Back to Journals » Patient Preference and Adherence » Volume 17
Patient Perspectives on the Burden of Heart Failure with Preserved Ejection Fraction in a US Commercially Insured and Medicare Advantage Population: A Survey Study
Authors Nguyen C , Bamber L, Willey VJ, Evers T , Power TP, Stephenson JJ
Received 16 November 2022
Accepted for publication 1 April 2023
Published 3 May 2023 Volume 2023:17 Pages 1181—1196
DOI https://doi.org/10.2147/PPA.S395242
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Johnny Chen
Chi Nguyen,1 Luke Bamber,2 Vincent J Willey,1 Thomas Evers,2 Thomas P Power,3 Judith J Stephenson1
1HealthCore, Inc., Wilmington, DE, USA; 2Bayer AG, Wuppertal, 42096, Germany; 3AIM Specialty Health, Chicago, IL, USA
Correspondence: Chi Nguyen, HealthCore, Inc., 123 Justison Street, Suite 200, Wilmington, DE, USA, Tel +1- 302-230-2000, Fax +1-302-230-2020, Email [email protected]
Background: Patient-reported health related quality of life (HRQOL) is not routinely assessed in clinical practice. Little is known about health status outcomes reported by patients with heart failure with preserved ejection fraction (HFpEF) in non-clinical trial settings.
Purpose: To better understand patient burden of HFpEF in terms of HF-specific functional and symptom status, HRQOL, healthcare resource utilization (HCRU) and costs in a US-based commercial and Medicare Advantage insured population.
Patients and methods: We conducted a cross-sectional survey of patients with HFpEF and linked their survey and administrative claims data. Consenting, eligible patients completed a survey that included the 23-item Kansas City Cardiomyopathy Questionnaire (KCCQ-23) and the PROMIS Global Health-10 (GH-10) questionnaire, as well as clinical and demographic questions. HF medication use, HCRU and costs during the 12-month baseline period before the survey were determined from claims data. Generalized linear regression was used to assess the associations between baseline characteristics and the KCCQ-23 overall summary score.
Results: Of 598 survey respondents with survey and claims data, 54.7% were female with mean age 74.0 years. The KCCQ-23 overall summary and clinical summary scores were 64.8 and 63.0, respectively, and the GH-10 physical and mental health summary scores were 39.9 and 45.5. Factors related to lower KCCQ-23 overall summary scores were HF treatment and symptom changes during the past 4-weeks before the survey, hospital admission during the past year, low household income, high comorbidity index, and morbid obesity (BMI> 40). Total all-cause healthcare costs were $38,243 during the year prior to the survey, of which 42% were HF-related.
Conclusion: Patient-reported outcome measure scores indicated impairment due to HF symptoms and physical limitations in this real-world sample of patients with HFpEF, highlighting a need to assess patient-reported outcomes as well as the clinical and economic outcomes traditionally assessed by clinicians, health systems and payers.
Keywords: heart failure with preserved ejection fraction, HFpEF, patient-reported health related quality of life, healthcare resource utilization, healthcare costs
Introduction
According to the American Heart Association (AHA), approximately 6 million adults in the United States (US) lived with heart failure (HF) from 2015 to 2018, and this figure is projected to rise to over 8 million by 2030.1 HF is the leading cause of hospitalization in older adults with nearly one million hospitalizations in the US annually, and is characterized by a high rate of mortality with one in every eight deaths related to HF.1 HF patients typically experience physical and mental health symptoms that have a significant impact on their health-related quality of life (HRQOL). Common symptoms of HF include dyspnea, fatigue, shortness of breath, edema, chest pain, depression, and anxiety,2 resulting in lower HRQOL and physical functioning limitations in carrying out activities of daily living. In addition to the clinical burden, HF is also associated with a substantial economic burden on both patients and the health care system. Healthcare expenditures are expected to increase to $70 billion by the end of the next decade due to the growing prevalence of HF and the number of hospital admissions.1
Patients with HF are classified according to their left ventricular ejection fraction (LVEF) as HF with reduced ejection fraction (HFrEF), HF with preserved ejection fraction (HFpEF) and borderline/intermediate pEF. HFpEF accounts for approximately 50% of HF cases and its prevalence relative to HFrEF is increasing.1,3 There are currently few effective therapies for HFpEF and guideline-indicated therapeutic options for HFpEF are limited to the management of comorbidities and the use of diuretics to alleviate symptoms.4,5 The US Food and Drug Administration (FDA) recently approved the use of sacubitril/valsartan for patients with HFpEF, making it the first drug available for patients with HFpEF in the US.6 Other HFpEF clinical trials have not led to therapies that show a meaningful reduction in morbidity or mortality in HFpEF patients.7–10
