Back to Journals » Journal of Inflammation Research » Volume 14
Spermidine Inhibits Joints Inflammation and Macrophage Activation in Mice with Collagen-Induced Arthritis
Authors Yuan H , Wu SX, Zhou YF, Peng F
Received 28 March 2021
Accepted for publication 12 June 2021
Published 24 June 2021 Volume 2021:14 Pages 2713—2721
DOI https://doi.org/10.2147/JIR.S313179
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Ning Quan
Hao Yuan,1 Si-Xian Wu,1 Yi-Feng Zhou,2 Fang Peng1
1Department of Clinical Laboratory, Hunan Provincial People’s Hospital, The First Affiliated Hospital of Hunan Normal University, Changsha, 410005, People’s Republic of China; 2Operating Room, Hunan Provincial People’s Hospital, The First Affiliated Hospital of Hunan Normal University, Changsha, 410005, People’s Republic of China
Correspondence: Yi-Feng Zhou
Operating Room, Hunan Provincial People’s Hospital, The First Affiliated Hospital of Hunan Normal University, Changsha, 410005, People’s Republic of China
Tel +86-13677366519
Fax +86-731-82278012
Email [email protected]
Purpose: Spermidine (SPD) is a naturally occurring polyamine. In this study, we examined the role and possible mechanism of SPD in collagen-induced arthritis (CIA) mice.
Materials and Methods: CIA mice were intraperitoneally injected with SPD (2 and 50 mg/kg), dexamethasone (0.5 mg/kg), or saline daily for 21 days. The severity of the disease and inflammatory responses in the serum and joint tissue were assessed through macroscopic, immunohistochemical, and histological analyses.
Results: Macroscopic and histological results indicated that SPD protected against the development of CIA. SPD suppressed the levels of the pro-inflammatory cytokines IL-6 and IL-1β and increased the levels of the anti-inflammatory factor IL-10 in the serum. Immunohistochemical staining showed that 50 mg/kg SPD inhibited iNOS expression in synovial macrophages in the ankle joints of CIA mice.
Conclusion: These results suggest that SPD may protect CIA mice by inhibiting the polarization of M1 macrophages in the synovial tissue, reducing pro-inflammatory cytokines, and promoting anti-inflammatory factor release.
Keywords: rheumatoid arthritis, collagen-induced arthritis mice, spermidine, macrophage polarization, inflammation
Introduction
Rheumatoid arthritis (RA) is an autoimmune disease characterized by chronic symmetrical synovitis and extra-articular disease, with an incidence of 0.1–1.1% in the population.1–3 To date, the cause of RA has not been clarified, and no drug can completely relieve RA.
Excessive production of pro-inflammatory mediators is an important factor that promotes RA pathogenesis.1 Macrophages are one of the main effector cells involved in the development of RA. Macrophage-lineage cells in RA patients or animal models tend to polarize to pro-inflammatory macrophages (M1). M1 cells secrete a large number of pro-inflammatory cytokines, such as interferon-γ (IFN-γ), tumor necrosis factor α (TNF-α), interleukin-1 (IL-1), and IL-6, which can activate fibroblasts and osteoclasts, recruit neutrophils, monocytes, and lymphocytes, trigger a series of inflammatory reactions, accelerate the inflammatory process, and articular cartilage.4–9
Spermidine (SPD) is a naturally occurring polyamine.10 Studies have found that exogenous SPD supplementation is beneficial for aging,11–13 cancer,13,14 neurodegenerative diseases,15 cardiovascular diseases,16,17 and inflammatory diseases.18,19 SPD can inhibit the activation of macrophages and exert anti-inflammatory effects through macrophages.20 Yang et al21 demonstrated that SPD can improve experimental autoimmune encephalomyelitis by regulating the infiltration of macrophages in the central nervous system and inducing the polarization of macrophages to the M2 phenotype. The depletion of polyamines in macrophages increases the gene expression of LPS-induced pro-inflammatory mediators, such as TNF-α, IL-12, IL-6, and chemokines, indicating that polyamines are inhibitors of inflammatory gene expression.22 During infection, trauma, cancer, and autoimmune diseases, damaged or dying cells at the site of inflammation release polyamines, increasing the concentration of polyamines, which is believed to play an important regulatory role in limiting inflammatory damage.21,23 A previous study showed that SPD and spermine (SPM) accumulate in the urine, synovial fluid, and synovial tissue of patients with RA.24 Iezaki et al25 demonstrated that oral supplementation of SPD or SPM can suppress the increase in the expression of receptor activator of nuclear factor-κB ligand (RANKL) in the synovial tissue and prevent RANKL-induced osteoclast differentiation in collagen-induced arthritis (CIA) rats. However, Silva et al26 found that inhibiting the synthesis of endogenous polyamines can reduce inflammatory pain in arthritic rats, whereas administration of exogenous polyamines under the surface of the hind paw can cause pain and edema in non-arthritic rats. Whether SPD supplementation alleviates the condition of RA or aggravates its clinical symptoms remains controversial.
