Back to Journals » Journal of Inflammation Research » Volume 14
Evaluation of Adjunctive Photobiomodulation (PBMT) for COVID-19 Pneumonia via Clinical Status and Pulmonary Severity Indices in a Preliminary Trial
Authors Vetrici MA , Mokmeli S , Bohm AR, Monici M, Sigman SA
Received 12 January 2021
Accepted for publication 26 February 2021
Published 19 March 2021 Volume 2021:14 Pages 965—979
DOI https://doi.org/10.2147/JIR.S301625
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Ning Quan
Mariana A Vetrici,1,* Soheila Mokmeli,2,* Andrew R Bohm,3 Monica Monici,4 Scott A Sigman5,*
1Department of Biological Sciences, University of Lethbridge, Lethbridge, AB, Canada; 2Training Institute, Canadian Optic and Laser Center, Victoria, BC, Canada; 3Department of Orthopedics, Lenox Hill Hospital, New York, NY, USA; 4ASA Campus J.L., ASA Res. Division – Department of Experimental and Clinical Biomedical Sciences, University of Florence, Florence, Italy; 5Department of Orthopedics, Lowell General Hospital, Lowell, MA, 01863, USA
*These authors contributed equally to this work
Correspondence: Scott A Sigman
Department of Orthopedics, Lowell General Hospital, 295 Varnum Avenue, Lowell, MA, 01863, USA
Tel +1 978 856 7676
Email [email protected]
Purpose: Evidence-based and effective treatments for COVID-19 are limited, and a new wave of infections and deaths calls for novel, easily implemented treatment strategies. Photobiomodulation therapy (PBMT) is a well-known adjunctive treatment for pain management, wound healing, lymphedema, and cellulitis. PBMT uses light to start a cascade of photochemical reactions that lead to local and systemic anti-inflammatory effects at multiple levels and that stimulate healing. Numerous empirical studies of PBMT for patients with pulmonary disease such as pneumonia, COPD and asthma suggest that PBMT is a safe and effective adjunctive treatment. Recent systematic reviews suggest that PBMT may be applied to target lung tissue in COVID-19 patients. In this preliminary study, we evaluated the effect of adjunctive PBMT on COVID-19 pneumonia and patient clinical status.
Patients and Methods: We present a small-scale clinical trial with 10 patients randomized to standard medical care or standard medical care plus adjunctive PBMT. The PBMT group received four daily sessions of near-infrared light treatment targeting the lung tissue via a Multiwave Locked System (MLS) laser. Patient outcomes were measured via blood work, chest x-rays, pulse oximetry and validated scoring tools for pneumonia.
Results: PBMT patients showed improvement on pulmonary indices such as SMART-COP, BCRSS, RALE, and CAP (Community-Acquired Pneumonia questionnaire). PBMT-treated patients showed rapid recovery, did not require ICU admission or mechanical ventilation, and reported no long-term sequelae at 5 months after treatment. In the control group, 60% of patients were admitted to the ICU for mechanical ventilation. The control group had an overall mortality of 40%. At a 5-month follow-up, 40% of the control group experienced long-term sequelae.
Conclusion: PBMT is a safe and effective potential treatment for COVID-19 pneumonia and improves clinical status in COVID-19 pneumonia.
Keywords: COVID-19, low-level laser therapy, pneumonia, SMART-COP, BCRSS, RALE
Introduction
The COVID-19 pandemic spread rapidly throughout the world, causing millions of infections, hundreds of thousands of deaths, and overloaded hospitals and intensive care units, with patients in need of critical care management. The initial hallmarks of COVID-19 were cytokine storm and acute respiratory distress syndrome (ARDS). Some patients are asymptomatic and recover spontaneously while others experience progressive symptoms leading to mild, moderate, or serious cases. Many serious cases require admission to intensive care units (ICUs) and ventilation support. The exceptional number of patients who died while receiving optimal medical care and ventilator support remains an enigma and successful treatment strategies remain to be found.