While ongoing trials with existing and new therapies continue to examine HFpEF relative to clinical endpoints, understanding health status from a patient’s perspective and economic burden from a health plan’s point of view are equally important in assessing the burden of disease, improving patient-centered care and properly allocating healthcare resources. With today’s enhanced focus on patient-centered care, the use of validated patient-reported outcome measures (PROMs) is becoming more important. For example, the Centers for Medicare and Medicaid Services (CMS) has launched the Meaningful Measures initiative which seeks to improve outcomes though a variety of strategies, including “unleashing (the) voice of (the) patient through use of patient-reported outcome measures” and aligning measures across all payers.11
This study aimed to better understand the self-reported burden of HFpEF from a patient’s perspective and assess its association with the economic burden in a US-based commercial and Medicare Advantage insured population through a patient survey and linked administrative claims data. The study focused on HFpEF as the treatment options for this group of patients are being actively explored but have been very limited until recently. Data in this HFpEF population will help describe the burden in these patients prior to the availably of approved therapies. More specifically, the study objectives were to: (1) Describe patients’ self-reported health status, including HF-specific functional and symptom status, and overall well-being using the 23-item Kansas City Cardiomyopathy Questionnaire (KCCQ-23)12 and the Patient-Reported Outcomes Measurement Information System Global Health 10-item Short Form (PROMIS Global-10) Questionnaire;13 (2) Assess the impacts of key baseline factors on the KCCQ-23 overall summary score; and (3) Describe the healthcare resource utilization (HCRU) and healthcare costs during the 12-month baseline period prior to and including the survey date using linked administrative claims data.
Methods
Study Design
This study consisted of a cross-sectional patient survey of adults with HFpEF followed by linkage of respondents’ survey data with their administrative claims data for the 12-month period prior to and including the survey date, referred to as the baseline period. The sample population consisted of patients who had at least 1 medical claim with an ICD-9-CM or ICD-10-CM diagnosis code for diastolic HF as a specific ICD-10-CM code does not exist for HFpEF during the patient identification period from January 1, 2006 to July 31, 2019 and satisfied all other inclusion and exclusion criteria.
Data Source and Patient Population
The HealthCore Integrated Research Database (HIRD®), a large administrative claims database that contains longitudinal medical and pharmacy claims from 14 US health plans, was utilized as the sampling frame to identify the eligible survey population from their administrative claims.
Patients were required to meet the following inclusion criteria: (1) Currently active, survey-eligible health plan members with commercial or Medicare Advantage health insurance; (2) At least 1 medical claim with an ICD-9-CM or ICD-10-CM diagnosis code in any position for diastolic HF (ICD-9-CM = 428.3, 428.31, 428.32, 428.33; ICD-10-CM = I50.30, I50.31, I50.32, I50.33) in an inpatient or emergency department setting OR at least 2 medical claims with diagnosis codes in an outpatient setting at least 30 days apart between January 1, 2006 and the patient identification period end date (July 31, 2019); (3) At least 1 evaluation of left ventricular ejection fraction (LVEF) to provide more certainty of the diagnosis given the important role of LVEF in the classification of HF, (4) At least 12 months health plan continuous enrolment (including both medical and pharmacy coverage) prior to the patient identification period end date and (5) at least 45 years of age on the patient identification period end date, July 31, 2019.
Recruitment and Data Collection Process
Recruitment emails and letters were sent to patients meeting the study inclusion criteria to inform them of the study and solicit their participation. If no response was received after a maximum of 5 attempts, interviewers called patients with dialable telephone numbers and recruited them over the telephone. Survey administration was either via the internet or over the telephone with an interviewer.
Patients who responded or were contacted and agreed to participate provided verbal or electronic consent prior to study screening and start of the survey. Patients were screened to ensure they still met study inclusion criteria; qualified patients who completed the survey were compensated for their time.
