Back to Journals » ClinicoEconomics and Outcomes Research » Volume 15
Assessing the Health Economic Outcomes from Commercially Insured Relapsing Multiple Sclerosis Patients Who Switched from Other Disease-Modifying Therapies to Teriflunomide, in the United States
Authors Araujo L, Kyatham S, Bzdek KG, Higuchi K, Greene N
Received 27 January 2023
Accepted for publication 2 May 2023
Published 20 May 2023 Volume 2023:15 Pages 361—373
DOI https://doi.org/10.2147/CEOR.S401687
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Dean Smith
Lita Araujo,1 Srikanth Kyatham,2 Kristen G Bzdek,1 Keiko Higuchi,1 Nupur Greene1
1Neurology and Immunology, Sanofi, Cambridge, MA, USA; 2Axtria Inc, Berkeley Heights, NJ, USA
Correspondence: Lita Araujo, Neurology and Immunology, Sanofi, 450 Water Street, Cambridge, MA, 02141, USA, Tel +1 617 937 9928, Email [email protected]
Objective: Assess patient characteristics, healthcare resource utilization (HCRU), and relapses in patients with multiple sclerosis (MS) who switched to teriflunomide from other disease-modifying therapies (DMTs).
Methods: Retrospective study of US Merative™ MarketScan® claims database (Jan 1, 2012–July 31, 2020,) including HIPAA-compliant, deidentified data. Patients ≥ 18 years with MS diagnosis (based on ICD-9/ICD-10 codes), receiving ≥ 1 DMT prior to teriflunomide and ≥ 12 months continuous enrollment pre and post index (date of teriflunomide initiation). Outcomes included inpatient and emergency room claims coinciding with MS diagnosis, MS-related healthcare costs, and annualized relapse rates (ARRs) (indirectly assessed using hospitalization/outpatient claims and steroid use coinciding with MS diagnosis).
Results: The analyzed cohort (N=2016) was primarily female (79%); age (mean ± standard deviation) 51.4 ± 9.3 years; MS duration 4.7± 2.8 years (at index). The majority (89.2%) were treated with one DMT before switching to teriflunomide. Use of outpatient services (event rate/100 person-years) increased post vs pre index; however, MRI visits significantly reduced over the same period (both P< 0.0001). Costs for MS-specific outpatient visits decreased by $371 per patient per year (PPPY) after switching to teriflunomide. Despite an increase in use post index (0.024 to 0.033 rate/100 person-years; P< 0.0001), costs for MS-specific laboratory services reduced (pre-index: $271 vs $248 PPPY post-index; P=0.02). Fewer patients had relapses after switching (pre-index: n=417 [20.7%]; post-index: n=333 [16.5%]). ARR was significantly lower after switching (pre-index: 0.269 vs post-index: 0.205; P=0.000).
Conclusion: Switching to teriflunomide from existing DMTs in patients with relapsing MS resulted in a reduction in outpatient HCRU in this analysis of US claims data. The real-world effectiveness of teriflunomide was generally consistent with efficacy reported in clinical trials, showing a reduction in relapse following a switch to teriflunomide.
Keywords: DMTs, healthcare resource utilization, healthcare costs, prescription, relapsing–remitting multiple sclerosis
Introduction
In 2020, it was estimated that 2.8 million people worldwide (35.9 per 100,000) were living with multiple sclerosis (MS),1 with nearly 1 million living with MS in the United States (US) alone, and prevalence is on the rise.2,3 As MS is an incurable neurodegenerative disease, where disability can progress with time,4,5 there is a significant impact on patients’ health-related quality of life and a substantial economic burden.6,7 For example, the total economic burden of MS in 2019 was estimated at $85.4 billion in the US, with direct medical costs of $63.3 billion and indirect and nonmedical costs of $22.1 billion.8
Various disease-modifying therapies (DMTs) are available to decrease the frequency, duration and severity of relapses in patients with MS,9 and some DMTs can also stabilize and reduce disease progression.10,11 However, patients may need to switch between DMTs over the course of their disease.12,13 For example, of the ~68% of patients who discontinued their DMTs over 10-years’ follow-up, the vast majority (87%) switched to an alternative DMT within 6 months of discontinuation, in the Big MS data network study.14 Common reasons for discontinuing treatment included lack of efficacy (23%), adverse events or side effects (16%), and intolerance (14%).14 Other reasons for switching could include but not limited to convenience with the dosing or treatment satisfaction.15 These studies suggest switching between DMTs is both common in MS, and occurs early in treatment.14,15 It is important that patients find a well-tolerated DMT early in their treatment trajectory,9 not only for the patient, but also for healthcare providers.
