Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 17
Association Between Empirical Anti-Pseudomonal Antibiotics for Recurrent Lower Respiratory Tract Infections and Mortality: A Retrospective Cohort Study
Authors Shiroshita A , Yamamoto S, Anan K, Suzuki H, Takeshita M, Kataoka Y
Received 20 August 2022
Accepted for publication 13 November 2022
Published 17 November 2022 Volume 2022:17 Pages 2919—2929
DOI https://doi.org/10.2147/COPD.S386965
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Min Zhang
Akihiro Shiroshita,1– 3 Shungo Yamamoto,4 Keisuke Anan,3,5 Hokuto Suzuki,1 Masafumi Takeshita,1 Yuki Kataoka3,6– 8
1Department of Respiratory Medicine, Ichinomiyanishi Hospital, Ichinomiya, Japan; 2Division of Epidemiology, Department of Medicine, Vanderbilt University School of Medicine, Nashville, TN, USA; 3Scientific Research Works Peer Support Group (SRWS-PSG), Osaka, Japan; 4Department of Infectious Disease, Kyoto City Hospital, Kyoto, Japan; 5Division of Respiratory Medicine, Saiseikai Kumamoto Hospital, Kumamoto, Japan; 6Department of Internal Medicine, Kyoto Min-Iren Asukai Hospital, Kyoto, 606-8226, Japan; 7Section of Clinical Epidemiology, Department of Community Medicine, Kyoto University Graduate School of Medicine, Kyoto, 606-8501, Japan; 8Department of Healthcare Epidemiology, Kyoto University Graduate School of Medicine/Public Health, Kyoto, 606-8501, Japan
Correspondence: Akihiro Shiroshita, Department of Respiratory Medicine, Ichinomiyanishi Hospital, 1 Kaimeihira, Ichinomiya, Aichi Prefecture, 494-0001, Japan, Tel +81-80-3807-4960, Fax +81-586-48-0055, Email [email protected]
Purpose: Whether the empirical use of anti-pseudomonal antibiotics actually improves patient outcomes is unclear. Hence, we aimed to determine whether empirical anti-pseudomonal antibiotics are better than anti-pseudomonal antibiotics in treating patients with recurrent lower respiratory tract infections (LRTIs).
Patients and Methods: We extracted data from the Japanese nationwide database of the Real World Data Co., Ltd. Our target population was patients with LRTIs, defined as chronic obstructive pulmonary disease exacerbation and pneumonia. We included patients aged ≥ 40 years who were admitted for lower respiratory tract infections ≥ 2 times within 90 days. We excluded patients who had an event (death or transfer) within 24 h after admission. We ran a frailty model adjusted for the following confounding factors: number of recurrences, age, body mass index, activities of daily living, Hugh-Johns classification, altered mental status, oxygen use on admission, blood urea nitrogen, and systemic steroid use.
Results: We included 893 patients with 1362 observations of recurrent LRTIs. There were 897 (66%) observations in the non-anti-pseudomonal antibiotic group and 465 (34%) in the anti-pseudomonal group; the numbers of in-hospital deaths were 86/897 (10%) and 63/465 (14%), respectively. Our frailty model yielded an adjusted hazard ratio (HR) (anti-pseudomonal group/non-anti-pseudomonal group) of 1.49 (95% confidence interval, 1.03– 2.14).
Conclusion: The empirical use of anti-pseudomonal antibiotics was associated with a higher HR of in-hospital mortality than the use of non-anti-pseudomonal antibiotics. Physicians might need to consider limiting the prescription of anti-pseudomonal antibiotics based on background factors such as the patient’s baseline function and disease severity. Further studies are needed to evaluate the causal relationship between empirical anti-pseudomonal antibiotics and mortality, and identify specific patient population who benefit from empirical anti-pseudomonal antibiotics.
Keywords: clinical epidemiology, COPD, Emphysema, infection and inflammation, pneumonia
Plain Language Summary
Pseudomonas aeruginosa is associated with recurrent lower respiratory tract infections (LRTIs). However, to date, it remains unclear whether anti-pseudomonal antibiotics should be empirically used in recurrent lower respiratory tract infections (LRTIs) including COPD exacerbation and bacterial pneumonia. Our database research showed the empirical use of anti-pseudomonal antibiotics was associated with a high risk of in-hospital mortality. Physicians might need to carefully consider the use of anti-pseudomonal antibiotics depending on factors such as baseline function and disease severity. Further studies are needed to evaluate the causal relationship between empirical anti-pseudomonal antibiotics and mortality, and identify an appropriate patient population for empirical anti-pseudomonal antibiotics.