Patients completed a survey that included the 23-item Kansas City Cardiomyopathy Questionnaire (KCCQ-23), PROMIS Global Health 10-item (GH-10) Short Form, as well as demographic and clinical questions (age, gender, race/ethnicity, marital status, education, employment status, household income, height, weight, smoking status, and activity level), HF diagnosis, HF treatments, and recent changes in medications. After allowing sufficient time for the administrative claims data to overlap the dates of the survey, patients’ survey data were linked with their administrative claims data for determination of the comorbidity index, HF medication use, HCRU and healthcare costs during the baseline period.
Patient Reported Outcome Measures
The patient survey was developed to collect data regarding the real-world burden of HF on HRQoL, symptoms and physical functioning. The primary outcomes of interest were the burden of HF as measured by the KCCQ-23 and HRQoL impairment as measured by the GH-10.
The KCCQ-23 is a validated, reliable 23-item questionnaire that assesses HF-specific symptoms, function, and quality of life (QoL) in patients with HFpEF.12,14 The KCCQ has seven domains: symptom frequency, symptom burden, symptom stability, physical limitations, social limitations, quality of life, and self-efficacy. The following summary scores are formed by combining domain scores: total symptom score (symptom frequency score and symptom burden score); clinical summary score (total symptom score and physical limitations score); and overall summary score (total symptom score, physical limitations score, social limitations score, and QoL score). All raw domain and summary scores are transformed into scores that range from 0–100, with higher scores indicating better HF-specific health status; the 0–100 scores are frequently summarized in 25-point ranges where scores represent health statuses as follows: 0–24: “very poor to poor”; 25–49: “poor to fair”; 50–74: “fair to good”; and 75–100: “good to excellent”.15
The PROMIS Global Health 10-item Short Form (GH-10) is a validated HRQoL measure consisting of 10 global items that assess an individual’s physical, mental, and social health.13 The measure is generic, rather than disease-specific, and is intended to globally reflect an individual’s assessment of their health. Two summary scores are calculated from the GH-10: Physical Health Summary (PHS) score and Mental Health Summary (MHS) score. Raw PHS and MHS scores are computed and standardized (norm scored) relative to the US general population norm score of 50 and standard deviation of 10 with lower scores indicating more impaired health compared to the US general population. PHS and MHS scores may be interpreted as: >50: “same or better than US general population norm”; 40 - < 50: “below US general population norm”; <40: “well below US general population norm”.
A secondary PROM of interest was the patient self-assessment of the severity of their current HF symptoms based on the traditional, clinician-assessed New York Heart Association (NYHA) functional classification system.16 The self-reported NYHA functional classification classifies the extent HF limits physical activity on a four-point scale that ranges from Class I-No limitations on patient physical activity to Class IV-Severe limitations.
Administrative Claims Linkage
Administrative claims submitted during the 12 month baseline period were extracted from the HIRD for patients who completed the patient survey and had at least 11 months of continuous enrollment during the baseline period. Patient demographics, clinical characteristics, comorbidities, and medication use were determined from the claims data. In addition, all cause and HF-related HCRU and costs were assessed.
Statistical Analysis
A respondent versus non-respondent analysis compared administrative claims data characteristics of survey respondents and non-respondents using standardized differences. The standardized difference represented the magnitude of the difference between the two groups and was independent of sample size. A standardized difference of less than 0.2 indicated a small and negligible difference between the two comparison groups.17,18
All survey and claims data variables were described with univariate statistics using means, standard deviations, and medians for continuous variables and relative frequencies and percentages for categorical variables, respectively. The KCCQ-23 and GH-10 were scored according to instructions from measure developers and reported with descriptive statistics (means and standard deviations). A generalized linear regression model was developed to assess the associations between the KCCQ-23 overall summary score and key baseline factors, including patient demographics (age, gender, race/ethnicity), household annual income, comorbidity index, body mass index (BMI), HF-related HCRU during 12 months prior to the survey, and HF treatment and symptoms changes in the 4 weeks prior to the survey. The model assumed a normal distribution and model variables were selected based on clinical relevance and model fit. A complete-case analysis approach was used, and individuals with missing data on any variable in the generalized linear regression model were excluded. A two-sided alpha-level of p≤0.05 was considered significant for all analyses. Statistical analyses were performed using SAS Enterprise Guide 7.1 (SAS Institute; Cary, NC).