COVID-19 is caused by the Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2). SARS-CoV-2 enters cells via the ACE2 entry receptor, activates alveolar macrophages and neutrophils, and thereby enhances inflammation and vascular permeability. The immune response activates inflammatory cells and pathways and leads to cytokine storm.1,2 A single molecular marker associated with COVID-19 remains to be found, since many COVID-19 patients exhibited diverse levels of inflammatory markers and blood cell counts within normal limits. COVID-19 patients with severe symptoms have significantly higher levels of plasma pro-inflammatory cytokines such as IL-2, IL-6, TNF-α.3–6 The most common clinical manifestations of COVID-19 are ground glass opacities on chest x-ray, cytokine storm, and acute respiratory distress syndrome.3–6 The exaggerated immune response remains the main cause of morbidity or mortality. Prevention or modulation of this exaggerated inflammatory state could be the key to managing COVID-19 patients.6
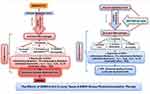
PBMT is an adjunctive treatment option that has demonstrated significant anti-inflammatory effects in pain management, lymphedema, wound healing, and musculoskeletal injuries.7–10 Other terms that fall under the category of PBMT include low-level laser therapy (LLLT), cold laser, and biostimulatory laser therapy.11 PBMT differs from the lasers used in cosmetic and surgical procedures, which destroy or cut tissue.12,13 Instead, PBMT uses non-ionizing, non-thermal light sources in the visible and infrared spectra (600–1200 nm), which in turn, reduce inflammation and stimulate healing.7 Light is applied through the skin and targets the damaged or inflamed tissues. Light energy absorbed by intracellular photoreceptors starts a cascade of photochemical intracellular reactions that improve cellular activity and increase the tissue’s healing process.12,13 Furthermore, PBMT is cost-effective, non-invasive, and has no reported adverse side effects in over 50 years of human experience. Experimental and animal models of pulmonary disease, ARDS and infection revealed that PBMT has cellular and molecular effects at multiple levels against both cytokine and bradykinin storms14 (Figure 1). PBMT downregulates proinflammatory Interleukins (IL-1b levels, IL-6, MIP-2 mRNA expression, etc.), prostaglandins, and TNF-alpha. PBMT also upregulates anti-inflammatory cytokines such as IL-10. PBMT decreases pulmonary microvascular leakage, activates macrophages, T cells, and neutrophil influx.15–26 The P2X7 receptor 7 (P2X7r) has been identified as a new potential therapeutic target in COVID‐19 pathogenesis.17 P2X7r is constitutively expressed in many cells and is a major factor involved in activation of cytokine storm and lung pathology in response to viruses.20,27–29 PBMT downregulates the P2X7r expression and decreases collagen deposition.20,22,30
The laser equipment required to deliver PBMT is already available throughout the world, is approved by countries' health authorities such as the FDA and Health Canada, and is readily accessible in pain clinics, and physiotherapy and rehabilitation centers. The Multiwave Locked System (MLS) laser used in this study was donated from a local pain center. The MLS laser increases the number of photons penetrating the tissue per unit of time, allowing for the treatment of deep tissues such as the lung more effectively and in shorter times.31 Investigation of the mechanism of action underlying the anti-inflammatory effects of MLS lasers demonstrated an increase in the production of NLRP 10, a potent inhibitor of the inflammasome.32,33 NLRP 10 inhibits the conversion of pro-IL 1β and pro-IL 18 in IL1β and IL-18 which blocks the production of many other cytokines and inflammation mediators.32,33
PBMT has been used to treat respiratory disorders such as pneumonia, asthma or COPD in children, adults, and elderly patients.34–39 Clinical studies of more than 1000 patients show positive effects of PBMT on pulmonary conditions, including shortened recovery times, decreased need for medications, reduced respiratory symptoms, and improved parameters in radiological, immunological and blood indicators.34–39 Recent publications and systematic reviews provide the theory and potential mechanism of action of PBMT in fighting COVID-19.14,18,40–43 Our experience in treating pain, inflammation and respiratory conditions motivated this trial to use PBMT in COVID-19 patients. COVID-19 continues to claim lives while some of the patients who recover show long-term sequelae. There is no current effective therapy available to the public. Human experience with PBMT suggests that it is a feasible solution for reducing inflammation, cytokine storm and lung pathology, and may be easily applied to a high volume of patients at low cost per patient. We designed a small-scale clinical trial to assess the effects of PBMT on COVID-19 pneumonia. For the evaluation of patient outcomes, we used validated objective and subjective pulmonary assessments designed for acute pneumonia and COVID-19.
Patients and Methods
Patients
During the period from March 2020 to May 2020, we performed a preliminary clinical trial with a parallel design for the evaluation of PBMT on COVID-19 pneumonia (ClinicalTrials.gov identifier: NCT04391712). Before obtaining Institutional Review Board (IRB) approval, the US Food and Drug Administration assessed the MLS scanner-equipped laser and deemed it was a nonsignificant risk device. Subsequently, the IRB, and the Clinical Research Review Committee of the Lowell General Hospital (Massachusetts, USA) approved the clinical protocol for PBMT treatment of COVID-19 pneumonia. All patients provided written informed consent for participation in this trial. A preliminary 10 patient study was approved by the hospital. Patients were assigned to the PBMT group (standard medical care plus adjunctive PBMT) or control group (standard medical care) using the Sealed Envelope computer application (Table 1). There was no masking of the treatment group, and the study was performed in an open-label fashion. Inclusion criteria were: SARS-CoV-2 infection confirmed by nasopharyngeal swab and RT-PCR on an Abbott ID system upon hospitalization, age 18–90 years, and pulmonary compromise requiring oxygen support. Patients had to be able to self-prone or support themselves in a self-sitting position to facilitate the administration of PBMT. Exclusion criteria included patients who required ventilator management, those with autoimmune disorders or inflammatory conditions not related to COVID-19, and pregnancy. The trial was conducted in accordance with the Declaration of Helsinki.
 |
Table 1 Demographic Characteristics of PBMT and Control Patients Were Not Statistically Different at the Beginning of the Trial |
Device and Treatment Protocol
The MLS scanner-equipped laser (ASA Laser, Nogarazza, Vincenza, Italy) was used to administer PBMT. This system consists of two laser sources classified as class IV laser hazards and emitting two different near-infrared wavelengths (808 nm and 905 nm). The MLS pulse is composed of two wavelengths that work simultaneously and synchronously. The device is maintained and calibrated biannually by a laser engineer and the parameters confirmed at 2 months prior to the trial were as follows:
- Three GaAlAs diode laser, 808 nm, peak power of 1 W for each diode, average power 500 mW each diode, in total 1.5 W for three diode lasers, power density 75 mW/cm2, frequency of 1500 Hz, duty cycle of 50%, pulse duration of 330 µs, spot size of 19.6 cm2.
- Three superpulsed GaAs laser diodes, 905 nm, peak power 75 W, average power 203 mW each diode, in total 610 mW for three diode lasers, power density 31 mW/cm2, frequency of 1500 Hz (train pulses 90 kHz modulated at 1 Hz ÷ 2 kHz), pulse duration of 100 ns, spot size of 19.6 cm2.