Teriflunomide (first approved in the US 2012) is available in >80 countries for treating relapsing MS, including patients with relapsing–remitting MS, active secondary progressive MS, and clinically isolated syndrome.16,17 Evidence from randomized controlled trials and real-world effectiveness studies have demonstrated that oral teriflunomide is effective and generally well-tolerated for patients with relapsing MS,18–25 and furthermore, switching to once-daily teriflunomide improves patient satisfaction with treatment.25,26 At present, data investigating the health economic impact of changing therapeutic regimen from another DMT to teriflunomide is limited. Understanding how a change in MS therapy impacts health economic outcomes provides useful information for different stakeholders (ie, payers, healthcare professionals [HCPs], and patients), particularly given the chronic nature of MS and the high disability burden imposed on healthcare systems. This retrospective study of US claims data assessed patient characteristics, healthcare resource utilization (HCRU), and relapses in patients with MS treated with teriflunomide who were previously treated with a different DMT.
Methods
Study Design
This was a retrospective, observational study using the Merative™ (formerly IBM®) MarketScan® Research Database between Jan-1-2012, and July-31-2020, which identified patients with MS who were treated with a DMT before changing (being switched) to teriflunomide (Figure S1). Merative™ MarketScan® database includes data from large employers, health plans, and government and public organizations within the US, and captures expenditures and enrollments and person-specific clinical utilizations across inpatient, outpatient, prescription drug, and other carve-out services.
This study was performed in accordance with relevant guidelines and regulations. Data in the Merative™ MarketScan® Research databases27 are fully deidentified and statistically validated to meet privacy requirements set out by the Health Insurance Portability and Accountability Act (HIPAA). The study did not involve collection, use, or transmittal of individually identifiable data. As such, this study only analyzed de-identified data which are a priori exempt from IRB approval according to the Federal Policy for the Protection of Human Subjects “Common Rule” (1991, revised 2018) [45CFR: Subtitle A Subchapter A, Part 46.104(d)(4)(ii): Existing Data & Specimens—No Identifiers].28
The index date was the date of teriflunomide initiation (prescription filled). A 1-year pre-index (“look-back”) period was used to identify patient characteristics. Within the pre-index period, 7-day grace periods were used for gaps in prescriptions of oral and weekly injectable medications, 21-day grace periods for prescriptions of monthly injectable medications, and 91-day grace periods for infusions. Patients were followed-up for 1-year post-index (Figure S1).
Sample Selection
Patients (≥18 years) with an MS diagnosis according to the International Classification of Diseases, 9th/10th Revision (ICD-9/ICD-10) codes 340 or G35, respectively, were eligible if they received ≥1 DMT before switching to teriflunomide and had ≥12 months continuous enrollment (allowing up to a 30-day gap during the enrollment period) before and after index (ie, date of teriflunomide prescription fill). Patients were excluded if they had <1 year of continuous enrollment prior to or after index, and if they had not received any DMTs before receiving teriflunomide. A grace period of 60 days was used for gaps in teriflunomide prescriptions.
Outcome Measures
Demographics and clinical characteristics, including comorbidities and MS symptoms, were extracted alongside outcomes of interest which included DMTs and concomitant medications (ie, longitudinal prescription claims) and inpatient and emergency room (ER) claims coinciding with a primary or secondary MS diagnosis. MS-related healthcare costs (HCC) were captured based on inpatient claims, procedure claims for DMTs, or pharmacy claims for concomitant medications for MS-related treatments, captured using J-codes or national drug codes. Intravenous infusion therapies administered in-office or other infusion procedures were captured via current procedural terminology codes.
MS relapses were indirectly assessed by surrogate parameters, as described elsewhere.29–32 A relapse was recorded if ≥1 of these criteria were met: i) hospitalization, where MS was the primary discharge diagnosis (if cystitis, pneumonia, or a urinary tract infection was documented as a secondary diagnosis, a pseudo relapse was assumed and it was therefore not considered a relapse and criteria were not met); ii) hospitalization, where MS was a secondary diagnosis and corticosteroid therapy was recorded during the hospital stay; and iii) an outpatient MS diagnosis and, within 7-days one of the following: a pharmacy claim for corticosteroid therapy; a medical claim for injectable adrenocorticotropic hormone; or, a medical claim for IV methylprednisolone. If within the same month any of the relapse criteria were met more than once, only one relapse was recorded.