Human Subject Projection
As protected health information (PHI) was required for the conduct of this study, a Health Insurance Portability and Accountability Act of 1996 (HIPAA) Waiver of Authorization was applied for and obtained from the New England Institutional Review Board (NEIRB) prior to any PHI being identified and the start of survey fielding. In addition, survey respondents gave verbal or electronic informed consent prior to starting the survey.
Results
Survey Sample Disposition
Of the 19,405 eligible patients with diastolic HF who were identified from administrative claims in the HIRD, 15,908 patients were sent recruitment emails and/or letters, 2662 patients responded to the recruitment materials, 1286 patients provided verbal or electronic consent to participate, 734 patients met all screening questions and qualified, and 610 patients completed the surveys (list completion rate was 3.8%). Of these 610 survey respondents, we were able to link the survey data and claims data of 598 patients (Figure 1).
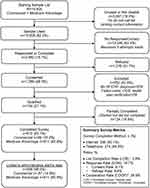 |
Figure 1 Illustrates the flow of survey sample disposition. |
A survey respondent and non-respondent analysis indicated that there were no major differences between the two groups as all standardized differences were less than 0.2, except resident region where survey respondents had a slightly greater percentage of patients living in the Midwest region than survey non-respondents (67.5% vs 59.9%) (Supplemental Table 1).
Patient Characteristics
Of the 598 patients with linked survey and claims data, 54.7% were female, with a mean age of 74.0 years. The majority of respondents were white, non-Hispanic (88.6%). Most respondents reported they were retired or disabled (81.1%) and had an annual household income less than $50,000 per year (66.4%). Average body mass index (BMI) was 32.3 and more than half (53.0%) of respondents were obese (BMI ≥30). On average, respondents had HF for approximately 9 years (Table 1).
 |
Table 1 Patient Demographic and Clinical Characteristics |
When asked about any changes to their HF treatment, lifestyle, and symptoms (ie, diuretic dose increase, noticeable weight gain, unscheduled doctor visits, ER visits, and diet change) during the 4 weeks before the survey, 57.7% of respondents reported no changes, 42.1% reported at least one change and 9.9% reported 3 or more changes. Specifically, 19.2% experienced edema indicated by a greater than 5 pound weight gain in a single week, and 20.1% of respondents reported an increase in diuretic dose over the past 4 weeks (Table 1).
Respondents reported their physical function status that most closely corresponded to the NYHA functional classifications, where Level I represented no limitation on physical activity and Level IV represented severe limitations. Approximately 22.2% of respondents classified themselves as NYHA Level I, 37.0% as Level II, and 31.1% as Level III; only 8.7% of respondents classified themselves as Level IV indicating severe limitation on their physical activity (Table 1). The correlation between self-reported NYHA and the KCCQ-23 overall summary score was −0.73 (p<0.01).
The mean Quan-Enhanced Charlson comorbidity index (QCI) score was 3.8. The most commonly occurring comorbidities were hypertension (96.0%), hyperlipidemia (81.8%), and chronic kidney disease (46.0%) (Table 1).
Patient’s Self-Reported Health Status
The KCCQ-23 mean (SD) overall summary and clinical summary scores were 64.8 (SD 25.4) and 63.0 (SD 24.3), respectively. The mean (SD) total symptom score, which quantified the frequency and burden of HF clinical symptoms such as fatigue, shortness of breath, and edema, was 67.7 (SD 24.8). The mean (SD) physical limitations score and the mean (SD) quality of life score per KCCQ-23 were 58.3 (SD 27.8) and 70.4 (SD 30.2), respectively. The mean (SD) social limitation score was 63.8 (SD 31.6). The symptom stability score, which was not part of the symptom domain score, measured recent changes in HF symptoms for the 2 weeks before the survey date. The mean (SD) symptom stability score was 51.7 (SD 16.8). On a categorical scale, 10.3% of respondents reported their HF symptoms on the survey date were worse than two weeks ago, 50% reported their HF symptoms were unchanged, 13.3% reported their HF symptoms were better, and 26.4% reported they had no HF symptoms at all during the two week period prior to the survey date. Respondents mean (SD) self-efficacy score was 86.4 (SD 18.1) (Table 2).
 |
Table 2 Patient-Reported Outcome Measure (PROM) Scores |
The mean (SD) GH-10 physical health summary score was 39.9 (SD 9.2), equivalent to one standard deviation lower than the general US population norm of 50. The mean (SD) mental health summary score was 45.5 (SD 9.4) (Table 2).
