Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 18
Association Between C-Reactive Protein and Albumin Ratios and Risk of Mortality in Patients with Chronic Obstructive Pulmonary Disease
Received 23 March 2023
Accepted for publication 14 September 2023
Published 18 October 2023 Volume 2023:18 Pages 2289—2303
DOI https://doi.org/10.2147/COPD.S413912
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 6
Editor who approved publication: Dr Richard Russell
Shenghua Shen, Yuan Xiao
Department of Pulmonary and Critical Care Medicine, The Affiliated Changzhou Second People’s Hospital of Nanjing Medical University, Changzhou, Jiangsu, 213004, People’s Republic of China
Correspondence: Yuan Xiao, Department of Pulmonary and Critical Care Medicine, The Affiliated Changzhou Second People’s Hospital of Nanjing Medical University, 29 Xinglong Lane, Tianning District, Changzhou, Jiangsu, 213004, People’s Republic of China, Tel +86 13775118679 ; +86 519-88125317, Email [email protected]
Purpose: This study was to evaluate the association between C-reactive protein (CRP) to albumin (ALB) ratio (CAR) and the risk of mortality in patients with chronic obstructive pulmonary disease (COPD).
Methods: In this retrospective cohort study, clinical data were extracted from the National Institutes of Health National Health and Nutrition Examination Survey (NHANES) database between 2007 and 2010. Cox proportional hazard regressions were performed to assess the association between CAR and 5-year mortality in COPD patients. Subgroup analyses were applied to identify the consistency of the association based on the severity of COPD, gender, body mass index (BMI), smoking status, cardiovascular disease (CVD), and chronic kidney disease (CKD), and diabetes. The area under the curve (AUC) of the receiver operator characteristic (ROC) curve analysis was used to evaluate the predictive performance of CAR.
Results: A total of 1210 COPD patients were included, of which 110 COPD patients (9.09%) had 5-year mortality. The mean follow-up was 57.76 (0.33) months. A higher CAR was associated with an increased risk of 5-year mortality in COPD patients [hazard ratio (HR): 1.94, 95% confidence interval (CI): 1.07 to 3.50, P =0.029)]. Subgroup analysis showed that the association between CAR and mortality was especially suitable for COPD patients with mild COPD, in COPD patients who were still smoking, in COPD patients with BMI <=29.9 kg/m2, in COPD patients who were without CVD, in COPD patients who were without diabetes, and COPD patients who were without CKD. The AUCs of CAR for predicting 1-year, 3-year, and 5-year mortality in COPD patients were 0.735, 0.615, and 0.608, respectively.
Conclusion: CAR is strongly correlated with mortality in patients with COPD and CAR could be served as a prognostic biomarker for patients with COPD. This study may provide a promising prognostic biomarker for risk stratification and clinical management of patients with COPD.
Keywords: C-reactive protein, albumin, mortality, chronic obstructive pulmonary disease
Introduction
Chronic obstructive pulmonary disease (COPD) is a devastating lung disease characterized by incomplete and reversible airflow restriction,1 affecting more than 250 million people worldwide.2 COPD is a common and prevalent disease that poses a serious threat to human health, ranking as the fourth cause of mortality worldwide, and representing a challenge to public health.3 With an increasing smoking population, the prevalence and mortality of COPD are increasing every year, and it is expected that more than 4.5 million people will die from COPD annually by 2030.4 The World Health Organization (WHO) predicts that COPD will become the third leading cause of death worldwide by 2030.5,6 Therefore, the search for factors closely associated with the risk of death in patients with COPD may be crucial for the management of COPD.
Factors independently related to an increase in mortality of COPD include age, race, gender, cigarette smoking, the number of comorbidities, body mass index (BMI), physical activity, air pollution, arterial carbon dioxide tension (PaCO2), and hospitalization characteristics.7–13 In addition, inflammation plays an important role in the pathogenesis and prognosis of COPD.14 C-reactive protein (CRP), an acute-phase protein produced by the liver, is a marker of inflammation in the body.15 According to a previous study, higher levels of CRP were observed in COPD patients compared with healthy controls.16 Another study suggests that high baseline CRP levels are significantly associated with higher late mortality in patients with COPD.17 In addition to inflammation, the nutritional status of the body is also an important factor that influences the prognosis of patients with COPD.18 Albumin (ALB) has been used as a biochemical indicator of nutritional status.19 Qiu et al reported that a low level of serum ALB might be one of the poor prognostic markers in COPD patients admitted to the intensive care unit (ICU).20 Nevertheless, ALB was also found not related to the nutritional status but was associated with systemic inflammation.21 Moreover, serum ALB level can be affected by age, nutritional status, proteinuria, fluid status, and liver dysfunction.22,23 On the other hand, the CRP level is susceptible to a variety of physiological and pathological factors, such as acute or chronic infections, anti-infective and anti-inflammatory drugs and autoimmune diseases.24 Therefore, it may be more useful to find combined indicators to assess the prognosis of patients with COPD. Recently, a new scoring index, the CRP to ALB ratio (CAR) has been applied to evaluate the prognosis of several diseases,25–27 including lung cancer.26 Additionally, a study has found that a higher CAR was associated with a higher risk of malnutrition in hospitalized COPD patients.28 Further research is needed to investigate the relationship between CAR and mortality risk in patients with COPD.