Statistical Analysis
Categorical variables were summarized by frequency and percentage. Continuous variables were summarized using mean (standard deviation [SD]), 95% confidence intervals, or by median (range) or interquartile range. Descriptive analyses were carried out to summarize the number of patients by DMT use prior to index, and by treatment sequence, and by type of DMT. Continuous variables were compared using a paired t-test or if the data are not normally distributed, a Wilcoxon rank-sum test, as appropriate. Categorical variables were compared using McNemar-Bowker’s test of symmetry or exact tests, where appropriate. P<0.05 was considered significant for all analyses.
Event rates were expressed per 100 patient-years and calculated by dividing (mean # of events)/(mean # years follow-up), within each treatment arm. All-cause and MS-related HCC were adjusted using the annual medical care component of the Consumer Price Index with inflation to 2020 US dollars.33 Mean event costs were calculated by dividing: (cumulative costs for all events)/(total # events occurring over follow-up). Total HCC included inpatient and outpatient costs, pharmacy and medical costs. Per patient per year (PPPY) costs were estimated by dividing: (mean costs for each individual patient)/(mean # years individual patient in each cohort contributed to follow-up).
Annualized relapse rate (ARR), for 1-year pre- and post-index, were compared using a repeated negative binomial regression model, where the total number of relapses was the dependent variable and the treatment period (1-year pre or post-index) was the covariate. The Log time of follow-up prior to index (years), up to 1-year post-index, was the offset variable. Relapse rates were calculated as number of relapses/time on treatment and were compared using Poisson regression.
Results
Patient Selection and Characteristics
The final study sample included 2016 patients with MS who received ≥1 DMT before switching to teriflunomide and were followed for 1-year post-index (Figure 1). The majority of patients were female (n=1599 [79%]), and at initiation of teriflunomide, patients had a mean (±SD) age of 51.4±9.3 years, and had had MS for an average of 4.7±2.8 years (Table 1). Comorbidities most commonly reported in the pre-index period included hypertension (21.2%), hyperlipidemia (12.9%), and urinary tract infections (6.8%) (Table 1). Symptoms most commonly reported in the pre-index period were headache (7.8%), anxiety (7.4%), and malaise/fatigue (6.3%) (Table 1). Charlson Comorbidity Index (CCI) was (mean±SD) 0.4±1.0 at index.
 |
Table 1 Patient Demographics at Index (Initiation of Teriflunomide Treatment), and Comorbidities Recorded in the 1-Year Pre-Index Period |
 |
Figure 1 Patient attrition from Merative™ (formerly IBM®) MarketScan® Research Database. |
Treatment Patterns Prior to Switching to Teriflunomide
Of patients who had received DMTs prior to teriflunomide (n=1741), the majority (89.2%; n=1577) were treated with one DMT before switching to teriflunomide, 10% (n=160) received two DMTs; fewer than 1% (n=4) received three DMTs. DMTs most commonly used prior to switching were glatiramer acetate (26.1%), dimethyl fumarate (24.6%), and interferon ß1A (23.6%) (Figure S2A). DMTs used for the longest duration were daclizumab and interferon ß1A (Figure S2B).
HCRU and Costs Following Switching to Teriflunomide
Utilization of outpatient services increased significantly post-index for both all-cause and MS-specific events (Table 2). This was due to all-cause and MS-specific therapy visits and laboratory visits increasing significantly from the pre to post-index period. However, magnetic resonance imaging (MRI) visits significantly reduced for both all-cause and MS-specific services over the same period (Table 2). The number of patients visiting the ER (all-cause and MS-specific visits) was generally lower post-index, particularly for those visiting 1–2 times (Figure 2).