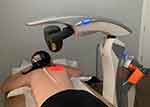
The scanner was positioned 20 cm above the skin, according to the manufacturer’s instructions. Each lung was scanned for 14 minutes, from apex to base, over an area of 250 cm2 of the posterior thorax, resulting in 28 minutes of PBMT with a dosage of 7.18 J/cm2 and a total energy of 3590 J. Each patient received once-daily treatments on 4 consecutive days. The patients were treated in the prone position with hands under their head for maximal scapular protraction to reduce the muscle and bone barrier and improve laser penetration (Figure 2). Control patients were observed and monitored for 4 days to replicate the treatment group protocol. All patients in the study including the PBMT and the control group were encouraged to spend 10–16 hours per day in the prone position. Patients in both groups were managed based on standard clinical care (oxygen supplementation, fluid and electrolyte balance, standard nursing care), and no additional corticosteroid, antiviral, pharmacological or antibody treatment was provided. At the time that this study was implemented, these modalities were not available at the hospital during the treatment protocol.
Data Collection
Response to treatment was evaluated by a series of outcome measures. Our primary outcome measure was an improvement in patient clinical status, which was measured using a series of scoring tools used in critical care. These tools are easy to use and available online.
- The SMART-COP score includes systolic blood pressure, multilobar infiltrates, albumin levels, respiratory rate, tachycardia, confusion, oxygen, and pH, and was assessed to determine the severity of pneumonia, and to evaluate changes in SMART-COP scores before and after treatment.44 This score provides detailed clinical parameters, but it is difficult to use rapidly on high volumes of critical patients.
- The Brescia-COVID respiratory severity scale (BCRSS) is a practical and simplified stepwise process for evaluating the respiratory status of COVID-19 patients.45 The score is calculated according to the patient’s respiratory condition, respiratory rate, partial pressure of oxygen in arterial blood (PaO2) or peripheral oxygen saturation (SpO2), and chest X-Ray, and it was developed in Italy during the peak of the pandemic. The scale was assessed pre- and post-treatment.45
- The Community-Acquired Pneumonia (CAP) assessment tool is a subjective, patient-reported outcomes score in adults, which uses a short and sensitive questionnaire to evaluate changes in respiratory symptoms and well-being before and after treatment of CAP.46
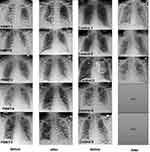
- Chest X-rays (CXR) were compared pre- and post-treatment using the radiographic assessment of lung edema (RALE) chest X-ray evaluation scale.47 The RALE score evaluates edema and CXR in ARDS patients via ground glass opacities and consolidation in each lung field. RALE scores were calculated by the investigators in a blinded fashion.
Arterial blood gases (ABGs) were not obtained during the trial, however SpO2 measurements and oxygen requirements were recorded. To compare each patient’s ability to oxygenate, or to estimate their lung function, we utilized the peripheral oxygen saturation divided by fraction of inspired oxygen (SpO2/FiO2) ratio and adjusted individual SpO2 measurements for the amount of oxygen supplementation that they required by using an FiO2 quotient. By convention, every 1L/min of oxygen delivered by low flow devices (nasal cannulas) is equivalent to a 4% increase in oxygen. Thus, supplementation of 1L/min provides 1.04 times the amount of oxygen present in room air, therefore the FiO2 quotient is 1.04. The adjusted SpO2 was calculated from (SpO2 obtained by pulse oximetry)/(FiO2 quotient).
Complete blood count (CBC), C-reactive protein (CRP), electrolytes and albumin were obtained before and after treatment. Adverse events were monitored using standard protocols.
Statistical Analyses
Statistical analyses were performed to evaluate for any variation in demographics and comorbid conditions using successive chi-squared tests for dichotomous variables and t-tests for continuous variables (recommended for two groups). Numerous statistical tests were conducted to determine the efficacy of the treatment. Paired t-tests were performed for each functional outcome measure stratified by PBMT or control group (within-group testing). We also compared the overall change in levels for functional outcomes between PBMT and control groups using traditional t-tests (between-group testing). Finally, we evaluated clinical lab findings for WBC, neutrophils, lymphocytes, platelets, CRP, hematocrit, hemoglobin, glucose, sodium, albumin using successive paired t-tests to evaluate for pre-to-post changes. Missing data were handled with listwise deletion. There was no more than 1 patient missing from any analysis, and most analyses contained all 10 patients. All statistics were performed with Stata 15 (College Point, TX). P-values of <0.05 and 95% confidence intervals were considered statistically significant.
Results
The experimental group received PBMT and standard supportive inpatient care (N = 5, average age 53.4), and the control group received standard supportive inpatient care (N = 5, average age 53.2). Comorbidity indices were measured by the Elixhauser Comorbidity Index.48 Comparison of the groups at baseline demonstrated no significant difference in average age, gender ratio, weight, or pre-existing conditions (Table 1). Patient clinical status was evaluated via the SMART-COP, BCRSS, CAP and RALE pulmonary severity index criteria summarized in Table 2. The comparison of the pre- and post-treatment average pulmonary indices both within and between the PBMT group and the control group demonstrated an improvement in the PBMT group versus the control group.