In this study, we established a CIA mouse model and administered different concentrations of SPD by intraperitoneal injection to observe the effects of SPD on joint swelling, expression of serum inflammatory factors IL-10, IL-6, and IL-1β, synovial pathology, and expression of F4/80, inducible nitric oxide synthase (iNOS), and CD206 in membrane tissues to explore the role and possible mechanism of SPD in CIA mice.
Materials and Methods
Experimental Animals
Six-week-old male C57BL/6 mice (20–22 g) were purchased from Hunan SJA Laboratory Animal Co., Ltd. and maintained under specific pathogen-free conditions. After one week of adaptive feeding, the mice were randomly divided into five groups. One group was the normal control group, and the remaining four groups were CIA models. On day 27, the CIA mice were randomly divided into four groups (n = 5/group) as follows: CIA control group, SPD-high dose (50 mg/kg SPD, SPD-H) group, SPD-low dose (2 mg/kg SPD, SPD-L) group, and dexamethasone (DEX; 0.5 mg/kg DEX) group. SPD and DEX were diluted in saline and abdominally injected daily from days 27 to 48. The normal control and CIA control groups were administered an equal volume of saline on the same schedule. On day 49, the mice were euthanized, and their ankle joints were harvested for histological and immunohistochemical analyses.
Ethics Approval
The study was approved by the Ethics Committee of Hunan Provincial People’s Hospital (approval number: 2020S16). The experiments were conducted in compliance with institutional review board regulations. We attempted to minimize animal suffering and reduce the number of experimental animals used.
Induction of CIA Animal Model
Chicken type II collagen (CII) (Chondrex, Inc., Redmond, WA, USA) was dissolved at 2 mg/mL in 0.05 M acetic acid and gently stirred overnight at 4 °C. It was then emulsified with an equal volume of complete Freund’s adjuvant (CFA; Chondrex, Inc.) in an ice-cold water bath. The emulsion was kept at 4 °C and injected within 1 h. Arthritis was induced by an intradermal injection (0.01 mL) at the base of the mouse tail. Twenty-one days later, mice were administered a booster immunization with CII emulsified in CFA in the same manner.27
Determination of Paw Swelling and Arthritis Score
During the course of the experiment, the thickness of both the left and right hind paws of mice was measured using a cursor caliper every five days. The increase in paw swelling was calculated using the following formula: rate of paw swelling change (%) = [paw thickness on day 48 after immunization] – [paw thickness on day 0 prior immunization]/[paw thickness prior to immunization] × 100.28 Mice were assessed every three days between days 21 and 48 for signs of arthritis. Individual paws were recorded on an ordinal scale of 1–4 as follows: 0 = no swelling and redness, 1 = slight swelling and redness in small or large joints, 2 = moderate swelling and redness in at least one joint, 3 = severe swelling and redness in large joints and moderate swelling and redness in small joints; and 4 = entire paw affected and maximal erythema and swelling. The arthritis score was equal to the sum of the limb scores, with a maximum score of 16. An arthritis index score of ≥ 4 indicated that the model was successfully established.27
Measurement of Cytokine Levels in Mice Serum
Blood was collected after all mice were sacrificed, clotted for 2 h at room temperature, and centrifuged at 3500 × g for 10 min at 4 °C. Serum levels of IL-6, IL-1β, and IL-10 were measured using commercially available enzyme-linked immunosorbent assay kits (Multi Sciences, Hangzhou, China) according to the manufacturer’s instructions.