 |
Table 2 Healthcare Resource Utilization Rates and Healthcare Costs per Patient per Year at 1 Year Pre Index Vs 1 Year Post Index (n=2016) |
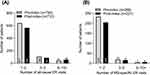 |
Figure 2 Healthcare resource utilization in the 1-year pre- and 1-year post-index periods. (A) Frequency of all-cause and (B) MS-specific emergency room visits. |
Despite the increase in outpatient utilization, PPPY costs for all-cause and MS-specific outpatient costs decreased significantly post-index (vs 1-year pre-index) (Table 2). For example, costs for MS-specific outpatient visits reduced by $371 PPPY following switching to teriflunomide (from $3580 [1-year pre-index] to $3209 [1-year post-index]) (Table 2). Reflecting the overall decrease in MRI visits, costs associated with MRI visits were significantly reduced (all-cause and MS-specific) 1-year post-index (Table 2). Despite an increase in utilization, costs for all-cause and MS-specific laboratory services were reduced 1-year post-index by $61 and $23 PPPY, respectively (Table 2), indicating that laboratory test services were less expensive following a switch to teriflunomide, despite being used more frequently. There was no change in the number of inpatient visits or costs following switching to teriflunomide (Table 2).
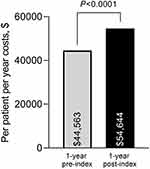
Overall rates of MS-specific durable medical equipment (DME) was similar post-index (Table S1). The number of patients requiring no ambulatory aids increased by 22 over the post-index period, although the change was not significant (P=0.474). PPPY costs for DME use were lower post-index for all-cause costs and MS-specific costs, although nonsignificant for MS-specific costs (Table S1). Total PPPY DMT costs (including pharmacy and medical prescription claims [inpatient and outpatient]) significantly increased post-index (pre-index, $44,563 vs post-index, $54,644; P<0.0001; Figure 3).
Relapses Following Switching to Teriflunomide
The proportion of patients with relapses reduced following initiation of teriflunomide from 20.7% (n=417) 1-year pre-index to 16.5% (n=333) post-index. ARR was significantly lower post-index (Figure 4), and as a result, total costs associated with relapses were also reduced, from $4383 pre-index to $3217 post-index.
 |
Figure 4 Annualized relapse rate in the 1-year pre- and 1-year post-index periods. Relapse rates compared using Poisson regression. P-values calculated using Wilcoxon rank-sum test. |
Discussion
In this retrospective analysis of US claims data, MS-specific healthcare utilization and costs for outpatient services were significantly reduced in the 1-year period following a switch to teriflunomide from an existing DMT regimen; this was largely due to a reduction in the number of outpatient and MRI visits and associated laboratory services. A reduction in ARR was recorded following switching to teriflunomide, suggesting that clinical effectiveness was maintained or improved in the year following a treatment switch. Longer term studies would be needed to confirm reductions in HCRU and cost changes over more than 1-year, including the impact of direct medical costs over the longer term.
Analysis of real-world data allows us to explore how therapies are used in large populations. In this study, we helped to identify common treatment patterns used prior to switching to teriflunomide. We found the majority of patients (89%) were treated with one DMT prior to switching to teriflunomide, and the DMTs most commonly used were glatiramer acetate, dimethyl fumarate, and interferon ß1A. Although we cannot determine the reasons for a treatment switch using claims data, such as patients wanting to avoid an intravenous treatment or having a side-effect from a previous DMT, studying the impact of switching provides valuable information for patients, HCPs, and payers. Patterns of healthcare resource use seen in the current study also allowed us to determine how resource used in one area is often offset in another. For example, with switching to teriflunomide, there was an increase in healthcare utilization of laboratory services, which would be expected since patients receiving teriflunomide require more ongoing monitoring during the first year following treatment initiation, according to the therapeutic label.16 Despite the increase in laboratory services, the monitoring required during teriflunomide treatment is generally less costly than for some other DMTs, for example, the requirements for tuberculosis and pregnancy screening, transaminase and bilirubin levels prior to starting, and alanine aminotransferase levels monthly for 6 months. Other DMTs may also require or recommend more expensive monitoring, including screening for hepatitis, varicella-zoster virus, IgG levels, periodic John Cunningham virus testing, and lymphocyte counts and differentials that may drive up monitoring costs. This was reflected in the current study as, although utilization of laboratory services were higher post-index, costs of MS-specific laboratory services decreased during the same post-index period (by $23 PPPY). It should be noted that due to the nature of claims data, we are unable to know whether the changes in costs of services were due to changes in the price of goods and services or due to a change in their nature.