Health Resource Utilization (HCRU) and Costs
All-cause HCRU during the 12-month baseline period prior to the survey date was high among respondents with 48.2% of them having at least one inpatient admission, 20.1% having at least one 30-day readmission and 40% with at least one ER visit. Among respondents who were hospitalized, the average length of stay was 5 (SD 4.55) days. Almost all respondents had at least one office visit (99.7%) and approximately 10% of them had a stay in a skilled nursing facility (SNF) (Table 3).
 |
Table 3 Healthcare Resource Utilization (HCRU) for 12 Month Period Prior to Survey |
HF-related hospitalization, defined as a medical claim for an inpatient admission with a HF diagnosis code in any position, was 39.8%, accounting for 82.6% of all hospitalizations. During the baseline period, 93.6% of respondents filled a prescription for one or more classes of HF-related medications. Among those treated, respondents received approximately 2.7 classes of HF-related medications and were covered by HF-related medications 90% of the time during the year. The most commonly used medications were loop diuretics and beta blockers (76.1% and 53.7%, respectively). Patients also filled prescriptions for other medications such as angiotensin converting enzyme (ACEs) inhibitors (31.9%), angiotensin receptor blockers (ARBs) (24.6%), mineralocorticoid receptor antagonists (MRAs) (22.2%) and digoxin (6.5%). Only 3.7% of respondents used sacubitril/valsartan (Entresto) during the baseline period (Table 3).
The average annual all-cause healthcare costs per patient during the baseline period were $38,243, of which medical costs were $29,613 and pharmacy costs were $8630. Hospitalization expenses contributed to 38.4% of the all-cause total healthcare costs ($14,683). HF-related costs ($15,380) accounted for approximately 40.2% of the total healthcare costs (Figure 2).
 |
Figure 2 Illustrates the average annual all-cause and HF-related healthcare costs per patient during the baseline period, categorized by place of service. |
Baseline Determinant Factors of KCCQ-23 Summary Score
The linear regression model showed that current HF treatment and recent changes in HF treatments or symptoms during the 4 week period before the survey date, such as increasing diuretic dose, noticeable weight gain, unscheduled office visit, ER visit, and diet change, were strongly associated with the KCCQ-23 overall summary score. More specifically, after controlling for other baseline factors, the KCCQ-23 overall summary scores of respondents with 1, 2, 3+ changes in HF treatment, lifestyle, or symptoms were 6.6, 13.3 and 16.3 points lower than respondents with no change, respectively (p ≤0.01) (Table 4).
 |
Table 4 Generalized Linear Model* Examining the Impact of Baseline Characteristics and All-Cause HCRU to KCCQ Overall Summary Score |
Other factors related to lower KCCQ-23 overall summary scores were being admitted to the hospital for any reason during the baseline period (estimate −5.0, p=0.02), having a QCI score of 3 or higher (estimate −9.3, p<0.01), and being morbidly obese (estimate −16.1, p<0.01). In contrast, a higher household annual income appeared to be associated with an increased KCCQ-23 overall summary score. The KCCQ-23 overall summary scores of respondents with household annual incomes of $25,000 - $49,999, $50,000 - $74,999, and $75,000+ were 5.4, 9.6 and 10.8 points higher than those with low income (less than $25,000 annually), respectively (p<0.05). Patient demographic characteristics such as age, gender, and race/ethnicity were not associated with KCCQ-23 overall summary scores (Table 4).
Similarly, the model including HF-related hospitalizations showed they were also associated with lower KCCQ-23 overall summary scores after adjusting for other baseline factors (Table 5).
 |
Table 5 Generalized Linear Model* Examining the Impact of Baseline Characteristics and HF-Related HCRU to KCCQ-23 Overall Summary Score |
Discussion
This study provided a real-world picture of the HF burden on patients’ HRQoL and health status in a contemporary, real-world US setting. The findings in this commercial and Medicare Advantage population showed that HFpEF patients in the community setting experienced symptom burden and quality of life impairment, notably in physical functioning. KCCQ scores showed similar level of symptom, physical limitation, and quality of life impairment as in recent clinical trials in the HFpEF population. The population had a high disease burden in terms of healthcare utilization and costs. We used several validated instruments to assess functional and symptom status and overall health-related quality of life (KCCQ-23 and GH-10). The feature that distinguishes our study from previously published research was our ability to link patients’ survey data with their administrative claims data, which added information on HF prescription fill patterns, HCRU, and healthcare expenses during the 12-month baseline period.