Histological Analysis
The ankle joints of the mice were fixed in 10% neutral buffered formalin for 48 h and decalcified in EDTA for 30 days at 4 °C. After paraffin embedding, tissue sectioning (3 μm thick), and staining with hematoxylin and eosin (HE), two experienced pathologists examined the sections by light microscopy and determined synovitis scores according to the literature.29
Immunohistochemical Staining
Immunohistochemical staining was performed to observe the expression of F4/80, iNOS, and CD206 (Abcam, USA) in the synovial tissue of the ankle joint. Joint tissue slices were observed under the microscope, and the proportion of positive cells was counted in each sample over 10 fields of view.
Statistical Analysis
Statistical analyses were performed using SPSS (version 23.0; SPSS Inc., Chicago, IL, USA). Data are presented as the mean ± standard deviation (SD) or median with interquartile ranges. Data conforming to the normal distribution were analyzed using repeated-measure analysis of variance (ANOVA) or single-factor ANOVA followed by the least significant difference test for comparisons between two groups. Data that did not conform to normal distribution were compared using the Kruskal–Wallis test, and the Kruskal–Wallis single-factor ANOVA test was used for two comparisons between groups. Differences were considered significant at p < 0.05.
Results
Effect of SPD on Macroscopic Features of CIA
Initial signs of arthritis development were observed 24 days after the first immunization with CII. The paw thickness of the CIA control mice increased gradually after immunization and reached a maximum thickness by day 40 (Figure 1A). From the 35th day, the paw thickness of CIA mice treated with 50 mg/kg SPD was significantly lower than that of CIA control mice (p < 0.05, Figure 1A), whereas the paw thickness of DEX-treated CIA mice was significantly lower than that of CIA control mice until the 45th day (p < 0.05, Figure 1A).
The arthritis score of the SPD-H group decreased significantly after three days of treatment, which occurred earlier than that of the DEX group. From day 36, the arthritis score of the SPD-H group was significantly lower than that of the CIA control group (p < 0.05, Figure 1B). Although the arthritis scores of mice in the DEX and SPD-L groups showed a downward trend, there was no significant difference compared with those of the CIA control group (p > 0.05, Figure 1B).
CIA control mice showed significant paw swelling compared to normal control mice (p < 0.001, Figure 1C). There was no significant difference between normal control mice and SPD-H, SPD-L, and DEX mice with regard to the rate of paw swelling change (p > 0.05, Figure 1C).
Effect of SPD on the Expression of IL-6, IL-1β, and IL-10 in the Serum of CIA Mice
To evaluate the capacity of SPD to regulate cytokine production in CIA mice, the levels of the pro-inflammatory cytokines IL-6 and IL-1β and the anti-inflammatory cytokine IL-10 in the serum of each mouse were determined. As shown in Figure 2, the levels of IL-6 (p < 0.01) and IL-1β (p < 0.001) in CIA control mice significantly increased, whereas the expression of IL-10 (p < 0.001) was lower in CIA mice than in normal control mice. The release of IL-10 significantly increased, whereas that of IL-6 and IL-1β significantly decreased after SPD administration at a dose of 50 mg/kg (p < 0.001) or 2 mg/kg (p < 0.01) compared with those in CIA mice, demonstrating a concentration-dependent relationship.
Histopathological Examination
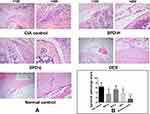
There were no pathological changes in the joints or cartilages of normal mice. HE staining of the ankle joints of CIA mice showed typical histopathological features of synovitis compared to the those of normal control mice (Figure 3A and Table 1): synovial cell proliferation (p < 0.01) with infiltration of a large number of neutrophils, lymphocytes, plasma cells, and other inflammatory cells; formation of lymphoid follicles and appearance of non-foreign-body giant cells; fibrinoid necrosis (p < 0.001); interstitial fibrosis cell proliferation accompanied by neovascularization (p < 0.001), with mild to moderate mesenchymal transformation; and villus-like synovium stretched into the joint cavity, adhered and invaded into the cartilage, destroyed the articular cartilage surface, and caused the joint space to be narrower than that in normal control mice.
 |
Table 1 Histological Scores of Each Group |
In mice treated with 50 mg/kg SPD or DEX, the formation of new blood vessels was significantly reduced compared with that in CIA control mice (p < 0.01, p < 0.01, respectively; Table 1). Treatment with 50 mg/kg or 2 mg/kg SPD or DEX improved fibrinoid necrosis in the joint synovial tissue of CIA mice (p < 0.001, p < 0.001, and p < 0.01, respectively; Table 1). Comprehensive evaluation was performed to obtain the total synovitis score. The ankle synovitis score was significantly lower in the SPD-H (p < 0.05) and DEX groups (p < 0.05) than in the CIA control group, indicating that 50 mg/kg SPD treatment improved joint synovitis in CIA mice.