Changing or discontinuing DMTs is common among people with MS,14,34,35 and is known to be associated with costs outside of the new treatment regimen,35 which has to be considered within the economic value of a treatment regimen. However, the largest driver of a reduction in outpatient costs in the present study was likely a reduction in MRI visits post-index, presumably due to patients having fewer relapses. MRI visits are costly but are recommended for routine monitoring, in particular for patients experiencing relapses or during changes of treatment.5,36 Our study extended to July 2020, 1-year following treatment switch to teriflunomide, and additional study would be needed to confirm if the number of MRIs, especially MRIs with contrast, become less frequent when patients stabilize on a new treatment regimen, and cost differences reduce as a result. Economic benefits have been reported for other studies within US claims data of DMTs switching used for MS.37,38 For example, reductions in non-prescription medical costs were associated with decreased use of outpatient services and inpatient hospital stays in one study of MS patients initiating different DMTs, including teriflunomide, and these reductions in outpatient services had the potential to partially offset DMT costs.38 In addition, initiating natalizumab significant decreased MS-related inpatient stays and decreased costs, in another study.37 In our study, the average cost of DMTs increased post-index, although costs and utilization for MS-specific outpatient services reduced over the same period, helping to offset some of these direct prescription costs. Given the inherent complexities with payment/co-pay within the US healthcare system, the total net economic benefit achieved may be hard to discern for patients themselves seeing out-of-pocket costs. The benefit may be felt more by healthcare systems, with a lower burden on inpatient and outpatient services, although how this changes beyond 1-year cannot be discerned from the current study. In addition, direct comparison of cost savings derived in the current study to earlier studies is challenging due to cost differences of various oral vs injectable therapies, and different availabilities of generic products within DMT comparator arms, at different times. Further study of direct medical costs is therefore warranted.
We also observed that switching to teriflunomide led to a reduction in relapses, and this is corroborated by the ARR and other proxy data including inpatient visits. These observations are consistent with findings from a real-world study using data from >10,000 patients.39 The probability of relapse was lower in patients starting teriflunomide vs an injectable DMT (ie, interferons/glatiramer acetate), but comparable with that of other oral DMTs (ie, dimethyl fumarate), for up to 24 months follow-up.39 Relapse rates are positively associated with higher HCRU and predictive of patients switching to other DMTs.30 Furthermore, DMT non-adherence is a significant predictor of relapse.40–42 With data suggesting improved satisfaction with teriflunomide following switching,25,26 another surrogate variable of adherence,15 we hypothesize that patients who switch to teriflunomide may be more likely to remain with their treatment after switching, which may aid adherence and therefore, management of MS symptoms, although long-term studies are needed to confirm these assumptions. It should be noted we cannot rule out the influence of regression to the mean, whereby switching between DMTs (even between interferons) has been suggested to improve ARR.43
When interpreting our findings, some limitations should be considered. We only looked at patients who switched to teriflunomide, with no comparison to patients who did not switch therapies nor patients who switched to another DMT. MS disease duration at initiation of teriflunomide (average 4.7 years) was shorter than noted in older trials involving teriflunomide (disease duration typically 7–8 years),20 but is consistent with current treatment patterns. Furthermore, patients diagnosed prior to revisions to the clinical course of MS in 2013,36 tended to be older than after 2013 (Table S2), possibly reflecting the impact of more detailed criteria improving recognition of people with MS.5,44 These changes to definitions and diagnosis criteria may impact recognition and diagnosis in years that cross these classifications. CCI scores were assessed via surrogate measures, and relapses were assessed via proxies, such as hospitalization and use of corticosteroids. These methods, well established in claims research, have been validated to capture moderate-to-severe relapses;32 therefore, mild relapses were not included in the calculation of ARR. General limitations of US claims research should be considered including the generalizability of data to individuals in the US without insurance or MS patients outside of the US. Although data should be generally representative to the US population, there was a lower proportion of patients from the West than would be expected by Census estimates. We cannot rule out inherent bias within the data set, or, as noted, influence of regression to the mean, which would be a factor in any study on patient switching DMTs.43 When healthcare professionals switch patient’s DMT in the real-world, this is done with consideration to the most suitable therapy, and this adds inherent variability which cannot be controlled for within our, or any claims-based analyses. As such, our study aims to be hypothesis-generating, to help stakeholders understand the impact of changes in MS therapy on HCRU and costs, to apply observations in a wider context.