Introduction
Patients with lower respiratory tract infection (LRTI) often experience recurrent episodes of the disease.1,2 Recurrent LRTI is a risk factor for the detection of drug-resistant organisms, such as Pseudomonas aeruginosa (PA).3,4 Infection with PA is associated with poor outcomes.5–7 Thus, international guidelines recommend the empirical use of anti-pseudomonal antibiotics for recurrent chronic obstructive pulmonary disease (COPD) exacerbation and pneumonia, which are the main causes of LRTI.8,9
Despite these recommendations, whether the empirical use of anti-pseudomonal antibiotics actually improves patient outcomes is unclear.6,10–12 Furthermore, the detection of PA in a sputum culture does not necessarily indicate infection with PA.13 In a previous cohort study, the empirical use of anti-pseudomonal antibiotics did not shorten the length of hospital stay of patients with recurrent COPD exacerbation,14 and in this study, PA was detected in 22% of the sputum cultures. Thus, we hypothesized that recurrent LRTI does not necessarily support the use of anti-pseudomonal antibiotics as an empirical therapy and escalation therapy from narrow-spectrum antibiotics to broad-spectrum antibiotics when empirical therapy fails would be adequate in most cases. Hence, the current study aimed to evaluate the effect of empirical use of anti-pseudomonal antibiotics on clinically important outcomes in recurrent LRTI using data from a large-scale database.
Materials and Methods
Study Design
The current study was a database research study. We extracted data from Real World Data ([RWD] Real World Data Co., Ltd., Kyoto, Japan), which is a Japanese nationwide database maintained by the Health, Clinic, and Education Information Evaluation Institute (Kyoto, Japan) with support from Real World Data Co., Ltd. The database is composed of patient data from electronic medical records at 215 hospitals, including demographic data, diagnoses, procedures, drug prescriptions, and laboratory data. To confirm the validity of our patient selection algorithm, we performed a validation study at a tertiary care hospital (Ichinomiyanishi Hospital) in advance. The Institutional Review Board (IRB) of Ichinomiyanishi Hospital approved our validation study (approval number: 2021028). The requirement for written informed consent was waived because of the study’s retrospective design and patients were offered an online opt-out option. The IRB of Kyoto City Hospital approved our main study (approval number: 665), and the requirement for written informed consent was waived because the data was already deidentified. Patient data was maintained with confidentiality throughout the study. The study adheres to the Declaration of Helsinki, and This article adhered to the Reporting of studies Conducted using Observational Routinely-collected Data Statement (Supplementary Table 1).15
Patient Selection
The inclusion criteria were age ≥40 years and admission for LRTI two or more times within 90 days.9 LRTI was defined as the presence of COPD exacerbation and pneumonia, and acute bronchitis was not included because antibiotics are generally not indicated for it. Our patient selection algorithm is described in Supplementary Table 2. The patient enrolment period was dependent on the storage terms of the RWD database.
In the validation study at Ichinomiyanishi Hospital, we selected patients aged ≥40 years who were admitted for LRTI, identified by the International Classification of Diseases 10th revision, classification codes, between October 1, 2020, and September 30, 2021, regardless of the department. One of the three authors (HS, MT, and AS) reviewed the patients’ medical records during hospitalization and confirmed the diagnosis of LRTI. We defined LRTI based on the description of COPD exacerbation and pneumonia in the medical records. A description of pneumonia other than that of bacterial aetiologies and bronchitis was not accepted unless we found the description of COPD exacerbation.
Data Extraction
In the RWD database, a patient is followed longitudinally in the same hospital. We extracted the following patient data: age, admission date, type of admission (from home, health care facility, or another hospital), sex, body mass index, exercise tolerability and dyspnoea as assessed by the Hugh-Johns classification, activities of daily living (ADL) as assessed by the Barthel index, presence of comorbidities, oxygen use on admission, mental status on admission as assessed by the Japan Coma Scale, medications, procedures, discharge date, and prognosis. For patients with pneumonia, we evaluated the systolic blood pressure (≤90 mmHg or not) and type of immune deficiency, ie, malignancy or immune deficiency, from the administrative claims data. The definitions of the variables are listed in Supplementary Table 3.