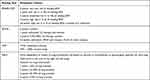 |
Table 2 Normal Ranges and Evaluation Criteria for SMART-COP, BCRSS, CAP and RALE |
The within-group changes for SMART-COP, BCRSS, CAP and RALE demonstrated improvement in the PBMT group and no significant changes in the control group (Table 3, Figure 3). The average SMART-COP scores within the PBMT group improved from 5.4 to 1.4 (p <0.001) while a non-significant increase from 2.8 to 4.4 (p=0.356) occurred in the control group. The average BCRSS scores within the PBMT group improved from 4.0 to 0.4 (p <0.001) while there was no statistically significant change within the control group 2.4 to 3.6 (p=0.324). The average CAP scores within the PBMT group improved from 41.5 to 82.0 (p <0.004) but showed negligible change within the control group 38.3 to 43.2 (p=0.0819). The average RALE Chest X-ray scores within the PBMT group improved from 8.0 to 5.2 (p <0.025) and worsened non-significantly within the control group 4.4 to 6.6 (p=0.141). All outcome measures showed a similar pattern of marked improvement in the treatment arm of the study which was statistically significant, and little to no change in the control arm, with patients tending to worsen as time went on and the disease progressed.
The between-group comparison of changes for SMART-COP, BCRSS, CAP and RALE revealed improvement in the PBMT group (Table 4, Figure 4). Comparison of the average change between the groups in SMART-COP, demonstrated a reduction in clinical risk of −4.0 in the PBMT group, and an increase of clinical risk by +1.6 in the control group (p=0.0065). The comparison of the average change in BCRSS between the groups, revealed a change in respiratory severity of −3.6 in PBMT group and a worsening by +1.2 in the control group (p=0.0023). The comparison of the average CAP score change between the groups, demonstrated an improvement of +40.5 in the PBMT group and an improvement of only +4.9 in the control group (p=0.0032). The chest x-rays, used for calculating RALE scores, showed increased lucency in all PBMT subjects following treatment, but increased opacity in control patients (Figure 5). The comparison of the average change in RALE scores between the groups, demonstrated an improvement of −2.8 in the PBMT group and a worsening by +2.2 in the control group (p=0.0085).
 |
Table 4 Between-Group Comparison of Change (Mean Difference) in Scores Following Treatment Reveals Significantly Improved Scores in SMART-COP, BCRSS, CAP and RALE in the PBMT Group |
Lab results, including WBC count, platelet, hemoglobin, hematocrit, lymphocyte count, glucose, albumin, BUN, Creatinine and CRP were measured pre- and post-treatment (Table 5). There was no significant difference between the groups for the lab results, except albumin, which decreased significantly in the control group at the end of the observation period (p=0.038). WBC, hemoglobin, hematocrit, platelet, neutrophil and lymphocyte count were within normal limits before and after treatment for all subjects in the trial.
 |
Table 5 Comparison of Baseline and Post-Treatment Hematological Parameters Within the Groups |
Both control and PBMT groups had similar clinical status at the beginning of the trial. The patients who received PBMT were being considered for ICU and intubation, but all recovered following PBMT without mechanical ventilation and the adjusted SpO2 (Table 6) of the PBMT patients remained above 80% (Table 7). An adjusted SpO2 of >90% is within normal range. All patients within the PBMT group showed increased oxygenation within 10 minutes of treatment during the PBMT session. This effect was observed for each PBMT session, and all PBMT patients achieved >90% adjusted SpO2 after the final treatments. In the PBMT group, patient 1 had a lengthy period of 5 days at 92% adjusted SpO2. Patients 2 and 3 reached 91% adjusted SpO2 by the end of day 2 (Table 7). Patient 4 reached a nadir of 84% adjusted SpO2 on day 2 and reached SpO2 of 92% by day 6. In patient 5, a sinusoidal pattern of O2 saturation was observed with a nadir of 80% adjusted SpO2 on day 2, but patient recovered to 91% by day 3 (Table 7).
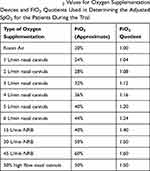 |
Table 6 Approximate FiO2 Values for Oxygen Supplementation Devices and FiO2 Quotients Used in Determining the Adjusted SpO2 for the Patients During the Trial |
 |
Table 7 Adjusted SpO2 Values Show Time Course of Lung Function for All Patients |
Control patients 1, 2 and 3 progressed to serious COVID-19 and were intubated by day 2 due to rapidly declining O2 saturation, and Control patients 1 and 3 died (Table 7). Control patient 2 was weaned off the ventilator after 7 days and recovered but continues to experience sequelae. Control patients 4 and 5 recovered spontaneously. Control patient 4 recovered over a 4-day hospitalization, was discharged on 2L of home oxygen with an adjusted SpO2 of 88% and weaned to room air by day 9. Control patient 5 recovered over a 9-day hospitalization and was discharged on home oxygen with an adjusted SpO2 of 89%.
At a 5-month follow-up, 2 of the 3 living control patients, one who recovered spontaneously and one who was on a ventilator, continue to experience aggravating pulmonary symptoms. All subjects in the PBMT group recovered without the need for mechanical ventilation or pharmacotherapy and were discharged from the hospital within 7 days of trial enrolment, and all were on room air by 9 days. No side effects were reported in the PMBT group after the treatment. All patients in the PBMT group were asymptomatic at a 5-month follow-up.
Discussion
The first report of PBMT for respiratory tract disorders occurred in 1978.49 Solid laboratory and experimental data and peer-reviewed studies support and demonstrate the anti-inflammatory effect of PBMT on lung tissue.20,22–26,30 This preliminary clinical trial demonstrated the benefits of adjunct PBMT in patients with severe COVID-19 symptoms. Due to the severity of the pandemic at the time of treatment, there was a tremendous lack of resources and manpower. Collection of more comprehensive clinical data was not feasible. The data collected for this study were deemed reasonable. In this study, the pulmonary severity indices BCRSS, SMART-COP and CAP scores after treatment improved in the PBMT group compared to the control group. Also, the RALE score, which monitors the severity of ARDS by quantifying pulmonary edema, was statistically improved in the PBMT group.