Immunohistochemical Staining Examination
The immunohistochemical results are shown in Figure 4 and Table 2. The synovial tissue of the normal control group had no inflammatory lesions, and a small number of F4/80-, iNOS-, or CD206-positive cells were observed. The CIA control group showed typical synovitis lesions, strong expression of F4/80 and iNOS, and weak CD206 expression. After treatment with different concentrations of SPD, the expression of F4/80 in the ankle synovial tissue was lower than that in the CIA control group. With the increase of SPD treatment concentration, the expression of iNOS in cytoplasm gradually decreased, the cytoplasm color changed from brown yellow to light yellow, and the expression of CD206 gradually increased.
 |
Table 2 Number of Immune-Positive Cells (%) in Each Group |
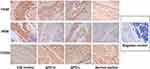 |
Figure 4 Immunohistochemical results of the ankle synovial tissue in each group (SP×400). |
Analysis of F4/80-, iNOS-, and CD206-positive cells in the ankle synovial tissue under high magnification showed that the number of F4/80- and iNOS-positive cells decreased with the increase in SPD treatment concentration, whereas the number of CD206-positive cells increased gradually. Statistical analysis showed that the number of iNOS-positive cells was different among the groups (p < 0.05), and the number of iNOS-positive cells in the ankle tissue of the SPD-H group was significantly lower than that of the CIA control group (p < 0.05). However, there was no significant difference in the number of F4/80- and CD206-positive cells between the groups (p > 0.05).
Discussion
The findings of this study indicate the therapeutic potential of SPD in CIA. Specifically, SPD may reduce joint swelling and synovitis in CIA mice by inhibiting the polarization of macrophages to the M1 type in the synovial tissue, downregulating the release of pro-inflammatory factors IL-6 and IL-1β, and increasing the level of the anti-inflammatory factor IL-10.
RA is a chronic inflammatory joint disease that can cause cartilage and bone damage. As the disease progresses, it can even lead to disability and systemic complications, resulting in decreased quality of life and increased mortality.1,30 The CIA model is an internationally recognized model for studying the pathogenesis of RA and therapeutic drugs.27 In this study, the administration of collagen emulsion to mice resulted in significant paw swelling. Treatment with 50 mg/kg SPD or DEX reduced paw swelling and lowered the arthritis score. However, these findings are not consistent with those of Iezaki et al,25 in that, they demonstrated that oral supplementation of SPM or SPD did not reduce paw volume and arthritis scores of CIA rats. The difference may be because (1) the two groups of studies had different administration methods. We intraperitoneally injected SPD, whereas they mixed SPM or SPD with the drinking water of CIA rats. Therefore, there may be fluctuations in the drug concentration, and the absorption of the drug may be affected by several factors such as the food in the stomach and pH of the digestive tract. (2) The timing of the administration was different. We administered SPD after the CIA model was successfully established, whereas they started oral treatment after the first immunization with collagen emulsion, and performed the second and third booster immunizations on days 7 and 14 of the experiment, respectively. Repeated inflammatory stimulation causes persistent swelling and joint inflammation in the hind paws of rats, which may cause no significant improvement in joint swelling after the treatment. However, their experiments indicated that SPM can inhibit the expression of RANKL mRNA in both the synovial tissue and cartilage tissue and reduce bone destruction of the toe joints of CIA rats, and they proposed that supplementation with specific polyamines may have a protective effect on the bone and cartilage of CIA rats.25 Silva et al26 injected SPD and SPM into the plantar of rats to cause pain and edema, which may be related to the stimulation of excessive local drug concentration. Although polyamines are necessary for cell survival and proliferation, if they cannot maintain their optimal state, they may be harmful to the body.31
Persistent synovitis is a sign of RA. The complex interaction between genetic factors and environmental factors induces abnormal activation of the innate and adaptive immune system, leading to a decline in immune tolerance, autoantigen presentation, antigen-specific T cell and B cell activation, and abnormal inflammatory cytokine production. These form a complex network that triggers the activation of resident fibroblast-like synovial cells, leading to synovitis, proliferation of synovium and cartilage, and destruction of subchondral bone.1,32 In our study, intraperitoneal injection of 50 mg/kg SPD improved neovascularization and fibrinoid necrosis, and reduced the synovitis score in CIA mice, showing good anti-synovitis effect of SPD. SPD can prevent bone loss by inhibiting osteoclast activation in mouse models.33 SPD supplementation activates autophagy and promotes cartilage formation, which may prevent the development of osteoarthritis.34 Kim et al20 found that intraperitoneal injection of 1 mg/kg or 10 mg/kg SPD can reduce tissue thickening, edema, hemorrhage, hyperkeratosis, and inflammatory cell infiltration in the inflamed area of the skin of mice with idiopathic dermatitis.