The PROM results from the varying instruments were congruent with each other. Both the KCCQ-23 scores and the patient self-reported NYHA indicated that HF patients experienced impairment due to symptoms and physical limitations. The KCCQ-23 mean overall summary scores were consistent with previous clinical trials in the HFpEF population.19–23 Specifically, the KCCQ-23 mean overall summary score of HFpEF patients at randomization was 71.4 (18.9) in the PARAGON-HF trial of sacubitril/valsartan22 and 58.1 (23.4) in the TOPCAT trial of spironolactone among the sub-group of patients enrolled in the US.23 In our study population, the KCCQ-23 mean overall summary score was 64.8 (25.4). The high mean self-efficacy sub-domain score (86.4) indicated that patients had knowledge about HFand knew what to do and who to contact if their HF symptoms worsened. The GH-10 physical and mental health summary scores were consistent with other scores in that they indicated a greater impact on physical health functioning than mental health functioning among patients with HF.
We saw a strong correlation between the KCCQ-23 overall summary scores and the patient-reported NYHA class, as consistent with the literature.14 While not surprising, it is important when taken in context with the results of recent research comparing the longitudinal changes, concordance and clinical outcomes associated with traditional clinician-assessed NYHA class and the KCCQ-23 overall summary scores. Greene et al24 reported that the KCCQ was more sensitive to clinically meaningful changes in health status over time and may have more prognostic value than changes in clinician-assessed NYHA class in patients with reduced ejection fraction HF. Our results suggest that valuable insights into a patient’s overall condition may be obtained through the use of a single question that asks patients to self-report their NYHA classification. Keeping the burden associated with the collection of PROMs at a minimum may facilitate their routine collection and use by clinicians.25
In our real-world, non-clinical trial population, after adjustment for confounding factors, markers of suboptimal HF status such as increased diuretic use, short term weight gain, diet changes and unscheduled healthcare visits in the past month were significantly associated with lower KCCQ-23 overall summary scores and KCCQ-23 symptom stability domain scores. These factors were asked within a 4-week recall period, indicating high correlations of KCCQ-23 scores and factors that occurred close to the assessment date. In this study, we found that socioeconomic factors played a role in HF-related QOL, as higher incomes had a direct and significant association with increased KCCQ-23 scores. The results aligned with prior research,26 highlighting the impact of socio-economic status on patients’ QOL. Morbid obesity, with its links to poorer clinical outcomes, was associated with lower KCCQ-23 overall summary scores.
The linkage to administrative claims data also allowed us to examine the impact of baseline healthcare resource utilization on PROM scores. Our data show that inpatient admissions during the baseline period for any indication significantly reduced the KCCQ-23 overall summary score by 5 points, after controlling for baseline factors. In clinical trials and observational registries, the KCCQ-23 has been shown to be an effective risk assessment for subsequent clinical events.15,24 Additional research is needed to assess the incremental predictive value of adding the use of the KCCQ-23 or KCCQ-12 to traditional clinical and clinician-reported parameters for predicting impending HF exacerbations.
Our analysis confirmed a high disease burden for patients and payers. In terms of HCRU, 48.2% of study respondents were admitted to the hospital during the year prior to their survey date and their average total annual healthcare costs were $38,243. HF-related costs were $15,380, accounting for 40% of the total costs. The cost figures were slightly higher than determined for a previous study,27 most likely due to our predominantly Medicare Advantage population. Both all-cause and HF-related hospitalizations in the baseline period were associated with worse/lower KCCQ-23 overall summary scores.
We also observed a significant proportion of patients on ACE inhibitors, ARBs, or MRA, most likely due to the treatment of HF-related comorbidities as these therapies lack evidence demonstrating their clinical benefits in patients with diastolic HF. The use of sacubitril/valsartan was low, indicating its recent approval in HFpEF. It is important to continue to assess treatment patterns in HFpEF patients as specific HFpEF therapy data, FDA approvals and guidelines continue to evolve.