The average pretreatment SMART-COP score in the PBMT group was 5.4, categorizing this group as high-risk and indicating a 1 in 3 chance of needing intensive respiratory or vasopressor support (IRVS). Following PBMT, the SMART-COP score decreased to 1.4, indicating an improved lung condition with minimal possibility of IRVS treatment. The average pretreatment SMART-COP score in the control group was 2.8, categorizing this group as moderate-risk, and indicating a 1 in 8 chance of needing IRVS. After the observation period, the SMART-COP score increased to 4.4 in the control group, implying a worsening lung condition and high-risk for IRVS. Comparison between the two groups’ post-treatment SMART-COP scores showed evidence for clinical improvement in the PBMT group (p = 0.0065).
The average pretreatment BCRSS was 4 in the PBMT group, which placed them at risk for ICU admission and mechanical ventilation. Post PBMT, the average BCRSS score decreased to 0.4, which implies improvement of lung function and requiring routine patient monitoring. The average pretreatment BCRSS was 2.4 in the control group, and patients needed supplemental O2 and routine patient monitoring with pulse oximetry. Following the 4-day observation period in the control group, the average BCRSS increased to 3.6. This increase in the BCRSS score implies worsening lung function, placing them at risk for ICU admission and mechanical ventilation. Again, comparison between the two groups’ post-treatment BCRSS scores showed evidence of clinical status improvement in the treatment group (p = 0.0023).
The CAP score evaluates the patient’s subjective respiratory condition via a self-reported questionnaire. Scores of ≥75% indicate normal respiratory condition. The average pretreatment CAP score in the PBMT group was 41.5, indicating respiratory distress. After PBMT, the CAP score improved to 82.0, indicating no further respiratory distress. The average pretreatment CAP score in the control group was 38.3 indicating respiratory distress. After the observation period, the CAP score was 43.2 in the control group, indicating no improvement in respiratory distress. Comparison of the post-treatment CAP scores between the two groups showed evidence for subjective improvement of patient-reported outcomes for those in the PBMT group (p = 0.0032).
The average pretreatment RALE score was 8.0 in the PBMT group, which indicates >75% involvement of both lungs on CXR. For the PBMT group, the average RALE score decreased to 5.2, which implies CXR improvement to 50–75% involvement of both lungs (p=0.025). The average pretreatment RALE score was 4.4 in the control group, indicating 25–50% involvement of both lungs. Following the observation period of 4 days in the control group, the average RALE score increased to 6.6, indicating more than 75% lung involvement (p=0.141). This increase in the RALE score implies worsening CXR. In accord with all other functional outcome measures studied, there was a statistically significant improvement (p = 0.0085) in lung involvement for those in the PBMT group. The imaging absorption phase of consolidation or ground glass opacities correlates with healing of the lung tissue. For severe COVID-19 patients, this is usually observed after ≥ 14 days,50 but in the PBMT group, the absorption phase was apparent at 5-7 days.
An unexpected observation occurred within 5–10 minutes of PBMT: SpO2 increased from 93–94% to 98–100% as detected by pulse oximetry after each PBMT session for all patients in the PBMT group. This phenomenon occurred during each treatment. After PBMT, oxygenation returned to the baseline in a sinusoidal pattern. In three of the PBMT patients there was a gradual improvement in oxygenation over the 4 days of treatment. Two of the PBMT patients required 3 days of treatment before seeing improvement in oxygenation. This result could imply a primary effect of PBMT in lung tissue and the benefit of PBMT, which was observed promptly. We believe that the immediate increase in SpO2 was not the effect of the prone position during treatment. Clinical trials have confirmed the positive effect of long-term (12–18 h per session) prone position ventilation in selected patients with ARDS.51,52 All patients in the PBMT and the control group were advised to use the prone position during the hospital stay for up to 12–18 hours per day. It is unlikely that an additional 28 minutes in the prone position during the treatment cycle improved the pulmonary severity indices, oxygenation, and CXR.
We utilized the adjusted SpO2 to compare lung function among all study patients and illustrate the time-course of treatment. This is a rapid and non-invasive estimation of lung function when ABG testing is not available.53 Approximate FiO2 values for all oxygen supplementation devices used for patients in this study are calculated or estimated (Table 6). Both control and PBMT patients showed fluctuations in their lung function; however, PBMT patients never required ICU admission or mechanical ventilation (Table 7). All PBMT patients recovered to room air within 9 days of entering the trial (Table 7). The control patients experienced longer recovery times, long-term sequelae, mechanical ventilation, and death (Table 7). One of the control patients was lost to follow-up after being discharged from the hospital on 2L/min oxygen supplementation (Table 7).
The average number of hospitalized days in the PBMT group was 7.6 days versus the control group for 12.2 days (p=0.292). The average ICU days in the PBMT group was 0.4 days versus the control group for 5.4 days (p=0.112). The average number of days of ventilator support in the PBMT group was 0 days versus the control group for 5.2 days (p=0.10). There is no statistically significant difference; however, our findings may be clinically meaningful since hospital, ICU and ventilator days were all reduced in the PBMT group. PBMT patient 1 spent 2 days in the ICU prior to enrolment in the trial; however, none of the PBMT subjects required ICU admission or mechanical ventilation after enrolment. SMART-COP and BCRSS scores predicted a 1 to 3 chance of ICU admission and ventilator support for the PBMT group. None of the patients in the PBMT group were admitted to the ICU nor required ventilator support. PBMT may have been effective in potentially reducing the need for ventilator support in the PMBT group. SMART-COP and BCRSS scores predicted a 1 to 10 chance of ICU admission and ventilator support for the control group. Unfortunately, 3 out of 5 patients (60%) in the control group were admitted to the ICU and required ventilator support. One patient expired after 4 days of ventilator support, another after 15 days of ventilator support and a third patient was weaned off the ventilator after 7 days.