Herein, the objective of this study was to evaluate the association between CAR and risk of mortality in patients with COPD and evaluate the predictive value of CAR for mortality risk in patients with COPD.
Methods
Data Sources and Study Participants
This study was designed to be a retrospective cohort study. Data were from the National Institutes of Health National Health and Nutrition Examination Survey (NHANES) database between 2007 and 2010. NHANES used a multistage stratified probability design in a sample population to obtain a nationally representative sample of the non-institutionalized civilian United States. Data from these samples included demographic information, dietary data, anthropometric data, laboratory data, and questionnaire data.
The inclusion criteria were: (1) age ≥ 18 years old; (2) patients diagnosed with COPD; (3) measurement of CRP and ALB. Exclusion criteria were (1) missing survival information. Of 77,478 participants, we excluded 75,540 participants who were not with COPD, 678 participants with missing CRP data, 21 participants with missing ALB, and 29 participants younger than 18 years. Finally, 1210 participants were used in the study. Data screening and population selection are shown in Figure 1. The study was approved by the National Center for Health Statistics’ Ethics Review Board (https://www.cdc.gov/nchs/nhanes/irba98.htm), and informed consent was obtained from all NHANES participants. Patient informed consent was not required for retrospective studies.
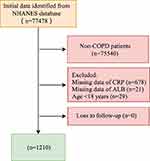 |
Figure 1 Diagram flow of data screening and population selection. |
Data Collection
All data were collected and recorded by uniformly trained investigators Data used in this study included (1) demographics: age (years), gender, race/ethnicity, education level, marital status, the poverty income ratio (PIR), BMI (kg/m2), and smoking status; (2) comorbidities: hypertension, diabetes, dyslipidemia, cardiovascular disease (CVD), whether close relative had a heart attack, chronic kidney disease (CKD), chronic bronchitis, asthma, intestines problem, and cancer; (3) characteristics of COPD: COPD severity, baseline forced expiratory volume in 1 second (FEV1, mL), and baseline forced vital capacity (FVC, mL); (4) information on treatment: bronchodilator, and inhaled corticosteroids (ICS); (5) biochemical indicators: segmented neutrophils num (1000 cell/UL), lymphocyte number (1000 cells/UL), eosinophils number (1000 cells/UL), alanine aminotransferase (ALT, U/L), aspartate aminotransferase (AST, U/L), ALB (g/L), CRP (mg/dL), and CAR.
Definitions and Measurements
We used the GOLD criterion: FEV1/FVC < 0.70 to define COPD.29 Gender was categorized as Male or Female. Race included Mexican Americans, non-Hispanic whites, non-Hispanic Blacks, and other races. Education level was defined as less than 9th grade, 9th-11th grade, high school grade, some college or associate of arts (AA) degree, college graduate or above. Marital status was grouped into married, widowed, divorced, separated, never married, and living with a partner. The PIR is an index of poverty status that is total family income divided by the poverty threshold. BMI was calculated as weight (kg) divided by the square of the height in meters (m2) and was classified into BMI <=24.99 kg/m2, ≥25–29.9 kg/m2, 25 to 29.9 kg/m2, and ≥30 kg/m2. Smoking status was grouped into never smoking, current smoking, and quit smoking. Hypertension, diabetes, dyslipidemia, CVD, whether a close relative had a heart attack, CKD, Chronic bronchitis, asthma, and cancer were reported and classified into two categories (no or yes). Intestine problems were classified as chronic constipation, chronic diarrhea, and No. Hypertension was defined as mean systolic blood pressure (BP) ≥140 and/or mean diastolic BP ≥90 mmHg and/or a positive response to the question “Are you currently taking medication to lower your BP?”.30 Diabetes was determined based on laboratory tests, self-reports, and medication history. Diabetes laboratory diagnostic criteria were fasting blood glucose ≥ 7.0 mmol/L or glycosylated hemoglobin (HbAlc) ≥ 6.5% or self-reported diabetes or receiving hypoglycemic therapy.31 Dyslipidemia was defined as having any one of the following: High triglyceride (TG) level: TG ≥ 150 mg/dl (3.89 mmol/l); Hypercholesterolemia: total cholesterol (TC) ≥ 200 mg/dl (5.18 mmol/l), low-density lipoprotein cholesterol (LDL-C) ≥130 mg/dl (3.37 mmol/l), and high-density lipoprotein cholesterol (HDL-C) <40 mg/dl (1.04 mmol/l [males]) and 50 mg/dl (1.30 mmol/l [females]).32 Participants were identified as having a history of CVD if they answered “Yes” to any of the 5 questions asking about a history of congestive heart failure, coronary heart disease, angina/angina pectoris, heart attack, and stroke.33 CKD was defined as an estimated glomerular filtration rate <60 mL/min/1.73 m2 or an albumin-to-creatinine ratio ≥30 mg/g.34 Chronic bronchitis was determined using a self-questionnaire from the NHANES.35 Asthma was defined as those who reported ever being told that they had asthma and who had an asthma attack in the past year (https://wwwn.cdc.gov/Nchs/Nhanes/). Asthma: participants ≥12 years of age that a) reported ever being told by a doctor or other health professional that they had or still have asthma or b) had an episode of asthma or an asthma attack during the past 12 months.36
Outcome Measures
The outcome of this study was 5-year overall mortality in patients with COPD. The mean follow-up was 57.76 (0.33) months.