Macrophages, as the key cells newly discovered in the pathogenesis of RA, have attracted increasing attention in recent years. Macrophages can be divided into classically activated M1 and alternatively activated M2 macrophages. M1 macrophages express major histocompatibility complex II, CD80, CD86, and iNOS molecules, and secrete a variety of pro-inflammatory cytokines, such as TNF-α, IL-1β, IL-6, monocyte chemoattractant protein, and vascular endothelial growth factor, which can promote the development of inflammation.35 It has been reported that the number of monocytes in the peripheral blood of RA patients increases, and these cells can infiltrate the joints and differentiate into synovial macrophages. In the local microenvironment, macrophages are polarized by different cytokines or interferons and are mainly inclined to the pro-inflammatory M1 phenotype, resulting in an increase in pro-inflammatory mediators and a decrease in anti-inflammatory cytokines, aggravating the inflammatory response in joints.9 Supplementing exogenous SPD has been shown to inhibit the production of these inflammatory mediators in cell and animal experiments.18,20,36 In line with previous reports, our study also demonstrated a significant increase in IL-1β and IL-6 levels in the serum and iNOS expression in the synovial tissue of CIA control mice compared with normal control mice, and SPD treatment significantly reduced these indicators. Combining the results of serology and immunohistochemistry, SPD may inhibit M1 polarization of synovial macrophages in CIA mice and exert anti-inflammatory effects.
M2 macrophages express mannose receptor (MR/CD206), CD163, arginase-1 (Arg-1), etc., secrete IL-10 and other cytokines, and have anti-inflammatory effects.35 Recent studies have shown that SPD can inhibit the production of inflammatory mediators (such as NO and PEG2) and cytokines (such as TNF-α and IL-6) at the cellular transcription level, induce macrophage polarization to the M2 phenotype, and reduce the damage caused by inflammation.19–21,36 Here, treatment with SPD significantly increased serum IL-10 levels in CIA mice. However, the expression of the M2 macrophage polarization marker CD206 in the synovial tissue of SPD-H and SPD-L groups was not significantly different from that of the CIA control group. In view of the fact that this study only conducted a preliminary study on the macrophage phenotype in the mouse ankle joint synovial tissue, it is necessary to further explore the effect of SPD on macrophage polarization in CIA mouse joint tissue and its mechanism of action.
Conclusions
In summary, SPD may alleviate joint swelling and synovitis in CIA mice by inhibiting macrophage polarization to the M1 phenotype, downregulating the release of the pro-inflammatory factors IL-6 and IL-1β, and increasing the level of the anti-inflammatory factor IL-10. In particular, 50 mg/kg SPD had a better curative effect on CIA mice. Our experiment provides a solid theoretical basis for animal experiments for the treatment of RA with SPD, and lays a foundation for further mechanistic research.
Abbreviations
ANOVA, analysis of variance; CFA, complete Freund’s adjuvant; CII, Chicken type II collagen; DEX, dexamethasone; RA, rheumatoid arthritis; IFN-γ, interferon-γ; TNF-α, tumor necrosis factor α; IL, interleukin; SPD, spermidine; SPM, spermine; RANKL, receptor activator of nuclear factor-κB ligand; CIA, collagen-induced arthritis; iNOS, inducible nitric oxide synthase; HE, hematoxylin and eosin.
Acknowledgments
This work was supported by Scientific Research Fund of Hunan Provincial Education Department (No.18A035), 2020 Science and Technology Project of Changsha Science and Technology Bureau (No. kq2004120), and Health Commission of Hunan Provincial Research Project (No.C2019052).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
All authors report no potential conflicts of interest with regard to this work.