Intervention
Antipseudomonal antibiotics are listed in Supplementary Table 4. We selected the anti-pseudomonal antibiotics administered at admission or the next day as an intervention, regardless of the dose and route of administration.
Outcomes
The primary outcome was in-hospital mortality. The secondary outcomes were hospital discharge, next hospitalization for LRTI, and development of Clostridioides difficile colitis (Supplementary Table 3).
Covariates
For the primary analysis, we selected clinically meaningful confounding factors from published literature: the number of recurrences, age, body mass index, ADL, Hugh-Johns classification, altered mental status, oxygen use at admission, blood urea nitrogen level, and systemic steroid use.16,17 The cut-off value of blood urea nitrogen was determined by the BAP-65 score, a validated clinical prediction score for COPD exacerbation.16 We set the cut-off values of ADL, Hugh-Johns classification, and altered mental status as approximate median values of each variable. Systemic steroid therapy was defined as oral or parenteral administration of systemic steroids on admission, regardless of the dose.
Statistical Analysis
Categorical variables were presented as numbers and percentages, and continuous variables, as medians and interquartile ranges (IQRs), except for age. In the survival analyses of the primary and secondary outcomes, we used cluster analyses because patient outcomes were nested within the patients, and the outcomes of the same patient were correlated to each other. Next, we used the one-component frailty model. We assumed that unobserved heterogeneity specific to a patient has a log-normal distribution. We imputed missing data with chained equations multiple imputation and performed statistical analyses within each imputed dataset. Then, we combined each result using Rubin’s combining rule.
We performed the following sensitivity analyses for the primary outcome: (1) a frailty model based on the gamma distribution of the unobserved heterogeneity specific to a patient, (2) additional adjustment for length of hospital stay, tracheal ventilation, mechanical ventilation and use of vasopressors during previous hospitalization, (3) Cox regression using robust standard error, (4) a complete case analysis, (5) another definition of the target population as patients with intervals ≤365 days, (6) another definition of empirical antibiotics therapy as the use of antibiotics only on the day of admission, and (7) a frailty model targeting patients who were administered empirical antibiotics.
In the subgroup analyses, we evaluated the association of empirical anti-pseudomonal antibiotics with COPD exacerbation and pneumonia. For patients with pneumonia, we incorporated information on systolic blood pressure and immunodeficiency status as additional confounding factors because these data were collected routinely in the database. In addition, we performed a subgroup analysis of patients who had at least one of the following risk factors for the presence of multidrug-resistant organisms: residence in a healthcare facility, dialysis, previous antibiotic use within 90 days, previous use of a systemic steroid or an immunosuppressive agent within 30 days, or previous use of antacids within 30 days.4,18,19 Statistical analysis was performed using the R software version 4.1.2.
Results
Validation Study
During the study period between October 1, 2020, and September 30, 2021, our patient selection algorithm selected 363 patients with LRTI. Among the selected patients, 335 patients were clinically diagnosed with COPD exacerbation or bacterial pneumonia. The positive predictive value of our patient selection algorithm was 92% (95% confidence interval [CI], 89–96%).
Descriptive Analysis
After excluding patients who died or were transferred to another hospital within 24 h after admission, our patient selection algorithm selected 893 patients and 1362 observations of recurrent LRTIs (Figure 1). There were 897 observations (66%) in the non-anti-pseudomonal antibiotic group and 465 (34%) in the anti-pseudomonal antibiotics group; the median intervals between repeat hospitalizations were 41 days (IQR, 24–62 days) and 41 days (IQR, 25–62 days), respectively. Approximately 80% (1085/1362) of the observations were from hospitals with more than 300 beds. There were no missing data on the length of hospital stay or outcomes.