There are some study limitations that should be acknowledged. Identification of a HFpEF population in claims data relies on the use ICD-9/10-CM codes that are based on the diastolic/systolic nomenclature description for HF. In addition, ejection fraction results are key to the HFpEF diagnosis, and these results were not available within our data. However, we did mandate the presence of at least 1 diagnostic test claim for the assessment of LVEF in our inclusion criteria and the demographic and clinical characteristics our study cohort were compatible with other HFpEF populations identified using standard clinical and diagnostic criteria.28,29 Our study was limited to US patients with commercial and Medicare Advantage health insurance, which could impact the generalizability of the results to other population segments such as traditional Medicare, Medicaid and the uninsured population. Also, the patient-reported survey data may be subject to self-selection and recall bias. The study design was cross-sectional, and therefore no causal inference could be made. Other potential confounders, such as genetics, mental health, and out-of-pocket costs, were not measured and included in the study.
Conclusion
PROM results indicated health status impairment due to HF symptoms and physical limitations in this real-world study. The study also highlighted the impact of proximal baseline factors such as self-reported HF treatment and changes in the past 4 weeks as well as inpatient admissions in the past year, household income and BMI on KCCQ-23 overall summary scores. The findings underscore the importance of including the patient’s voice in clinical practice.
Research Ethics and Consent
This study was approved by the New England Institutional Review Board (NEIRB) (IRB no. 120190292). Survey participants were fully informed about the nature and objectives of the study. All survey participants provided electronic or verbal consent prior to participating in the survey. The study was conducted in compliance with the principles of the Declaration of Helsinki.
Funding
This work was funded by Bayer AG, Wuppertal, Germany.
Disclosure
Chi Nguyen, Judith J Stephenson, and Vincent J Willey are employees of HealthCore, Inc. Wilmington, Delaware 19801, USA. HealthCore received funding from Bayer AG, Wuppertal, Germany to conduct this study. Luke Bamber and Thomas Evers are employees of Bayer AG. Thomas P Power is an employee of AIM Specialty Health in Chicago, Illinois, USA. The authors report no other conflicts of interest in this work.
References
1. Virani SS, Alonso A, Aparicio HJ, et al. Heart disease and stroke statistics-2021 update: a report from the American heart association. Circulation. 2021;143(8):e254–e743. doi:10.1161/CIR.0000000000000950
2. Alpert CM, Smith MA, Hummel SL, Hummel EK. Symptom burden in heart failure: assessment, impact on outcomes, and management. Heart Fail Rev. 2017;22:25–39. doi:10.1007/s10741-016-9581-4
3. Oktay AA, Rich JD, Shah SJ. The emerging epidemic of heart failure with preserved ejection fraction. Curr Heart Fail Rep. 2013;10:401–410. doi:10.1007/s10741-016-9581-4
4. Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37(27):2129–2200. doi:10.1093/eurheartj/ehw128
5. Gazewood JD, Turner PL. Heart failure with preserved ejection fraction: diagnosis and management. Am Fam Physician. 2017;96(9):582–588.
6. Solomon SD, McMurray JJV, Anand IS, et al. Angiotensin-neprilysin inhibition in heart failure with preserved ejection fraction. N Engl J Med. 2019;381(17):1609–1620. doi:10.1056/NEJMoa1908655
7. Yusuf S, Pfeffer MA, Swedberg K, et al. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-preserved trial. Lancet. 2003;362:777–781. doi:10.1016/S0140-6736(03)14285-7
8. Cleland JG, Tendera M, Adamus J, et al. The perindopril in elderly people with chronic heart failure (PEP-CHF) study. Eur Heart J. 2006;27(19):2338–2345. doi:10.1093/eurheartj/ehl250
9. Massie BM, Carson PE, McMurray JJ, et al. Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med. 2008;359:2456–2467. doi:10.1056/NEJMoa0805450
10. Pitt B, Pfeffer MA, Assmann SF, et al. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014;370(15):1383–1392. doi:10.1056/NEJMoa1313731
11. Centers for Medicare and Medicaid Services. Patient assessment instruments. Available from: https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/MMS/Downloads/Blueprint.pdf.