The mortality rate was 0% in the PBMT group compared to 40% (2 out of 5 patients) in the control group. There were no reported complications or side effects associated with the PBMT group during treatment. All the PBMT patients were asymptomatic at a 5-month follow-up and reported no side effects or complications. Supportive PBMT may improve the clinical status and reduce the need for ventilators in the treatment of COVID-19.
A strength of this study is that we evaluated the groups by both subjective and objective measures. Upcoming studies should measure and evaluate Il-6, Il-10, TNF-α, as well as inflammatory markers, arterial blood gases, and comprehensive blood tests. Collection of data before, during and after treatment will strengthen the results.
The facility and adaptability of PBMT enabled us to perform this study in a community hospital. The laser is on wheels and can easily be transported for patient care. Similar work could be performed in medical offices or other healthcare settings. Appropriate laser parameters were obtained by expert consultation to ensure laser energy would reach the lung tissue. Another potential benefit of PBMT for COVID-19 pneumonia is the ease of treatment. This is a safe, non-invasive, non-pharmacologic, painless, and cost-effective modality. The laser used in this study uses a mobile scanner so there is no contact with the patient. One laser device with an average 25,000-hour lifespan can treat 10,000 patients.
The small number of the patients in this study is a limitation. Clinical trials with larger groups are needed to confirm the effect of PBMT in COVID-19. This was the first trial of PBMT in COVID-19 patients. The initial promising results of this study will stimulate more advanced studies at academic and university hospitals. The limited sample size represents a significant issue to statistical interpretation and should be considered when evaluating any findings of this research.
Conclusion
This study demonstrates the potential benefits of adjunctive PBMT in COVID-19 pneumonia. The use of PBMT in the early stages of severe ARDS in COVID-19 patients may improve pulmonary and clinical status and reduce the need for ventilator support and ICU stay. Adjunctive PBMT may decrease hospital stay and enhance the recovery process. Clinical status improvement was supported by an increase in SpO2 during the treatment sessions, the rapid relief of respiratory symptoms, and improved CXR findings. There was no incidence of mortality or major reported side effects in the PBMT group. The mortality rate was 40% in the control group and 40% of patients continue to experience pulmonary sequelae. The patients in the PBMT group recovered without needing ICU admission or mechanical ventilation. Conversely, 60% of patients in the control group required ICU admission and ventilation. Clinical trials with larger group sizes are necessary to confirm the effects of PBMT on COVID-19 pneumonia.
Data Sharing Statement
Data is available by directly contacting the primary investigator of this study, Dr. Scott A Sigman at [email protected].
Acknowledgments
We would like to offer acknowledgement to Jan Tuner, member of The Scientific Board of Swedish Medical Laser Society, for scientific advice and guidance.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosure
MM is an employee of and receives financial support from ASA (laser manufacturer) for her lab at the University of Florence. The authors report no other conflicts of interest in this work.
References
1. Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi:10.1038/s41586-020-2012-7
2. Fu Y, Cheng Y, Wu Y. Understanding SARS-CoV-2-mediated inflammatory responses: from mechanisms to potential therapeutic tools. Virol Sin. 2020;35(3):266–271. doi:10.1007/s12250-020-00207-4
3. Wang C, Xie J, Zhao L, et al. Aveolar macrophage activation and cytokine storm in the pathogenesis of severe COVID-19. Research Square. 2020. doi:10.21203/rs.3.rs-19346/v1
4. Liang T. Handbook of COVID-19 Prevention and Treatment. Zhejiang University School of Medicine; 2020.
5. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi:10.1016/S0140-6736(20)30183-5
6. Khadke S, Ahmed N, Ahmed N, et al. Harnessing the immune system to overcome cytokine storm and reduce viral load in COVID-19: a review of the phases of illness and therapeutic agents. Virol J. 2020;17(1):154. doi:10.1186/s12985-020-01415-w
7. Hamblin MR. Mechanisms and applications of the anti-inflammatory effects of photobiomodulation. AIMS Biophys. 2017;4(3):337–361. doi:10.3934/biophy.2017.3.337
8. Cotler HB, Chow RT, Hamblin MR, Carroll J. The use of low level laser therapy (LLLT) for musculoskeletal pain. MOJ Orthop Rheumatol. 2015;2(5):00068. doi:10.15406/mojor.2015.02.00068
9. Tata DB, Waynant RW. Laser therapy: a review of its mechanism of action and potential medical applications. Laser Photonics Rev. 2011;5(1):1–12. doi:10.1002/lpor.200900032
10. Bjordal JM, Rab L-M, Joensen J, Iversen VV. The anti-inflammatory mechanism of low level laser therapy and its relevance for clinical use in physiotherapy. Phys Ther Rev. 2010;15(4):286–293. doi:10.1179/1743288X10Y.0000000001
11. Anders JJ, Lanzafame RJ, Arany PR. Low-level light/laser therapy versus photobiomodulation therapy. Photomed Laser Surg. 2015;33(4):183–184. doi:10.1089/pho.2015.9848
12. Jang H, Lee H. Meta-analysis of pain relief effects by laser irradiation on joint areas. Photomed Laser Surg. 2012;30(8):405–417. doi:10.1089/pho.2012.3240
13. Woodruff LD, Bounkeo JM, Brannon WM, et al. The efficacy of laser therapy in wound repair: a meta-analysis of the literature. Photomed Laser Surg. 2004;22(3):241–247. doi:10.1089/1549541041438623
14. Mokmeli S, Vetrici M. Low level laser therapy as a modality to attenuate cytokine storm at multiple levels, enhance recovery, and reduce the use of ventilators in COVID-19. Can J Respir Ther. 2020;56:25–31. doi:10.29390/cjrt-2020-015
15. Hojyo S, Uchida M, Tanaka K, et al. How COVID-19 induces cytokine storm with high mortality. Inflamm Regen. 2020;40:37. doi:10.1186/s41232-020-00146-3
16. Mahmudpour M, Roozbeh J, Keshavarz M, Farrokhi S, Nabipour I. COVID-19 cytokine storm: the anger of inflammation. Cytokine. 2020;133:155151. doi:10.1016/j.cyto.2020.155151
17. Pacheco PAF, Faria RX. The potential involvement of P2X7 receptor in COVID-19 pathogenesis: a new therapeutic target? Scand J Immunol. 2020;14:e12960.