The patients’ characteristics did not show any substantial differences between the anti-pseudomonal antibiotic group and the non-antipseudomonal antibiotic group (Table 1, Figure 2). In 1051/1362 (78%) of the observations, the patients used triple inhalers of inhaled corticosteroids, long-acting beta-agonists, and long-acting muscarinic antagonists before hospitalization. The most frequently prescribed agents in each group were ampicillin/sulbactam (40%) and piperacillin/tazobactam (45%) (Table 2). In 10 (2%) observations, non-anti-pseudomonal was switched to anti-pseudomonal therapy between 1 and 7 days after the initial therapy. Regarding empirical antibiotics, atypical respiratory pathogens such as Mycoplasma pneumonia, Legionella spp., and Chlamydia spp. were covered in 78/513 (15%) observations in the non-anti-pseudomonal antibiotic group and in 147/521 (28%) observations in the anti-pseudomonal antibiotics group. Subsequent coverage between 1 and 7 days after the initial therapy occurred in only nine observations. Subsequent coverage of methicillin-resistant Staphylococcus aureus (MRSA) between 1 and 7 days after the initial therapy occurred only in one observation.
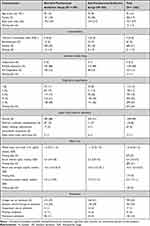 |
Table 1 Patient Characteristics per Observation |
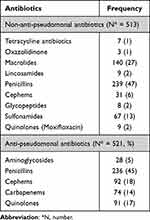 |
Table 2 Empirical Antibiotics Used in the Included Observations |
 |
Figure 2 The interval between hospitalizations and the number of recurrences in the non-anti-pseudomonal antibiotics group and the anti-pseudomonal antibiotics group. |
Primary Outcome
Table 3 summarizes the results of our statistical analyses of the risk of in-hospital mortality. The one-component frailty model found that the empirical use of anti-pseudomonal antibiotics was associated with a higher HR of in-hospital mortality than non-anti-pseudomonal antibiotics (crude hazard ratio [HR], 1.53; 95% CI, 1.03–2.25, and adjusted HR, 1.49; 95% CI, 1.03–2.14), and the sensitivity analyses showed similar trends. The coefficients of covariates in the main analysis are summarized in Supplementary Table 5.
 |
Table 3 Summary of the Statistical Analyses Evaluating the Risk of in-Hospital Mortality |
Secondary Outcomes
The one-component frailty model showed that the HRs (anti-pseudomonal antibiotics/non-anti-pseudomonal antibiotics) of hospital discharge and next hospitalization for LRTI were 0.772 (95% CI, 0.65–0.92) and 0.991 (95% CI, 0.87–1.13), respectively. Eleven patients (1.2%) in the non-anti-pseudomonal antibiotics group and 6 (1.3%) in the anti-pseudomonal antibiotics group were diagnosed with Clostridioides difficile colitis (risk ratio, 1.05; 95% CI, 0.39–2.83). We did not find any patient who was administered vancomycin orally.
Subgroup Analyses
Subgroup analysis of the patients with COPD exacerbation did not reveal a substantial difference in HR (HR, 0.68; 95% CI, 0.16–2.84). On the other hand, a subgroup analysis of patients with pneumonia who had at least one risk factor for the presence of multidrug-resistant organisms revealed an association between the empirical use of anti-pseudomonal antibiotics and in-hospital mortality (HRs [95% CI], 1.63 [1.04–2.54] and 1.59 [1.07–2.37], respectively).
Discussion
Summary of the Principal Findings
In our study, among patients with recurrent LRTI, the empirical use of anti-pseudomonal antibiotics was associated with a higher HR of in-hospital mortality than the use of non-anti-pseudomonal antibiotics. In addition, the use of anti-pseudomonal antibiotics was associated with a lower HR of hospital discharge. Empirical anti-pseudomonal antibiotics were used in only 34% of the observations, and non-anti-pseudomonal antibiotics were switched to anti-pseudomonal antibiotics in only 2% of the cases. These results suggest that physicians may not necessarily administer anti-pseudomonal antibiotics empirically to all patients with recurrent LRTIs.
Findings of the Present Study in Light of Published Evidence
The current study focused on populations susceptible to multi-drug resistant organisms. A previous cohort study revealed that for patients with pneumonia, the empirical use of broad-spectrum antibiotics was associated with a higher rate of 30 day-mortality than the use of narrow-spectrum antibiotics.12 However, the study excluded patients with two or more episodes of pneumonia within 12 months. Thus, in the current study, we focused on populations that are highly susceptible to multidrug-resistant organisms.3,4 Similar to the previous study,12 the results of this study favoured the empirical use of non-anti-pseudomonal antibiotics over that of anti-pseudomonal antibiotics.