References
1. Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet. 2016;388:2023–2038. doi:10.1016/S0140-6736(16)30173-8.
2. Myasoedova E, Crowson CS, Kremers HM, et al. Is the incidence of rheumatoid arthritis rising?: Results from Olmsted county, Minnesota, 1955–2007. Arthritis Rheum. 2010;62:1576–1582. doi:10.1002/art.27425.
3. Doran MF, Pond GR, Crowson CS, et al. Trends in incidence and mortality in rheumatoid arthritis in Rochester, Minnesota, over a forty-year period. Arthritis & Rheumatism. 2010;46:625–631. doi:10.1002/art.509.
4. Mulherin D, Fitzgerald O, Bresnihan B. Synovial tissue macrophage populations and articular damage in rheumatoid arthritis. Arthritis Rheum. 1996;39:115–124. doi:10.1002/art.1780390116.
5. Yang X, Li S, Zhao Y, et al. GRK2 mediated abnormal transduction of PGE2-EP4-cAMP-CREB signaling induces the imbalance of macrophages polarization in collagen-induced arthritis mice. Cells. 2019;8:1596. doi:10.3390/cells8121596.
6. Kuo D, Ding J, Cohn IS, et al. HBEGF+ macrophages in rheumatoid arthritis induce fibroblast invasiveness. Sci Transl Med. 2019;11(491):eaau8587. doi:10.1126/scitranslmed.aau8587.
7. Tardito S, Martinelli G, Soldano S, et al. Macrophage M1/M2 polarization and rheumatoid arthritis: a systematic review. Autoimmun Rev. 2019;18:102397. doi:10.1016/j.autrev.2019.102397.
8. Woo SJ, Noh HS, Lee NY, et al. Myeloid sirtuin 6 deficiency accelerates experimental rheumatoid arthritis by enhancing macrophage activation and infiltration into synovium. EBioMedicine. 2018;38:228–237. doi:10.1016/j.ebiom.2018.11.005.
9. Zhu W, Li X, Fang S, et al. Anti-citrullinated protein antibodies induce macrophage subset disequilibrium in RA patients. Inflammation. 2015;38(6):2067–2075. doi:10.1007/s10753-015-0188-z.
10. Muñoz-Esparza NC, Latorre-Moratalla ML, Comas-Basté O, et al. Polyamines in food. Front Nutr. 2019;6:108. doi:10.3389/fnut.2019.00108.
11. Eisenberg TT, Abdellatif M, Schroeder S, et al. Cardioprotection and lifespan extension by the natural polyamine spermidine. Nat Med. 2016;22:1428–1438. doi:10.1038/nm.4222.
12. Eisenberg T, Knauer H, Schauer A, et al. Induction of autophagy by spermidine promotes longevity. Nat Cell Biol. 2009;11(11):1305–1314. doi:10.1038/ncb1975.
13. Fei Y, Li W, Jing Z, et al. Spermidine prolongs lifespan and prevents liver fibrosis and hepatocellular carcinoma by activating MAP1S-mediated autophagy. Cancer Res. 2017;77(11):2938–2951. doi:10.1158/0008-5472.CAN-16-3462.
14. Vargas AJ, Ashbeck EL, Wertheim BC, et al. Dietary polyamine intake and colorectal cancer risk in postmenopausal women. Am J Clin Nutr. 2015;102(2):411–419. doi:10.3945/ajcn.114.103895.
15. Sigrist SJ, Carmona-Gutierrez D, Gupta VK, et al. Spermidine-triggered autophagy ameliorates memory during aging. Autophagy. 2014;10(1):178–179. doi:10.4161/auto.26918.
16. Eisenberg T, Abdellatif M, Zimmermann A, et al. Dietary spermidine for lowering high blood pressure. Autophagy. 2017;13(4):1280225. doi:10.1080/15548627.2017.
17. Nilsson BO, Persson L. Beneficial effects of spermidine on cardiovascular health and longevity suggest a cell type-specific import of polyamines by cardiomyocytes. Biochem Soc Trans. 2019;47(1):265–272. doi:10.1042/BST20180622.
18. Jeong J, Cha HJ, Han MH, et al. Spermidine protects against oxidative stress in inflammation models using macrophages and zebrafish. Biomol Ther. 2018;26(2):146–156. doi:10.4062/biomolther.2016.272.