Statistical Analysis
Continuous variables were expressed as mean +- standard error (S.E.) and a comparison between the two groups was performed using the independent sample t-test. The category data were in the form of the number of cases and constituent ratio n (%) and the chi-square test was adopted for intergroup comparison. In this study, we used multiple interpolation to solve the problem of missing data. The weighted variable was used in this study. The analysis included the following variables: masked variance pseudo-stratum (SDMVSTRA), masked variance pseudo-cluster (SDMVPSU), and full sample two-year MEC exam weight (WTMEC2YR). Missing data before and after interpolation were compared between groups as a sensitivity analysis. Statistical analysis was performed using SAS 9.4 (SAS Institute Inc., Cary, NC, USA).
COPD patients were grouped into CAR quartiles to compare the upper quartiles (CAR Q2-Q4) with the lower quartile (CAR Q1). The Kaplan-Meier (KM) method was used to estimate the survival of different CAR groups. The univariate Cox proportional hazards model was performed to screen influence factors of 5-year survival in COPD patients. The multivariate Cox proportional hazards model was applied to explore the association between CAR and 5-year mortality in COPD patients. Model 1 was an unadjusted model; model 2 adjusted for education level, marital status, BMI, smoking status, diabetes, CKD, and segmented neutrophils num; model 3 adjusted for education level, marital status, BMI, smoking status, segmented neutrophils num, diabetes, CKD, chronic bronchitis, asthma, intestines problem, cancer, ALT, AST, hypertension, dyslipidemia, CVD, bronchodilator, and ICS. In addition, subgroup analyses were performed to further evaluate the association between CAR and 5-year mortality in COPD patients based on the severity of COPD, gender, BMI, smoking status, CVD, CKD, diabetes. Association analysis was carried out by figuring the hazard ratio (HR) and 95% confidence interval (95% CI). We draw the time-dependent receiver operator characteristic (ROC) curves and the area under the curve (AUC) was utilized to testify the predictive performance of CAR in predicting the 5-year mortality in COPD patients. We used DeLong’s test to evaluate the significance of the AUC differences between models for different years. The significance for all tests was set at an alpha level of 0.05.
Results
Characteristics of Study Participants
Of the 1210 participants included, 110 participants (9.09%) had 5-year mortality. The mean age was 54.84 (0.61) years, and the majority of participants were male (61.03%). The majority of included patients (82.89%) were Non-Hispanic White race. Only 5.04% had less than a 9th-grade education. Of the 1210 participants, 705 (63.06%) were married. There were significant differences between COPD patients who were alive and dead in education level, marital status, diabetes, CKD, segmented neutrophils num, COPD severity, CAR, and follow-up times (all P <0.05). Characteristics of study participants are presented in Table 1. The COPD patients with CAR ≥-0.11 had a lower survival rate compared to COPD patients with CAR <-1.80, CAR between −1.80 and −0.97, and CAR between −0.97 and −0.11. The KM curve showed the survival in different CAR groups (Figure 2).
 |
Table 1 Characteristics of Study Participants |
 |
Figure 2 The KM curve of the survival in different CAR groups. |
Association Between CAR and Risk of 5-Year Mortality in Patients with COPD
Supplementary Table 1 shows the results of covariate screening by univariate COX analysis. Considering the fact that a multicollinearity problem might cause the regression results to be biased, we adopted the variance expansion factor (VIF) to affirm whether the selected variables are highly correlated. Supplementary Table 2 reports multiple collinearity results. The largest VIF value was 2.70, suggesting that no serious multicollinearity issue existed in the model. From the results of model 1 in multivariable COX analysis (HR: 1.33, 95% CI: 1.16 to 1.51, P =0.001), model 2 (HR: 1.17, 95% CI: 1.01–1.36, P =0.038), and model 3 (HR: 1.19, 95% CI: 1.02 to 1.38, P =0.028), a higher CAR was associated with an increased risk of 5-year mortality in COPD patients. Compared with a lower CAR, a higher CAR was also associated with an increased risk of 5-year mortality in COPD patients (HR: 1.94, 95% CI: 1.07 to 3.50, P =0.029). The association between CAR and the risk of mortality in patients with COPD is shown in Table 2.