Implications for Practice or Policy
Considering the non-mortality outcomes with the empirical use of non-anti-pseudomonal antibiotics, it might be an acceptable treatment strategy. Furthermore, the use of narrow-spectrum antibiotics is associated with lower subsequent infections, fewer adverse reactions, and lower costs than the use of broad-spectrum antibiotics.12,20,21 Although we could not draw a valid conclusion regarding the difference in the number of Clostridioides difficile colitis cases, the association between the use of broad-spectrum antibiotics and subsequent Clostridioides difficile colitis is obvious.12,22 Currently, the increasing prevalence of multidrug-resistant organisms is a global concern, and our results show that the unnecessary use of broad-spectrum antibiotics might be avoided.23
It is unlikely that our results were influenced by the subsequent coverage of other organisms. Although atypical respiratory pathogens were covered in 17% of the observations of empirical therapy, subsequent antibiotic administration for atypical respiratory pathogens occurred only in nine observations. Considering the extremely low number of observations (one observation) of subsequent administrations of anti-MRSA agents, our study results were not influenced by MRSA coverage. Thus, it is unlikely that our conclusions were skewed by subsequent antibiotic coverage.
In our study, empirical anti-pseudomonal antibiotics were used in only 34% of the observations. This result contradicts the recommendation of international guidelines,8,9 and it may reflect the complexity of the physician’s decision-making process for prescribing anti-pseudomonal antibiotics, which is based on various patient (eg, baseline function, comorbidities, disease severity, previous detection of multidrug resistant organisms) and environmental factors (eg, proportion of multidrug resistant organisms and access to hospital). Our study could not clarify this complex interplay, thus highlighting the need for further studies to validate the result and identify specific patient populations to whom administered anti-pseudomonal antibiotics should be administered. The caveat is that our study does not support avoiding anti-pseudomonal antibiotics in all recurrent LRTIs. Physicians should use anti-pseudomonal antibiotics on a case-by-case basis.
Strengths and Limitations of the Study
Our study has several strengths. First, we used data from a large-scale database in Japan. The large sample size and the abundant number of variables enabled us to evaluate hard outcomes after adjusting for multiple known confounding factors. In a previous study, inappropriate coverage of PA was associated with high mortality, but the study was not adjusted for patient baseline function and severity of LRTI.6 Our study overcame this issue and drew a reliable conclusion.
Second, we performed several sensitivity analyses to confirm the robustness of our study results. A frailty model can account for the unobserved heterogeneity. Considering the violation of the model assumptions, such as the correlation between independent variables and unobserved heterogeneity, for the sensitivity analysis, we applied a conservative method using robust standard errors. In addition, considering the measurement errors, we set different definitions for the target population and interventions. The trends of all the results were the same. Thus, we obtained robust results.
However, our study had several limitations. First, we were unable to adjust our model for unmeasured confounding factors. For example, sputum culture results could not be collected from the database. The previous detection of PA is a risk factor for LRTI due to PA and could influence the physician’s decision regarding the antibiotic prescription.4,24 Some studies have suggested an association between PA isolation and poor clinical outcomes.25,26 Additionally, disease severity may not have been adequately considered. For example, because of the limited variables in our dataset, we could not adjust for disease severity scores as well as respiratory effort, heart rate, and arterial blood gas analysis results.27 We mitigated the confounding bias by adjusting for proxies of disease severity such as oxygen use on admission. Furthermore, we could not adjust for other baseline factors such as pulmonary hypertension, concomitant pulmonary fibrosis, and the duration of diseases. These factors could be residual confounding factors. Because an observational study cannot fully cope with the confounding by indication; large-scale randomized controlled trials are required to address this limitation. Second, many variables in our study were dependent on administrative claims data, and their validity was not sufficiently evaluated.28 To overcome this limitation, we confirmed the validity of the patient selection algorithm and performed multiple sensitivity analyses. Third, in the RWD database, we could only follow patients in the same hospital; hence, the number of recurrences may have been underestimated, and this may have skewed the estimates of the treatment effects. Fourth, despite conducting various subgroup analyses, we could not identify any subgroups who would specifically benefit from empirical anti-pseudomonal antibiotics. Therefore, further research is needed to identify potential treatment heterogeneity and patient populations who can benefit from empirical anti-pseudomonal antibiotics. Fifth, due to the limited sample size and the [potential influence of post-treatment factors, we could not evaluate whether the choice of antibiotics influenced the patient outcomes after discharge.