Conclusions
This retrospective analysis of US claims data indicates a reduction in HCRU following switching to teriflunomide from other DMTs in the treatment of relapsing MS. A reduction in ARR was seen following a switch to teriflunomide, suggesting real-world effectiveness in-line with efficacy observations from prior clinical trials. Further study is needed to confirm the overall economic benefits of switching to teriflunomide therapy from other DMTs over the longer-term.
Abbreviations
ARR, annualized relapse rate; CDHP, consumer-driven health plan; DME, durable medical equipment; DMT, disease-modifying treatment; ER, emergency room; HCC, healthcare costs; HCP, healthcare professional; HCRU, healthcare resource utilization; HDHP, high-deductible health plan; HMO, health maintenance organization; MRI, magnetic resonance imaging; MS, multiple sclerosis; PPO, preferred-provider organization; PPPY, per patient per year; SD, standard deviation; US, United States.
Acknowledgments
Medical writing support was provided by Karen Burrows of CURO (part of Envision Pharma Group) and was funded by Sanofi.
Author Contributions
All authors made a significant contribution to the work reported, whether that was in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was sponsored by Sanofi.
Disclosure
Lita Araujo, Keiko Higuchi, and Nupur Greene are employees of Sanofi and may hold stock or stock options. Kristen G Bzdek was an employee of Sanofi at the time of the study. Srikanth Kyatham was an employee of Axtria at the time the study was conducted. Axtria was a paid consultant to Sanofi in relation to this project.
References
1. Multiple Sclerosis International Federation (MSIF). Atlas of MS 3rd edition. Part 1: mapping multiple sclerosis around the world: key epidemiology findings; 2020. Available from: https://www.msif.org/wp-content/uploads/2020/10/Atlas-3rd-Edition-Epidemiology-report-EN-updated-30-9-20.pdf.
2. Walton C, King R, Rechtman L, et al. Rising prevalence of multiple sclerosis worldwide: insights from the Atlas of MS, third edition. Mult Scler. 2020;26(14):1816–1821. doi:10.1177/1352458520970841
3. Wallin MT, Culpepper WJ, Campbell JD, et al. The prevalence of MS in the United States: a population-based estimate using health claims data. Neurology. 2019;92(10):e1029–e1040. doi:10.1212/WNL.0000000000007035
4. Klineova S, Lublin FD. Clinical course of multiple sclerosis. Cold Spring Harb Perspect Med. 2018;8(9):a028928. doi:10.1101/cshperspect.a028928
5. Lublin FD, Coetzee T, Cohen JA, Marrie RA, Thompson AJ. The 2013 clinical course descriptors for multiple sclerosis: a clarification. Neurology. 2020;94(24):1088–1092. doi:10.1212/WNL.0000000000009636
6. Wallin MT, Culpepper WJ, Nichols E; GBD Multiple Sclerosis Collaborators. Global, regional, and national burden of multiple sclerosis 1990–2016: a systematic analysis for the global burden of disease study 2016. Lancet Neurol. 2019;18(3):269–285. doi:10.1016/S1474-4422(18)30443-5
7. Visser LA, Louapre C, Uyl-de Groot CA, Redekop WK. Health-related quality of life of multiple sclerosis patients: a European multi-country study. Arch Public Health. 2021;79(1):39. doi:10.1186/s13690-021-00561-z
8. Bebo B, Cintina I, LaRocca N, et al. The economic burden of multiple sclerosis in the United States: estimate of direct and indirect costs. Neurology. 2022;98(18):e1810–e1817. doi:10.1212/WNL.0000000000200150
9. Rae-Grant A, Day GS, Marrie RA, et al. Practice guideline recommendations summary: disease-modifying therapies for adults with multiple sclerosis. Report of the guideline development, dissemination, and implementation subcommittee of the American Academy of Neurology. Neurology. 2018;90(17):777–788. doi:10.1212/WNL.0000000000005347
10. Zhang J, Shi S, Zhang Y, et al. Alemtuzumab versus interferon beta 1a for relapsing-remitting multiple sclerosis. Cochrane Database Syst Rev. 2017;11(11):CD010968. doi:10.1002/14651858.CD010968.pub2
11. Coles AJ, Twyman CL, Arnold DL, et al. Alemtuzumab for patients with relapsing multiple sclerosis after disease-modifying therapy: a randomised controlled Phase 3 trial. Lancet. 2012;380(9856):1829–1839. doi:10.1016/S0140-6736(12)61768-1
12. Bergvall N, Petrilla AA, Karkare SU, et al. Persistence with and adherence to fingolimod compared with other disease-modifying therapies for the treatment of multiple sclerosis: a retrospective US claims database analysis. J Med Econ. 2014;17(10):696–707. doi:10.3111/13696998.2014.940422
13. Grand’Maison F, Yeung M, Morrow SA, et al. Sequencing of high-efficacy disease-modifying therapies in multiple sclerosis: perspectives and approaches. Neural Regen Res. 2018;13(11):1871–1874. doi:10.4103/1673-5374.239432
14. Hillert J, Magyari M, Soelberg Sorensen P, et al. Treatment switching and discontinuation over 20 years in the Big Multiple Sclerosis Data Network. Front Neurol. 2021;12:647811. doi:10.3389/fneur.2021.647811
15. Haase R, Kullmann JS, Ziemssen T. Therapy satisfaction and adherence in patients with relapsing-remitting multiple sclerosis: the THEPA-MS survey. Ther Adv Neurol Disord. 2016;9(4):250–263. doi:10.1177/1756285616634247
16. Sanofi. AUBAGIO® (teriflunomide) tablets [prescribing information]; 2022. Available from: https://products.sanofi.us/aubagio/aubagio.pdf.