 |
Table 2 Association Between CAR and Risk of 5-Year Mortality in Patients with COPD |
Subgroup Analyses of the Association Between CAR and the Risk of Mortality in Patients with COPD
Subgroup analyses of the association between CAR and the risk of mortality in patients with COPD are presented in Table 3. Regarding the COPD severity, CAR was related to an increased risk of 5-year mortality in COPD patients with mild COPD (HR: 1.46, 95% CI: 1.05–2.02, P =0.025); however, there was no statistical association between CAR and risk of 5-year mortality in COPD patients with moderate-severe COPD. A higher CAR correlated to a rise in 5-year mortality in COPD patients with BMI <=24.99 kg/m2 (HR: 2.20, 95% CI: 1.06–4.55, P =0.035). CAR was also associated with a rise in 5-year mortality in COPD patients with BMI between 25 kg/m2 and 29.9 kg/m2 (HR: 1.56, 95% CI: 1.11–2.19, P =0.012). Nevertheless, there was no statistical association between CAR and the risk of 5-year mortality in COPD patients with BMI ≥30 kg/m2. Regardless of gender, the higher the CAR, the higher the 5-year risk of death in patients with COPD. In term of smoking status, CAR was related to a higher risk of 5-year mortality in COPD patients who were still smoking (HR: 1.73, 95% CI: 1.43–2.11, P <0.001). Regarding the comorbidities, a higher CAR was associated with a higher risk of 5-year mortality in COPD patients who were without CVD (HR: 2.26, 95% CI: 1.06–4.82, P =0.035) and in COPD patients who were without diabetes (HR: 2.17, 95% CI: 1.13–4.18, P =0.022). A higher CAR was associated with a higher risk of 5-year mortality in COPD patients who were without CKD (HR: 2.59, 95% CI: 1.28–5.22, P =0.009). However, a higher CAR was related to a lower risk of 5-year mortality in COPD patients who were with CKD (HR: 0.07, 95% CI: 0.01–0.66, P =0.023).
 |
Table 3 Subgroup Analyses of the Association Between CAR and the Risk of Mortality in Patients with COPD |
Predictive Performance of CAR in Predicting the Mortality in COPD Patients
The AUC of CAR in predicting the 1-year, 3-year, and 5-year mortality in COPD patients was 0.735 (95% CI: 0.655 to 0.815), 0.615 (95% CI: 0.542 to 0.689), and 0.608 (95% CI: 0.553), respectively. The predictive performance of CAR in predicting mortality in COPD patients is shown in Figure 3. The significance of the AUC differences between models of different years is shown in Supplementary Table 1. The cut-off value of CAR in predicting mortality in COPD patients is presented in Supplementary Table 3. Supplementary Table 4 illustrates the significance of the AUC differences between models for different years.
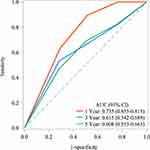 |
Figure 3 Predictive performance of CAR in predicting the mortality in COPD patients. |
Discussion
Recently, CAR has been used to assess the prognosis of several diseases.37–39 In this study, we evaluated the association between CAR and the risk of mortality in patients with COPD. A relationship between an increased CAR and 5-year mortality in COPD patients was observed. In addition, CAR was related to an increased risk of 5-year mortality in COPD patients with mild COPD, in COPD patients who were still smoking, in COPD patients with BMI <=29.9 kg/m2, in COPD patients who were without CVD, in COPD patients who were without diabetes, and COPD patients who were without CKD. However, a higher CAR was related to a lower risk of 5-year mortality in COPD patients who were with CKD. The AUC values in predicting 1-year, 3-year mortality, and 5-year mortality in COPD patients were 0.735, 0.615, and 0.608, respectively.
