RR, Wilkinson IB, Schiffrin EL, et al. Recommendations for improving and standardizing vascular research on arterial stiffness: a scientific statement from the American Heart Association. Hypertension. 2015;66(3):698–722. doi:10.1161/HYP.0000000000000033
39. Touboul PJ, Hennerici MG, Meairs S, et al. Mannheim Carotid Intima-Media Thickness and Plaque Consensus (2004–2006–2011): an update on behalf of the advisory board of the 3rd and 4th watching the risk symposium 13th and 15th European Stroke Conferences, Mannheim, Germany, 2004, and Brussels, Belgium, 2006. Cerebrovasc Dis. 2012;34(4):290–296. doi:10.1159/000343145
40. Orkaby A, Forman D. Physical activity and CVD in older adults: an expert’s perspective. Expert Rev Cardiovasc Ther. 2018;16(1):1–10. doi:10.1080/14779072.2018.1419062
41. Johnston E, Abbott R. Adipose tissue paracrine-, autocrine-, and matrix-dependent signaling during the development and progression of obesity. Cells. 2023;12(3):407. doi:10.3390/cells12030407
42. Ley K. Inflammation and atherosclerosis. Cells. 2021;10(5):1197. doi:10.3390/cells10051197
43. Colberg S, Hill L, Parson H, et al. Aerobic training increases skin perfusion by a nitric oxide mechanism in type 2 diabetes. Diabetes Metab Syndr Obes. 2010;3:275–280. doi:10.2147/DMSO.S12136
44. Fujie S, Hasegawa N, Horii N, et al. Aerobic exercise restores aging-associated reductions in arterial adropin levels and improves adropin-induced nitric oxide-dependent vasorelaxation. J Am Heart Assoc. 2021;10(10):e020641. doi:10.1161/JAHA.120.020641
45. Gao J, Pan X, Li G, et al. Physical exercise protects against endothelial dysfunction in cardiovascular and metabolic diseases. J Cardiovasc Transl Res. 2022;15(3):604–620. doi:10.1007/s12265-021-10171-3
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