19. Paul S, Kang SC. Natural polyamine inhibits mouse skin inflammation and macrophage activation. Inflammation Res. 2013;62:681–688. doi:10.1007/s00011-013-0620-5.
20. Kim GD, Kim TH, Park YS, et al. Immune response against 2,4-dinitrofluorobenzene-induced atopic dermatitis-like clinical manifestation is suppressed by spermidine in NC/ Nga mice. Scand J Immunol. 2015;81:221–228. doi:10.1111/sji.12274.
21. Yang Q, Zheng C, Cao J, et al. Spermidine alleviates experimental autoimmune encephalomyelitis through inducing inhibitory macrophages. Cell Death Differ. 2016;23:1850–1861. doi:10.1038/cdd.2016.71
22. Van den Bossche J, Lamers WH, Koehler ES, et al. Pivotal advance: arginase-1-independent polyamine production stimulates the expression of IL-4-induced alternatively activated macrophage markers while inhibiting LPS-induced expression of inflammatory genes. J Leukoc Biol. 2012;91:685–699. doi:10.1189/jlb.0911453
23. Weiss TS, Herfarth H, Obermeier F, et al. Intracellular polyamine levels of intestinal epithelial cells in inflammatory bowel disease. Inflamm Bowel Dis. 2010;10(5):529–535. doi:10.1097/00054725-200409000-00006
24. Yukioka K, Wakitani S, Yukioka M, et al. Polyamine levels in synovial tissues and synovial fluids of patients with rheumatoid arthritis. J Rheumatol. 1992;19(5):689.
25. Iezaki T, Hinoi E, Yamamoto T, et al. Amelioration by the natural polyamine spermine of cartilage and bone destruction in rats with collagen-induced arthritis. J Pharmacol Sci. 2012;119(1):107–111. doi:10.1254/jphs.11241SC
26. Silva MA, Klafke JZ, Rossato MF, et al. Role of peripheral polyamines in the development of inflammatory pain. Biochem Pharmacol. 2011;82(3):269–277. doi:10.1016/j.bcp.2011.04.015
27. Inglis JJ, Simelyte E, McCann FE, et al. Protocol for the induction of arthritis in C57BL/6 mice. Nat Protoc. 2008;3:612–618. doi:10.1038/nprot.2008.19
28. Han HM, Hong SH, Park HS, et al. Protective effects of Fructus sophorae extract on collagen-induced arthritis in BALB/c mice. Exp Ther Med. 2017;13:146–154. doi:10.3892/etm.2016.3929
29. Tsubaki T, Arita N, Kawakami T, et al. Characterization of histopathology and gene-expression profiles of synovitis in early rheumatoid arthritis using targeted biopsy specimens. Arthrit Res Ther. 2005;7:R825–836. doi:10.1186/ar1751
30. Calabresi E, Petrelli F, Bonifacio AF, et al. One year in review 2018: pathogenesis of rheumatoid arthritis. Clin Exp Rheumatol. 2018;36:175–184.
31. Li C, Brazill JM, Liu S, et al. Spermine synthase deficiency causes lysosomal dysfunction and oxidative stress in models of Snyder-Robinson syndrome. Nat Commun. 2017;8(1):1257. doi:10.1038/s41467-017-01289-7
32. Chen Z, Bozec A, Ramming A, et al. Anti-inflammatory and immune-regulatory cytokines in rheumatoid arthritis. Nat Rev Rheumatol. 2019;15:9–17.
33. Yamamoto T, Hinoi E, Fujita H, et al. The natural polyamines spermidine and spermine prevent bone loss through preferential disruption of osteoclastic activation in ovariectomized mice. Br J Pharmacol. 2012;166:1084–1096. doi:10.1111/j.1476-5381.2012.01856.x
34. Sacitharan PK, Lwin S, Gharios GB, et al. Spermidine restores dysregulated autophagy and polyamine synthesis in aged and osteoarthritic chondrocytes via EP300. Exp Mol Med. 2018;50:1–10.
35. Ardura JA, Rackov G, Izquierdo E, et al. Targeting macrophages: friends or foes in disease? Front Pharmacol. 2019;10:1255. doi:10.3389/fphar.2019.01255
36. Choi YH, Park HY. Anti-inflammatory effects of spermidine in lipopolysaccharide-stimulated BV2 microglial cells. J Biomed Sci. 2012;19:31. doi:10.1186/1423-0127-19-31
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.