CAR has been found to be associated with a wide range of diseases and disease prognosis. CAR predicts disease severity and 30-day survival in patients with sepsis.38 Researchers have used CAR to predict the clinical prognosis of patients with cancer39–41 and CVD.37 A meta-analysis suggested that high pretreatment CAR indicates poor prognosis in human malignancies except colorectal cancer, reporting pretreatment CAR could be used in the evaluation of prognosis in clinical work.42 An observational study demonstrated that CAR can be used as a reliable marker to predict the development of atrial fibrillation after coronary artery bypass grafting.43 He et al suggested that CAR can be used as a potential indicator for determining rheumatoid arthritis inflammation.44 A recent study by Uzum et al revealed that CAR is a potential parameter in distinguishing critically ill COVID-19 patients in need of intensive care.45 In the study by Baldemir et al, the cut-off value of 3.26 of CAR can be used in the nutritional risk assessment in patients with COPD.28 In our study, high CAR was significantly associated with 5-year mortality in COPD patients. The mechanism of CAR and mortality in COPD patients may be explained by high CRP and low ALB levels. CRP is an inflammatory protein first discovered by Toilet and Francis in 1930 and is now widely used in the assessment of systemic inflammation and infection status.46 A systematic review and meta-analysis suggested that high baseline CRP is significantly associated with higher mortality in patients with COPD.17 Liu et al found that a serum CRP concentration of >3 mg/dl was a worse prognostic variable for COPD than a CRP concentration of ≤3 mg/dl.47 Elevated circulating CRP levels are associated with poor lung function, systemic comorbidities, poorer quality of life, and higher late mortality in patients with COPD.48 ALB is a protein with important physiological functions such as maintenance of plasma colloid osmotic pressure, intravascular transport of certain substances, inflammatory response, thrombosis and lipid metabolism, is classically considered as a biomarker of malnutrition and poor health status.49 Malnutrition often leads to treatment failure and can be associated with a poor prognosis in COPD.50 We believe serum ALB is a marker of malnutrition, however, serum ALB level alone could not be used as a pure marker of the nutritional status in patients because low albumin levels are often associated with chronic systemic inflammation and other factors that are not nutrition-related.21 Further explanation is needed regarding the mechanism of CAR and mortality in COPD patients. Furthermore, the AUC value of CAR in predicting the mortality in COPD patients is greater than 0.6, indicating that future studies could examine whether CAR can be used to predict prognosis in COPD patients.
We observed that a higher CAR correlated with an increase in 5-year mortality in COPD patients with a BMI <=24.99 kg/m2. CAR was also associated with an increase in 5-year mortality in COPD patients with a BMI between 25 kg/m2 and 29.9 kg/m2. However, there was no statistical association between CAR and the risk of 5-year mortality in COPD patients with a BMI ≥30 kg/m2. We hypothesized that the different results at different BMIs might be due to the effect of BMI itself on the prognosis of COPD. Low BMI has long been recognized as a risk factor for increased mortality in patients with COPD.51 Keene et al found that lower BMI was associated with worsening disease in COPD patients.52 CAR was related to an increased risk of 5-year mortality in COPD patients with mild COPD, suggesting that CAR may correlate with the prognosis of disease in the early stage. In a study evaluating the regulation of CAR in health and disease, CAR may exert a role in the early stage of hepatic diseases, but this effect may be lost during disease progression.53 We found that an increase in CAR was associated with mortality in COPD patients in COPD patients who were still smoking. Long-term exposure to tobacco is known to cause lung damage and inflammation, resulting in an exponential systemic response that is related to the severity of COPD.54 Thus, high levels of CAR represent to some degree a high level of CRP that represents a high level of inflammation; therefore, CAR was associated with mortality in COPD patients with who were still smoking. Regarding the effect of comorbidities, CAR was related to an increased risk of 5-year mortality in COPD patients without CVD, without diabetes, and without CKD. This result implies that the relationship between CAR and prognosis of COPD patients may be more applicable to COPD patients without comorbidities. We did not perform a subgroup analysis based on marital status. However, different marital status may cause different prognosis. In a previous study by Lewis et al, mortality risk from COPD was increased significantly for those who were widowed or divorced.55 A further study evaluating the relationship between CAR and prognosis of COPD patients with different marital status is needed.
To the best of our knowledge, our study is the first to examine the association between CAR and mortality risk in patients with COPD based on a publicly available database. We found an association between the biomarker and mortality in COPD patients based on a simple calculation of CRP, and ALB. In addition, we conducted multiple subgroup analyses to further understand the specific populations in which CAR is associated with mortality in COPD. Routine blood testing is a very simple and inexpensive approach and can be performed in almost all medical institutions. The exploration of biomarkers related to mortality could help stratify high-risk patients in the future; this may facilitate early individualized treatment and optimal allocation of limited medical resources to reduce deaths. The findings of our study may help clinicians develop monitoring and treatment strategies for patients. However, there were limitations to this study that need to be acknowledged. First, in a retrospective study, recall bias and selection bias are difficult to avoid. Second, due to database limitations, confounding effects such as air pollution and occupational exposure cannot be excluded. Third, there may be selection bias due to the exclusion of patients with missing data. Fourth, although the detection technology of CRP and ALB is reliable, simple, and inexpensive, and are usually part of an inpatient examination in a general hospital. The level of CRP and ALB can be affected by multiple factors and diseases, suggesting that a single combination of CRP and ALB may not be able to accurately and objectively relate to the mortality in patients with COPD. Fifth, although patients were clinically stable, serum CRP and ALB may fluctuate slightly over time, affecting the association between CAR and mortality in COPD patients. Sixth, given that this study was a retrospective, database-based study, we were unable to track changes in CAR values over a period of 5 years, and the association of increasing CAR values during follow-up with mortality needs to be further explored. Seventh, we observed a significant difference in AUC between 1-year mortality and 5-year mortality, indicating the association of CAR with COPD mortality and the predictive value of CAR should be elaborated in different years. All of these limitations will be the subject of further investigation in the future.
Conclusion
This study demonstrated that high levels of CAR are associated with mortality in COPD patients. This study suggests CAR should be further studied in the future to determine its usefulness in predicting prognosis for COPD patients in practice.
