Back to Journals » Journal of Pain Research » Volume 13
IV Tramadol – A New Treatment Option for Management of Post-Operative Pain in the US: An Open-Label, Single-Arm, Safety Trial Including Various Types of Surgery
Authors Minkowitz H , Leiman D, Lu L, Reines S, Ryan M, Harnett M, Singla N
Received 3 March 2020
Accepted for publication 28 April 2020
Published 22 May 2020 Volume 2020:13 Pages 1155—1162
DOI https://doi.org/10.2147/JPR.S251175
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Michael Schatman
Harold Minkowitz,1 David Leiman,1 Lucy Lu,2 Scott Reines,2 Michael Ryan,2 Mark Harnett,2 Neil Singla3
1Clinical Research, HD Research, Bellaire, TX, USA; 2Clinical Operations, Avenue Therapeutics, New York, NY, USA; 3Clinical Research, Lotus Clinical Research, Pasadena, CA, USA
Correspondence: Lucy Lu Email [email protected]
Purpose: There is a need to reduce exposure to Schedule II opioids in the United States (US) due to the ongoing opioid epidemic. Schedule II opioids have higher potential for abuse and misuse than Schedule IV opioids. This Phase 3, multicenter, single-arm, open-label, multiple-dose US trial evaluated the safety and tolerability of intravenous tramadol 50 mg, a Schedule IV opioid, in the management of postoperative pain in a real-world setting, where intravenous tramadol is not yet approved for use.
Patients and Methods: Patients undergoing a range of soft-tissue and orthopedic surgeries were enrolled. Intravenous tramadol 50 mg was given at hours 0, 2, 4, and every 4 h thereafter through up to 7 days of treatment. Non-opioid medications per treating physicians’ discretion were allowed if additional pain relief was needed. Endpoints included treatment-emergent adverse events (TEAEs), laboratories, vital signs, electrocardiograms (ECGs), and patient global assessment (PGA) of effectiveness.
Results: A total of 251 patients were enrolled, with 4% discontinuing due to TEAE; no patient discontinued due to a lack of efficacy. Patients averaged 13 doses, resulting in average 48 h of exposure. Intravenous tramadol was well tolerated, with TEAEs consistent with known tramadol pharmacology. No unexpected findings were observed, with 95% of patients reporting study medication was good, very good, or excellent for controlling pain.
Conclusion: Outcomes from this real world use study demonstrated intravenous tramadol 50 mg was safe and well tolerated in the management of postoperative pain where intravenous conventional opioids are often used. Intravenous tramadol alone or coadministered with non-opioid medication (when needed) as a multimodal combination analgesia approach resulted in high patient satisfaction with their pain relief. In light of the US opioid epidemic, reducing the exposure to conventional opioids in these patients via use of IV tramadol may be possible.
Keywords: multimodal analgesia, abuse risk, Schedule II opioid, real-world study
Plain Language Summary
This was a clinical study performed in 251 patients who were undergoing a variety of elective surgeries in bone (such as a knee or hip replacement surgeries) and soft tissue (such as colon surgeries or breast augmentations). The study was designed to be a 'real-world' study in which safety information was collected for a new medication for pain relief. This medication, intravenous tramadol, is not yet available in the United States. Intravenous tramadol is a medication that may be used following a variety of surgeries where conventional opioids (such as morphone or oxycodone) are often used for pain relief. However, those conventional opioids carry higher risk of abuse potential compared to tramadol. This study demonstrated that few patients discontinued their intravenous tramadol treatment, and that the patients did not need to be given other opioid medications but rather the patients were satisfied with their pain relief. Notably, patients were able to complete their full course of pain treatment following the surgery without many side effects. Thus, intravenous tramadol may become a useful pain medication alternative to conventional opioids in patients with post-surgical pain in the United States.
Introduction
Tramadol is a centrally-acting, atypical opioid analgesic with a dual mechanism of action. It is an opioid agonist and inhibitor of norepinephrine and serotonin re-uptake. The analgesic effect of tramadol is believed to be due to both binding to μ-opioid receptors and weak inhibition of re-uptake of norepinephrine and serotonin. Opioid activity is due to both low-affinity binding of the parent compound and higher affinity binding of the O-demethylated metabolite M1 to μ-opioid receptors.1 Tramadol has been noted to have a low risk of abuse compared to conventional opioids such as morphine2,3 and is a Schedule IV controlled substance in the US The Drug Enforcement Administration (DEA) scheduling criteria indicate that Schedule IV drugs have a low potential for abuse and low risk of dependence.4 This contrasts with conventional opioids (Schedule II drugs) that have a high potential for abuse. The scheduling difference reflects the understanding that tramadol, as an atypical opioid with dual mechanism of action, does not carry the same abuse potential as Schedule II opioids.2
Oral tramadol was approved for use in the US by the FDA in 1995. Intravenous (IV) tramadol is not yet marketed in the US despite the fact that it has been in use for decade outside the United States and is currently approved in more than 70 countries.5 A novel dosing regimen for IV tramadol was recently developed for the US market.6 This regimen in which 50 mg IV tramadol is given at Hour 0, 2, 4, and every 4 h thereafter, provides a similar steady-state Cmax and AUC to that of approved oral tramadol regimen (100 mg Q6H), but with a more rapid increase during the first dosing interval. Furthermore, this dosing regimen of IV tramadol produces lower and slower exposure to M1 (the stronger µ opioid agonist than the parent compound) than does oral tramadol, and thus should result in lower likelihood of potential for abuse, independent of clinical setting. This is an important factor given the current US opioid epidemic, in which there is a strong desire to reduce risk of abuse to Schedule II opioid medications.