12. Green CP, Porter CB, Bresnahan DR, Spertus JA. Development and evaluation of the Kansas City cardiomyopathy questionnaire: a new health status measure for heart failure. J Am Coll Cardiol. 2000;35(5):1245–1255. doi:10.1016/s0735-1097(00)00531-3
13. Hays RD, Bjorner JB, Revicki DA, Spritzer KL, Cella D. Development of physical and mental health summary scores from the patient-reported outcomes measurement information system (PROMIS) global items. Qual Life Res. 2009;18:873–880. doi:10.1007/s11136-009-9496-9
14. Joseph SM, Novak E, Arnold SV, et al. Comparable performance of the Kansas City cardiomyopathy questionnaire in patients with heart failure with preserved and reduced ejection fraction. Circ Heart Fail. 2013;6(6):1139–1146. doi:10.1161/circheartfailure.113.000359
15. Spertus JA, Jones PG, Sandhu AT, Arnold SV. Interpreting the Kansas City cardiomyopathy questionnaire in clinical trials and clinical care: JACC state-of-the-art review. J Am Coll Cardiol. 2020;76:2379–2390. doi:10.1016/S0735-1097(00)00531-3
16. The Criteria Committee of the New York Heart Association. Nomenclature and Criteria for Diagnosis of Diseases of the Heart and Great Vessels.
17. Cohen J. Statistical Power Analysis for the Behavioral Sciences.
18. Faraone SV. Interpreting estimates of treatment effects: implications for managed care. P T. 2008;33(12):700–711.
19. Kosiborod MN, Bhatt AS, Claggett BL, et al. Effect of dapagliflozin on health status in patients with preserved or mildly reduced ejection fraction. J Am Coll Cardiol. 2023;81(5):460–473. doi:10.1016/j.jacc.2022.11.006
20. Chandra A, Polanczyk CA, Claggett BL, et al. Health-related quality of life outcomes in PARAGON-HF. Eur J Heart Fail. 2022;24(12):2264–2274. doi:10.1002/ejhf.2738
21. Armstrong PW, Lam CSP, Anstrom KJ, et al. Effect of vericiguat vs placebo on quality of life in patients with heart failure and preserved ejection fraction: the VITALITY-HFpEF randomized clinical trial. JAMA. 2020;324(15):1512–1521. doi:10.1001/jama.2020.15922
22. Chandra A, Vaduganathan M, Lewis EF, et al. Health-related quality of life in heart failure with preserved ejection fraction. JACC Heart Fail. 2019;7(10):862–874. doi:10.1016/j.jchf.2019.05.015
23. Flint KM, Shah SJ, Lewis EF, Kao DP. Variation in clinical and patient-reported outcomes among complex heart failure with preserved ejection fraction phenotypes. ESC Heart Fail. 2020;7(3):811–824. doi:10.1002/ehf2.12660
24. Greene SJ, Butler J, Spertus JA, et al. Comparison of New York heart association class and patient-reported outcomes for heart failure with reduced ejection fraction. JAMA Cardiol. 2021;6(5):522. doi:10.1001/jamacardio.2021.0372
25. Heidenreich PA. The growing case for routine collection of patient-reported outcomes. JAMA Cardiol. 2021;6(5):497. doi:10.1001/jamacardio.2021.0391
26. Khariton Y, Nassif ME, Thomas L, et al. Health status disparities by sex, race/ ethnicity, and socioeconomic status in outpatients with heart failure. JACC Heart Fail. 2018;6(6):465–473. doi:10.1016/j.jchf.2018.02.002
27. Nguyen C, Zhang X, Evers T, et al. Real-world treatment patterns, healthcare resource utilization, and costs for patients with newly diagnosed systolic versus diastolic heart failure. Am Health Drug Benefits. 2020;13(4):166.
28. Pfeffer MA, Shah AM, Borlaug BA. Heart failure with preserved ejection fraction in perspective. Circ Res. 2019;124(11):1598–1617. doi:10.1161/CIRCRESAHA.119.313572
29. Pfeffer MA, Claggett B, Assmann SF, et al. Regional variation in patients and outcomes in the Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist (TOPCAT) trial. Circulation. 2015;131:34–42. doi:10.1161/CIRCULATIONAHA.114.013255
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