Ethics Approval and Informed Consent
The requirement of ethical approval for this was waived by the Institutional Review Board of The Affiliated Changzhou Second People’s Hospital of Nanjing Medical University, because the data was accessed from NHANES (a publicly available database). The need for written informed consent was waived by the Institutional Review Board of The Affiliated Changzhou Second People’s Hospital of Nanjing Medical University due to retrospective nature of the study.
Funding
This study received no funding.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Huang X, Guan W, Xiang B, Wang W, Xie Y, Zheng J. MUC5B regulates goblet cell differentiation and reduces inflammation in a murine COPD model. Respir Res. 2022;23(1):11. doi:10.1186/s12931-021-01920-8
2. Alqahtani JS, Aquilina J, Bafadhel M, et al. Research priorities for exacerbations of COPD. Lancet Respir Med. 2021;9(8):824–826. doi:10.1016/S2213-2600(21)00227-7
3. Halpin DMG, Criner GJ, Papi A, et al. Global initiative for the diagnosis, management, and prevention of chronic obstructive lung disease. The 2020 GOLD science committee report on COVID-19 and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2021;203(1):24–36. doi:10.1164/rccm.202009-3533SO
4. Gupta N, Agrawal S, Chakrabarti S, Ish P. COPD 2020 guidelines - what is new and why? Adv Respir Med. 2020;88(1):38–40. doi:10.5603/ARM.2020.0080
5. Singh D, Agusti A, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease: the GOLD science committee report 2019. Eur Respir J. 2019;53(5):1900164. doi:10.1183/13993003.00164-2019
6. World Health Organization. Chronic respiratory diseases. Burden of COPD; 2019.
7. Esteban C, Castro-Acosta A, Alvarez-Martínez CJ, Capelastegui A, López-Campos JL, Pozo-Rodriguez F. Predictors of one-year mortality after hospitalization for an exacerbation of COPD. BMC Pulm Med. 2018;18(1):18. doi:10.1186/s12890-018-0574-z
8. Kim Y, Kim YJ, Cho WK. Effect of multiple comorbidities on mortality in chronic obstructive pulmonary disease among Korean population: a nationwide cohort study. BMC Pulm Med. 2021;21(1):56. doi:10.1186/s12890-021-01424-7
9. Hamadi H, Stallings-Smith S, Apatu E, Peterson B, Spaulding A. Smoke-Free Policies and 30-Day Mortality Rates for Chronic Obstructive Pulmonary Disease. Int J Health Policy Manag. 2022;11(9):1695–1702. doi:10.34172/ijhpm.2021.74
10. Lambert AA, Putcha N, Drummond MB, et al. Obesity is associated with increased morbidity in moderate to severe COPD. Chest. 2017;151(1):68–77. doi:10.1016/j.chest.2016.08.1432
11. Robichaux C, Aron J, Wendt CH, et al. Sociodemographic and geographic risk factors for all-cause mortality in patients with COPD. Int J Chron Obstruct Pulmon Dis. 2023;18:1587–1593. doi:10.2147/COPD.S406899
12. Nakanishi M, Minakata Y, Tanaka R, et al. Simple standard equation for daily step count in Japanese patients with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2019;14:1967–1977. doi:10.2147/COPD.S218705
13. Yin P, Wu J, Wang L, et al. The Burden of COPD in China and Its Provinces: findings From the Global Burden of Disease Study 2019. Front Public Health. 2022;10:859499. doi:10.3389/fpubh.2022.859499
14. Barnes PJ. Cellular and molecular mechanisms of chronic obstructive pulmonary disease. Clin Chest Med. 2014;35(1):71–86. doi:10.1016/j.ccm.2013.10.004
15. Sproston NR, Ashworth JJ. Role of C-reactive protein at sites of inflammation and infection. Front Immunol. 2018;9:754. doi:10.3389/fimmu.2018.00754
16. Pandey S, Garg R, Kant S, Gaur P. Vitamin D, C-reactive protein, and oxidative stress markers in chronic obstructive pulmonary disease. Tzu Chi Med J. 2021;33(1):80–86. doi:10.4103/tcmj.tcmj_198_19
17. Leuzzi G, Galeone C, Taverna F, Suatoni P, Morelli D, Pastorino U. C-reactive protein level predicts mortality in COPD: a systematic review and meta-analysis. Eur Respir Rev. 2017;26(143):160070. doi:10.1183/16000617.0070-2016
18. Yu H, Su X, Lei T, et al. Effect of Omega-3 fatty acids on chronic obstructive pulmonary disease: a systematic review and meta-analysis of randomized controlled trials. Int J Chron Obstruct Pulmon Dis. 2021;16:2677–2686. doi:10.2147/COPD.S331154