18. Enwemeka CS, Bumah VV, Masson-Meyers DS. Light as a potential treatment for pandemic coronavirus infections: a perspective. J Photochem Photobiol B. 2020;207:111891. doi:10.1016/j.jphotobiol.2020.111891
19. Garvin MR, Alvarez C, Miller JI, et al. A mechanistic model and therapeutic interventions for COVID-19 involving a RAS-mediated bradykinin storm. Elife. 2020;9:e59177. doi:10.7554/eLife.59177
20. da Cunha Moraes G, Vitoretti LB, de Brito AA, et al. Low-level laser therapy reduces lung inflammation in an experimental model of chronic obstructive pulmonary disease involving P2X7 receptor. Oxid Med Cell Longev. 2018;2018:6798238. doi:10.1155/2018/6798238
21. de Oliveira VL, Silva JA, Serra AJ, et al. Photobiomodulation therapy in the modulation of inflammatory mediators and bradykinin receptors in an experimental model of acute osteoarthritis. Lasers Med Sci. 2017;32(1):87–94. doi:10.1007/s10103-016-2089-2
22. Miran da da Silva C, Peres Leal M, Brochetti RA, et al. Low level laser therapy reduces the development of lung inflammation induced by formaldehyde exposure. PLoS One. 2015;10(11):e0142816. doi:10.1371/journal.pone.0142816
23. Oliveira MC, Greiffo FR, Rigonato-Oliveira NC, et al. Low level laser therapy reduces acute lung inflammation in a model of pulmonary and extrapulmonary LPS-induced ARDS. J Photochem Photobiol B. 2014;134:57–63. doi:10.1016/j.jphotobiol.2014.03.021
24. de Lima FM, Moreira LM, Villaverde AB, Albertini R, Castro-Faria-Neto HC, Aimbire F. Low-level laser therapy (LLLT) acts as cAMP-elevating agent in acute respiratory distress syndrome. Lasers Med Sci. 2011;26(3):389–400. doi:10.1007/s10103-010-0874-x
25. Aimbire F, Ligeiro de Oliveira AP, Albertini R, et al. Low level laser therapy (LLLT) decreases pulmonary microvascular leakage, neutrophil influx and IL-1beta levels in the airway and lung from rats subjected to LPS-induced inflammation. Inflammation. 2008;31(3):189–197. doi:10.1007/s10753-008-9064-4
26. Aimbire F, Albertine R, de Magalhães RG, et al. Effect of LLLT Ga-Al-As (685 nm) on LPS-induced inflammation of the airway and lung in the rat. Lasers Med Sci. 2005;20(1):11–20. doi:10.1007/s10103-005-0339-9
27. Rosli S, Kirby FJ, Lawlor KE, et al. Repurposing drugs targeting the P2X7 receptor to limit hyperinflammation and disease during influenza virus infection. Br J Pharmacol. 2019;176:3834–3844. doi:10.1111/bph.14787
28. Leyva-Grado VH, Ermler ME, Schotsaert M, et al. Contribution of the purinergic receptor P2X7 to development of lung immunopathology during influenza virus infection. mBio. 2017;8(2):e00229–17. doi:10.1128/mBio.00229-17
29. Kawano A, Tsukimoto M, Noguchi T, et al. Involvement of P2X4 receptor in P2X7 receptor-dependent cell death of mouse macrophages. Biochem Biophys Res Commun. 2012;419(2):374–380. doi:10.1016/j.bbrc.2012.01.156
30. de Brito AA, da Silveira EC, Rigonato-Oliveira NC, et al. Low-level laser therapy attenuates lung inflammation and airway remodeling in a murine model of idiopathic pulmonary fibrosis: relevance to cytokines secretion from lung structural cells. J Photochem Photobiol B. 2020;203:111731. doi:10.1016/j.jphotobiol.2019.111731
31. Monici M, Gnerucci A, Falconi T, et al. Laser therapy penetration depth: a near-infrared study on a horse tendon model. Muscles Ligaments Tendons J. 2018;8(2):222–228. doi:10.32098/mltj.02.2018.11
32. Micheli L, Cialdai F, Pacini A, et al. Effect of NIR laser therapy by MLS-MiS source against neuropathic pain in rats: in vivo and ex vivo analysis. Sci Rep. 2019;9(1):9297. doi:10.1038/s41598-019-45469-5
33. Monici M, Cialdai F, Ranaldi F, et al. Effect of IR laser on myoblasts: a proteomic study. Mol Biosyst. 2013;9(6):1147–1161. doi:10.1039/c2mb25398d
34. Arza RA. Upper and lower respiratory conditions. In: Riegel RJ, Godbold JC, editors. Laser Therapy in Veterinary Medicine. Hoboken: John Wiley & Sons, Inc; 2017:150–160.