17. European Medicines Agency. Aubagio (teriflunomide) tablets [summary of product characteristics]; 2022. Available from: https://www.ema.europa.eu/en/documents/product-information/aubagio-epar-product-information_en.pdf.
18. O’Connor P, Wolinsky JS, Confavreux C, et al. Randomized trial of oral teriflunomide for relapsing multiple sclerosis. N Engl J Med. 2011;365(14):1293–1303. doi:10.1056/NEJMoa1014656
19. O’Connor P, Comi G, Freedman MS, et al. Long-term safety and efficacy of teriflunomide: nine-year follow-up of the randomized TEMSO study. Neurology. 2016;86(10):920–930. doi:10.1212/WNL.0000000000002441
20. Confavreux C, O’Connor P, Comi G, et al. Oral teriflunomide for patients with relapsing multiple sclerosis (TOWER): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Neurol. 2014;13(3):247–256. doi:10.1016/S1474-4422(13)70308-9
21. Miller AE, Olsson TP, Wolinsky JS, et al. Long-term safety and efficacy of teriflunomide in patients with relapsing multiple sclerosis: results from the TOWER extension study. Mult Scler Relat Disord. 2020;46:102438. doi:10.1016/j.msard.2020.102438
22. Coyle PK, Khatri B, Edwards KR, et al. Patient-reported outcomes in relapsing forms of MS: real-world, global treatment experience with teriflunomide from the Teri-PRO study. Mult Scler Relat Disord. 2017;17:107–115. doi:10.1016/j.msard.2017.07.006
23. Coyle PK, Khatri B, Edwards KR, et al. Teriflunomide real-world evidence: global differences in the Phase 4 Teri-PRO study. Mult Scler Relat Disord. 2019;31:157–164. doi:10.1016/j.msard.2019.03.022
24. Papp V, Buron MD, Siersma V, et al. Real-world outcomes for a complete nationwide cohort of more than 3200 teriflunomide-treated multiple sclerosis patients in the Danish Multiple Sclerosis Registry. PLoS One. 2021;16(5):e0250820. doi:10.1371/journal.pone.0250820
25. Kallmann BA, Tiel-Wilck K, Kullmann JS, Engelmann U, Chan A. Real-life outcomes of teriflunomide treatment in patients with relapsing multiple sclerosis: TAURUS-MS observational study. Ther Adv Neurol Disord. 2019;12:1756286419835077. doi:10.1177/1756286419835077
26. Coyle PK, Khatri B, Edwards KR, et al. Patient-reported outcomes in patients with relapsing forms of MS switching to teriflunomide from other disease-modifying therapies: results from the global phase 4 Teri-PRO study in routine clinical practice. Mult Scler Relat Disord. 2018;26:211–218. doi:10.1016/j.msard.2018.09.017
27. Watson Health. IBM MarketScan Research Databases for life sciences researchers; 2023. Available from: https://www.ibm.com/downloads/cas/OWZWJ0QO.
28. Code of Federal Regulations. TITLE 45: PUBLIC WELFARE (subtitle A subchapter A part 46 - protection of human subjects) [§46.104(d)(4)(ii)]; 2018. Available from: https://www.ecfr.gov/current/title-45/subtitle-A/subchapter-A/part-46.