19. Hausman DB, Fischer JG, Johnson MA. Protein, lipid, and hematological biomarkers in centenarians: definitions, interpretation and relationships with health. Maturitas. 2012;71(3):205–212. doi:10.1016/j.maturitas.2011.12.002
20. Qiu Y, Wang Y, Shen N, et al. Association between red blood cell distribution width-albumin ratio and hospital mortality in chronic obstructive pulmonary disease patients admitted to the intensive care unit: a retrospective study. Int J Chron Obstruct Pulmon Dis. 2022;17:1797–1809. doi:10.2147/COPD.S371765
21. Ishida S, Hashimoto I, Seike T, Abe Y, Nakaya Y, Nakanishi H. Serum albumin levels correlate with inflammation rather than nutrition supply in burns patients: a retrospective study. The Journal of Med Invest. 2014;61(3–4):361–368. doi:10.2152/jmi.61.361
22. Lee CS, Devoe CE, Zhu X, Fishbein JS, Seetharamu N. Pretreatment nutritional status and response to checkpoint inhibitors in lung cancer. Lung Cancer Manag. 2020;9(2):Lmt31. doi:10.2217/lmt-2020-0008
23. Sivgin S, Baldane S, Ozenmis T, et al. The impact of pretransplant hypoalbuminemia on survival in patients with leukemia who underwent allogeneic hematopoietic stem cell transplantation (alloHSCT): a nutritional problem? Transplant Proc. 2013;45(9):3371–3374. doi:10.1016/j.transproceed.2013.02.144
24. Abolhassani AR, Schuler G, Kirchberger MC, Heinzerling L. C-reactive protein as an early marker of immune-related adverse events. J Cancer Res Clin Oncol. 2019;145(10):2625–2631. doi:10.1007/s00432-019-03002-1
25. Hizli O, Cayir S, Coluk Y, Kayabasi S, Yildirim G. The novel indicators of moderate to severe sleep apnea: fibrinogen to albumin ratio vs. CRP to albumin ratio. Eur Arch Otorhinolaryngol. 2021;278(3):851–855. doi:10.1007/s00405-019-05770-5
26. Frey A, Martin D, D’Cruz L, Fokas E, Rödel C, Fleischmann M. C-reactive protein to albumin ratio as prognostic marker in locally advanced non-small cell lung cancer treated with chemoradiotherapy. Biomedicines. 2022;10(3):598. doi:10.3390/biomedicines10030598
27. Martin D, Rödel F, Balermpas P, Winkelmann R, Fokas E, Rödel C. C-reactive protein-to-albumin ratio as prognostic marker for anal squamous cell carcinoma treated with chemoradiotherapy. Front Oncol. 2019;9:1200. doi:10.3389/fonc.2019.01200
28. Baldemir R, Öztürk A, Eraslan Doganay G, Cirik MO, Alagoz A. Evaluation of nutritional status in hospitalized chronic obstructive pulmonary disease patients and can c-reactive protein-to-albumin ratio be used in the nutritional risk assessment in these patients. Cureus. 2022;14(2):e21833. doi:10.7759/cureus.21833
29. Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD executive summary. Am J Respir Crit Care Med. 2017;195(5):557–582. doi:10.1164/rccm.201701-0218PP
30. Egan BM, Zhao Y, Axon RN. US trends in prevalence, awareness, treatment, and control of hypertension, 1988–2008. JAMA. 2010;303(20):2043–2050. doi:10.1001/jama.2010.650
31. Gong R, Liu Y, Luo G, Yin J, Xiao Z, Hu T. Using optimal subset regression to identify factors associated with insulin resistance and construct predictive models in the US adult population. Endoc Connect. 2022;11(7). doi:10.1530/EC-22-0066
32. Kammerlander AA, Mayrhofer T, Ferencik M, et al. Association of metabolic phenotypes with coronary artery disease and cardiovascular events in patients with stable chest pain. Diabetes Care. 2021;44(4):1038–1045. doi:10.2337/dc20-1760
33. Liao S, Zhang J, Shi S, et al. Association of aldehyde exposure with cardiovascular disease. Ecotoxicol Environ Saf. 2020;206:111385. doi:10.1016/j.ecoenv.2020.111385
34. Li F, Sun A, Wu F, Zhang D, Zhao Z. Trends in using of antihypertensive medication among US CKD adults, NHANES 2001–2018. Front Cardiovasc Med. 2023;10:990997. doi:10.3389/fcvm.2023.990997
35. Rahman HH, Niemann D, Munson-McGee SH. Urinary metals, arsenic, and polycyclic aromatic hydrocarbon exposure and risk of chronic bronchitis in the US adult population. Environ Sci Pollut Res Int. 2022;29(48):73480–73491. doi:10.1007/s11356-022-20982-9
36. Llanos JP, Ortega H, Germain G, et al. Health characteristics of patients with asthma, COPD and asthma-COPD overlap in the NHANES database. Int J Chron Obstruct Pulmon Dis. 2018;13:2859–2868. doi:10.2147/COPD.S167379