Conclusion
The empirical use of anti-pseudomonal antibiotics for recurrent LRTI was associated with higher mortality than the use of non-anti-pseudomonal antibiotics. Physicians might need to consider limiting the empirical use of anti-pseudomonal antibiotics depending on background factors such as the patient’s baseline function and disease severity. Further RCTs are needed to address residual confounding factors and evaluate the causal relationship between the empirical use of anti-pseudomonal antibiotics and mortality.
Abbreviations
ADL, activities of daily living; CI, confidence interval; COPD, chronic obstructive pulmonary disease; HR, hazard ratio; IQR, interquartile range; LRTI, lower respiratory tract infection; MRSA, methicillin-resistant Staphylococcus aureus; PA, Pseudomonas aeruginosa; RWD, Real World Data.
Data Sharing Statement
The data that support the findings of this study are available on request from the corresponding author, AS. The data are not publicly available due to restrictions of the Real World Data Co., Ltd.
Ethics Approval and Informed Consent
The Institutional Review Board (IRB) of Ichinomiyanishi Hospital approved our validation study (approval number: 2021028). The requirement for written informed consent was waived because of the study’s retrospective design and patients were offered an online opt-out option. The IRB of Kyoto City Hospital approved our main study (approval number: 665), and the requirement for written informed consent was waived because the data was already deidentified. Patient data was maintained with confidentiality throughout the study.
Consent for Publication
All authors agree to the publish statements.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.”.
Funding
Our study was funded by a grant from Real World Data, Co., Ltd. The funder played no role in the study design, study execution, data analyses, data interpretation, or decision to submit the results.
Disclosure
SY received a research grant from Real World Data, Co., Ltd for the study. The other authors have nothing to disclose in this study.
References
1. Dang TT, Majumdar SR, Marrie TJ, Eurich DT. Recurrent pneumonia: a review with focus on clinical epidemiology and modifiable risk factors in elderly patients. Drugs Aging. 2015;32(1):13–19. doi:10.1007/s40266-014-0229-6
2. Hurst JR, Vestbo J, Anzueto A, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363(12):1128–1138. doi:10.1056/NEJMoa0909883
3. Almirall J, Serra-Prat M, Bolíbar I, Balasso V. Risk factors for community-acquired pneumonia in adults: a systematic review of observational studies. Respiration. 2017;94(3):299–311. doi:10.1159/000479089
4. Garcia-Vidal C, Almagro P, Romaní V, et al. Pseudomonas aeruginosa in patients hospitalised for COPD exacerbation: a prospective study. Eur Respir J. 2009;34(5):1072–1078. doi:10.1183/09031936.00003309
5. Eklöf J, Sørensen R, Ingebrigtsen TS, et al. Pseudomonas aeruginosa and risk of death and exacerbations in patients with chronic obstructive pulmonary disease: an observational cohort study of 22 053 patients. Clin Microbiol Infect. 2020;26(2):227–234. doi:10.1016/j.cmi.2019.06.011
6. Sibila O, Laserna E, Maselli DJ, et al. Risk factors and antibiotic therapy in P aeruginosa community-acquired pneumonia. Respirology. 2015;20(4):660–666. doi:10.1111/resp.12506
7. Cillóniz C, Gabarrús A, Ferrer M, et al. Community-acquired pneumonia due to multidrug- and non-multidrug-resistant Pseudomonas aeruginosa. Chest. 2016;150(2):415–425. doi:10.1016/j.chest.2016.03.042
8. Global Initiative for Chronic Obstructive Lung Disease. Gold reports; 2022. Available from: https://goldcopd.org/2022-gold-reports/.
9. Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200(7):e45–e67. doi:10.1164/rccm.201908-1581ST
10. Attridge RT, Frei CR, Restrepo MI, et al. Guideline-concordant therapy and outcomes in healthcare-associated pneumonia. Eur Respir J. 2011;38(4):878–887. doi:10.1183/09031936.00141110
11. Rothberg MB, Zilberberg MD, Pekow PS, et al. Association of guideline-based antimicrobial therapy and outcomes in healthcare-associated pneumonia. J Antimicrob Chemother. 2015;70(5):1573–1579. doi:10.1093/jac/dku533
12. Webb BJ, Sorensen J, Jephson A, Mecham I, Dean NC. Broad-spectrum antibiotic use and poor outcomes in community-onset pneumonia: a cohort study. Eur Respir J. 2019;54(1):1900057. doi:10.1183/13993003.00057-2019
13. Sando E, Suzuki M, Ishida M, et al. Definitive and indeterminate Pseudomonas aeruginosa infection in adults with community-acquired pneumonia: a prospective observational study. Ann Am Thorac Soc. 2021;18(9):1475–1481. doi:10.1513/AnnalsATS.201906-459OC
14. Shiroshita A, Miyakoshi C, Tsutsumi S, et al. Effectiveness of empirical anti-pseudomonal antibiotics for recurrent COPD exacerbation: a multicenter retrospective cohort study. Sci Rep. 2021;11(1):20066. doi:10.1038/s41598-021-99640-y
15. Benchimol EI, Smeeth L, Guttmann A, et al. The REporting of studies Conducted using Observational Routinely-collected health Data (RECORD) statement. PLOS Med. 2015;12(10):e1001885. doi:10.1371/journal.pmed.1001885
16. Tabak YP, Sun X, Johannes RS, Gupta V, Shorr AF. Mortality and need for mechanical ventilation in acute exacerbations of chronic obstructive pulmonary disease: development and validation of a simple risk score. Arch Intern Med. 2009;169(17):1595–1602. doi:10.1001/archinternmed.2009.270
17. Fine MJ, Auble TE, Yealy DM, et al. A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med. 1997;336(4):243–250. doi:10.1056/NEJM199701233360402
18. Webb BJ, Dascomb K, Stenehjem E, Dean N. Predicting risk of drug-resistant organisms in pneumonia: moving beyond the HCAP model. Respir Med. 2015;109(1):1–10. doi:10.1016/j.rmed.2014.10.017
19. Shindo Y, Ito R, Kobayashi D, et al. Risk factors for drug-resistant pathogens in community-acquired and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2013;188(8):985–995. doi:10.1164/rccm.201301-0079OC
20. Baggs J, Jernigan JA, Halpin AL, Epstein L, Hatfield KM, McDonald LC. Risk of subsequent sepsis within 90 days after a hospital stay by type of antibiotic exposure. Clin Infect Dis. 2018;66(7):1004–1012. doi:10.1093/cid/cix947
21. Shehab N, Patel PR, Srinivasan A, Budnitz DS. Emergency department visits for antibiotic-associated adverse events. Clin Infect Dis. 2008;47(6):735–743. doi:10.1086/591126
22. Chalmers JD, Al-Khairalla M, Short PM, Fardon TC, Winter JH. Proposed changes to management of lower respiratory tract infections in response to the Clostridium difficile epidemic. J Antimicrob Chemother. 2010;65(4):608–618. doi:10.1093/jac/dkq038
23. Freedberg DE, Salmasian H, Cohen B, Abrams JA, Larson EL. Receipt of antibiotics in hospitalized patients and risk for Clostridium difficile infection in subsequent patients who occupy the same bed. JAMA Intern Med. 2016;176(12):1801–1808. doi:10.1001/jamainternmed.2016.6193
24. Gross AE, Van Schooneveld TC, Olsen KM, et al. Epidemiology and predictors of multidrug-resistant community-acquired and health care-associated pneumonia. Antimicrob Agents Chemother. 2014;58(9):5262–5268. doi:10.1128/AAC.02582-14
25. Almagro P, Salvadó M, Garcia-Vidal C, et al. Pseudomonas aeruginosa and mortality after hospital admission for chronic obstructive pulmonary disease. Respiration. 2012;84(1):36–43. doi:10.1159/000331224
26. Fine MJ, Smith MA, Carson CA, et al. Prognosis and outcomes of patients with community-acquired pneumonia. A meta-analysis. JAMA. 1996;275(2):134–141. doi:10.1001/jama.275.2.134
27. Quintana JM, Esteban C, Unzurrunzaga A, et al. Predictive score for mortality in patients with COPD exacerbations attending hospital emergency departments. BMC Med. 2014;12(1):66. doi:10.1186/1741-7015-12-66
28. Yamana H, Moriwaki M, Horiguchi H, Kodan M, Fushimi K, Yasunaga H. Validity of diagnoses, procedures, and laboratory data in Japanese administrative data. J Epidemiol. 2017;27(10):476–482. doi:10.1016/j.je.2016.09.009
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