29. Ohlmeier C, Gothe H, Haas J, et al. Epidemiology, characteristics and treatment of patients with relapsing remitting multiple sclerosis and incidence of high disease activity: real world evidence based on German claims data. PLoS One. 2020;15(5):e0231846. doi:10.1371/journal.pone.0231846
30. Raimundo K, Tian H, Zhou H, et al. Resource utilization, costs and treatment patterns of switching and discontinuing treatment of MS patients with high relapse activity. BMC Health Serv Res. 2013;13:131. doi:10.1186/1472-6963-13-131
31. Ollendorf DA, Jilinskaia E, Oleen-Burkey M. Clinical and economic impact of glatiramer acetate versus beta interferon therapy among patients with multiple sclerosis in a managed care population. J Manag Care Pharm. 2002;8(6):469–476. doi:10.18553/jmcp.2002.8.6.469
32. Chastek BJ, Oleen-Burkey M, Lopez-Bresnahan MV. Medical chart validation of an algorithm for identifying multiple sclerosis relapse in healthcare claims. J Med Econ. 2010;13(4):618–625. doi:10.3111/13696998.2010.523670
33. U.S. Bureau of Labor Statistics. CPI Medical care services (2013–2020); 2020. Available from: https://www.bls.gov/cpi/data.htm.
34. Duquette P, Yeung M, Mouallif S, Nakhaipour HR, Haddad P, Schecter R. A retrospective claims analysis: compliance and discontinuation rates among Canadian patients with multiple sclerosis treated with disease-modifying therapies. PLoS One. 2019;14(1):e0210417. doi:10.1371/journal.pone.0210417
35. Reynolds MW, Stephen R, Seaman C, Rajagopalan K. Healthcare resource utilization following switch or discontinuation in multiple sclerosis patients on disease modifying drugs. J Med Econ. 2010;13(1):90–98. doi:10.3111/13696990903579501
36. Lublin FD, Reingold SC, Cohen JA, et al. Defining the clinical course of multiple sclerosis: the 2013 revisions. Neurology. 2014;83(3):278–286. doi:10.1212/WNL.0000000000000560
37. Bonafede MM, Johnson BH, Watson C. Health care-resource utilization before and after natalizumab initiation in multiple sclerosis patients in the US. Clinicoecon Outcomes Res. 2013;6:11–20. doi:10.2147/CEOR.S55779
38. Nicholas J, Boster A, Wu N, et al. Comparison of disease-modifying therapies for the management of multiple sclerosis: analysis of healthcare resource utilization and relapse rates from US insurance claims data. Pharmacoecon Open. 2018;2(1):31–41. doi:10.1007/s41669-017-0035-2
39. Vermersch P, Suchet L, Colamarino R, Laurendeau C, Detournay B. An analysis of first-line disease-modifying therapies in patients with relapsing-remitting multiple sclerosis using the French nationwide health claims database from 2014–2017. Mult Scler Relat Disord. 2020;46:102521. doi:10.1016/j.msard.2020.102521
40. Yermakov S, Davis M, Calnan M, et al. Impact of increasing adherence to disease-modifying therapies on healthcare resource utilization and direct medical and indirect work loss costs for patients with multiple sclerosis. J Med Econ. 2015;18(9):711–720. doi:10.3111/13696998.2015.1044276
41. Lizán L, Comellas M, Paz S, Poveda JL, Meletiche DM, Polanco C. Treatment adherence and other patient-reported outcomes as cost determinants in multiple sclerosis: a review of the literature. Patient Prefer Adherence. 2014;8:1653–1664. doi:10.2147/PPA.S67253
42. Burks J, Marshall TS, Ye X. Adherence to disease-modifying therapies and its impact on relapse, health resource utilization, and costs among patients with multiple sclerosis. Clinicoecon Outcomes Res. 2017;9:251–260. doi:10.2147/CEOR.S130334
43. Río J, Tintoré M, Sastre-Garriga J, et al. Change in the clinical activity of multiple sclerosis after treatment switch for suboptimal response. Eur J Neurol. 2012;19(6):899–904. doi:10.1111/j.1468-1331.2011.03648.x
44. Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17(2):162–173. doi:10.1016/S1474-4422(17)30470-2
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.