35. Vatankhah Z, Mokmeli S, Boshbishe S. Evaluation of the effect of low-level laser therapy (LLLT) in the treatment of asthma, added to conventional drug therapy (crossover, case control clinical trial). Photodiagnosis Photodyn Ther. 2008;5(Suppl. 1):S22. doi:10.1016/S1572-1000(08)70063-2
36. Ostronosova NS. [Outpatient use of laser therapy in bronchial asthma]. Ter Arkh. 2006;78(3):41–44. Russian.
37. Erkinovna TB, Tulkunovna MH. Efficacy of laser therapy in infants with infectious-inflammatory respiratory diseases. European Science Review. ISSN: Печатный: 2310–5577; 2006. Available from: https://cyberleninka.ru/article/n/efficacy-of-laser-therapy-in-infants-with-infectious-inflammatory-respiratory-diseases.
38. Amirov NB. [Parameters of membrane permeability, microcirculation, external respiration, and trace element levels in the drug-laser treatment of pneumonia]. Ter Arkh. 2002;74(3):40–43. Russian.
39. Derbenev VA, Mikhailov VA, Denisov IN. Use of low-level laser therapy (LLLT) in the treatment of some pulmonary diseases: ten-year experience.
40. Fekrazad R. Photobiomodulation and antiviral photodynamic therapy as a possible novel approach in COVID-19 management. Photobiomodul Photomed Laser Surg. 2020;38(5):255–257. doi:10.1089/photob.2020.4868
41. Sigman SA, Mokmeli S, Monici M, Vetrici MA. A 57-year-old African American man with severe COVID-19 pneumonia who responded to supportive photobiomodulation therapy (PBMT): first use of PBMT in COVID-19. Am J Case Rep. 2020;21:e926779. doi:10.12659/AJCR.926779
42. Sigman SA, Mokmeli S, Vetrici MA. Adjunct low level laser therapy (LLLT) in a morbidly obese patient with severe COVID-19 pneumonia: a case report. Can J Respir Ther. 2020;56:52–56. doi:10.29390/cjrt-2020-022
43. Nejatifard M, Asefi S, Jamali R, Hamblin MR, Fekrazad R. Probable positive effects of the photobiomodulation as an adjunctive treatment in COVID-19: a systematic review. Cytokine. 2020;137:155312. doi:10.1016/j.cyto.2020.155312
44. Charles PG, Wolfe R, Whitby M, et al. SMART-COP: a tool for predicting the need for intensive respiratory or vasopressor support in community-acquired pneumonia. Clin Infect Dis. 2008;47(3):375–384. doi:10.1086/589754
45. Duca A, Piva S, Focà E, Latronico N, Rizzi M. Calculated decisions: Brescia-COVID respiratory severity scale (BCRSS)/algorithm. Emerg Med Pract. 2020;22(5Suppl):CD1–CD2.
46. El moussaoui R, Opmeer BC, Bossuyt PM, Speelman P, de Borgie CA, Prins JM. Development and validation of a short questionnaire in community acquired pneumonia. Thorax. 2004;59(7):591–595. doi:10.1136/thx.2003.015107
47. Warren MA, Zhao Z, Koyama T, et al. Severity scoring of lung oedema on the chest radiograph is associated with clinical outcomes in ARDS. Thorax. 2018;73(9):840–846. doi:10.1136/thoraxjnl-2017-211280
48. van Walraven C, Austin PC, Jennings A, Quan H, Forster AJ. A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data. Med Care. 2009;47(6):626–633. doi:10.1097/MLR.0b013e31819432e5
49. Danilova IN, Kamenetskaia TM, Minenkov AA, Aĭrapetova NS. Vliianie monokhromaticheskogo kogerentnogo izlucheniia geliĭ-neonovogo lazera na bol’nykh khronicheskoĭ pnevmonieĭ [Effect of monochromatic coherent helium-neon laser radiation on chronic pneumonia patients]. Vopr Kurortol Fizioter Lech Fiz Kult. 1978;3:36–40. Russian
50. Pan F, Ye T, Sun P, et al. Time course of lung changes at chest CT during recovery from coronavirus disease 2019 (COVID-19). Radiology. 2020;295(3):715–721. doi:10.1148/radiol.2020200370
51. Henderson WR, Griesdale DE, Dominelli P, Ronco JJ. Does prone positioning improve oxygenation and reduce mortality in patients with acute respiratory distress syndrome? Can Respir J. 2014;21(4):213–215. doi:10.1155/2014/472136
52. Guérin C, Reignier J, Richard JC, et al.; PROSEVA Study Group. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013;368(23):2159–2168. doi:10.1056/NEJMoa1214103
53. Batchinsky AI, Wendorff D, Jones J, et al. SpO2/FiO2 ratio as surrogate for PaO2/FiO2 ratio during simulated prolonged field care and ground and high-altitude evacuation. J Trauma Acute Care Surg. 2020;89(2SSuppl 2):S126–S131. doi:10.1097/TA.0000000000002744
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.