37. Dereli S, Çerik İB, Kaya A, Bektaş O. Assessment of the relationship between C-reactive protein-to-albumin ratio and the presence and severity of isolated coronary artery ectasia. Angiology. 2020;71(9):840–846. doi:10.1177/0003319720930983
38. Yu Y, Wu W, Dong Y, Li J, Steel HC. C-reactive protein-to-albumin ratio predicts sepsis and prognosis in patients with severe burn injury. Mediators Inflamm. 2021;2021:6621101. doi:10.1155/2021/6621101
39. Lin N, Li J, Ke Q, Wang L, Cao Y, Liu J. Clinical significance of C-reactive protein to albumin ratio in patients with hepatocellular carcinoma: a meta-analysis. Dis Markers. 2020;2020:4867974. doi:10.1155/2020/4867974
40. Tsujino T, Komura K, Hashimoto T, et al. C-reactive protein-albumin ratio as a prognostic factor in renal cell carcinoma - A data from multi-institutional study in Japan. Urol Oncol. 2019;37(11):812.e811–812.e818. doi:10.1016/j.urolonc.2019.04.002
41. Uchimoto T, Komura K, Fujiwara Y, et al. Prognostic impact of C-reactive protein-albumin ratio for the lethality in castration-resistant prostate cancer. Med Oncol. 2019;37(1):9. doi:10.1007/s12032-019-1332-7
42. Xu HJ, Ma Y, Deng F, Ju WB, Sun XY, Wang H. The prognostic value of C-reactive protein/albumin ratio in human malignancies: an updated meta-analysis. Onco Targets Ther. 2017;10:3059–3070. doi:10.2147/OTT.S137002
43. Aksoy F, Uysal D, Ibrişim E. Relationship between c-reactive protein/albumin ratio and new-onset atrial fibrillation after coronary artery bypass grafting. Rev Assoc Med Bras. 2020;66(8):1070–1076. doi:10.1590/1806-9282.66.8.1070
44. He Y, Tang J, Wu B, Yang B, Ou Q, Lin J. Correlation between albumin to fibrinogen ratio, C-reactive protein to albumin ratio and Th17 cells in patients with rheumatoid arthritis. Clinica chimica acta. 2020;500:149–154. doi:10.1016/j.cca.2019.10.009
45. Uzum Y, Turkkan E. Predictivity of CRP, albumin, and CRP to albumin ratio on the development of intensive care requirement, mortality, and disease severity in COVID-19. Cureus. 2023;15(1):e33600. doi:10.7759/cureus.33600
46. Tillett WS, Francis T. Serological reactions in pneumonia with a non-protein somatic fraction of pneumococcus. J Exp Med. 1930;52(4):561–571. doi:10.1084/jem.52.4.561
47. Liu SF, Wang CC, Chin CH, Chen YC, Lin MC. High value of combined serum C-reactive protein and BODE score for mortality prediction in patients with stable COPD. Arch Bronconeumol. 2011;47(9):427–432. doi:10.1016/j.arbres.2011.04.011
48. Aksu F, Capan N, Aksu K, et al. C-reactive protein levels are raised in stable Chronic obstructive pulmonary disease patients independent of smoking behavior and biomass exposure. J Thorac Dis. 2013;5(4):414–421. doi:10.3978/j.issn.2072-1439.2013.06.27
49. Nazha B, Moussaly E, Zaarour M, Weerasinghe C, Azab B. Hypoalbuminemia in colorectal cancer prognosis: nutritional marker or inflammatory surrogate? World J Gastrointest Surg. 2015;7(12):370–377. doi:10.4240/wjgs.v7.i12.370
50. Hallin R, Gudmundsson G, Suppli Ulrik C, et al. Nutritional status and long-term mortality in hospitalised patients with chronic obstructive pulmonary disease (COPD). Respir Med. 2007;101(9):1954–1960. doi:10.1016/j.rmed.2007.04.009
51. Yamauchi Y, Hasegawa W, Yasunaga H, et al. Paradoxical association between body mass index and in-hospital mortality in elderly patients with chronic obstructive pulmonary disease in Japan. Int J Chron Obstruct Pulmon Dis. 2014;9:1337–1346. doi:10.2147/COPD.S75175
52. Keene SJ, Adab P, de Vries F, et al. The stability of the ADO score among UK COPD patients from the health improvement network. ERJ Open Res. 2020;6(1):00196–2019. doi:10.1183/23120541.00196-2019
53. Daujat-Chavanieu M, Gerbal-Chaloin S. Regulation of CAR and PXR expression in health and disease. Cells. 2020;9(11):2395. doi:10.3390/cells9112395
54. Hogg JC. Pathophysiology of airflow limitation in chronic obstructive pulmonary disease. Lancet. 2004;364(9435):709–721. doi:10.1016/S0140-6736(04)16900-6
55. Lewis DR, Clegg LX, Johnson NJ. Lung disease mortality in the United States: the national longitudinal mortality study. Int J Tuberc Lung Dis. 2009;13(8):1008–1014.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