Because IV tramadol has not been approved in the US, the purpose of this study was to evaluate the safety of IV tramadol 50 mg in the management of postoperative pain following a variety of surgical procedures where intravenous conventional opioids are often used. The goal of this study was to understand if IV tramadol can provide adequate pain relief in a real-world standard of care setting. This open-label safety study was conducted in parallel with two Phase 3 efficacy studies, one in an orthopedic model (in press) and the other in a soft tissue model (in press). Unlike the Phase 3 efficacy studies, which were double-blind and strictly controlled use of other analgesia modalities, this current real-world study allowed patients to be given other forms of pain medication (eg, NSAIDs) in a multi-modal analgesia manner (which consistent with the current standard in post-surgical pain care).7 The only limitations on concomitant analgesia was the exclusion of conventional (Schedule II) opioids, which allowed for assessment of intravenous tramadol as an alternative to pure mu opioid analgesics in the hospital setting.
Patients and Methods
Study Design
This study was a Phase 3, multicenter, single-arm, open-label, uncontrolled, repeat dose trial to assess the safety of IV tramadol 50 mg in the management of postoperative pain, performed at two investigational centers in the United States. The study was performed in accordance with ethical principles that have their origin in the Declaration of Helsinki and are consistent with International Conference on Harmonization (ICH)/Good Clinical Practice (GCP), applicable regulatory requirements and the Sponsor or its delegate’s policy on Bioethics. All study materials were reviewed by institutional review boards (Western Institutional Review Board®, Puyallup, WA, USA, and Aspire IRB, Santee, CA, USA). The study was registered at Clinicaltrials.gov, identifier NCT03395808. First patient was enrolled December 21, 2017, and last patient completed on May 6, 2019. All patients provided written informed consent prior to participation in the study.
Patients must have met the definition of American Society of Anesthesiologists (ASA) Physical Class 1, or 2 and were excluded if they have used chronic opioid therapy or had a recent history of substance dependence, or had a seizure disorder or could not be withdrawn from medications that may lower seizure threshold or increase serotonergic tone. Eligible patients underwent elective surgery, including both orthopedic procedures such as knee replacement and hip replacement, and soft-tissue procedures such as abdominoplasty, colon, hernia, breast augmentation, and hysterectomy surgeries. The target enrollment was 250 patients.
Because this was a “real world” study, there were no restrictions on the surgical agents used for induction, neuromuscular blockade, and maintenance of anesthesia and no post-surgical restrictions on hypnotics, sedatives, or anxiolytics. Treating clinicians were allowed to use what they consider as standard-of-care in the U.S., such as propofol, midazolam, lidocaine, fentanyl, and IV acetaminophen. Following surgery, eligible patients received their study drug infusion (IV tramadol 50 mg) at T0 (Hour 0 or first dose), Hour 2, and Hour 4, and then every 4 h for up to 7 days. Following the first dose of study drug, patients were allowed to receive non-opioid pain medication, such as intravenous acetaminophen, celecoxib, ketorolac and bupivacaine-based local blocks, per the treating physician’s discretion, if additional pain relief was required. Schedule II opioids were specifically excluded, to assess how IV tramadol, in a multi-modal analgesic setting, would provide satisfactory pain relief after these surgeries.
Patients were to continue study treatment until it was no longer needed; however, some patients consented in advance to remain on treatment for 5 days to allow for collection of safety outcomes over a longer duration of treatment. Patients were housed in a healthcare facility during study medication administration. Upon discharge, patients were given a prescription for pain management at home per the physician’s discretion. Pain medications prescribed at discharge included Percocet, oxycodone, oral tramadol, and acetaminophen. A final safety assessment via telephone call was conducted on Day 14 (±2 days).
Endpoints
This was an open-label, single-arm study, and thus the primary objective was to evaluate safety and patient satisfaction with the therapy. Safety endpoints included adverse events (AEs), clinical laboratory, vital sign, and electrocardiogram (ECG) changes, and local tolerability.
- Treatment-emergent AEs (TEAEs) were classified by the Medical Dictionary for Regulatory Activities (MedDRA) System Organ Class (SOC) and preferred term (PT).
- Clinical laboratory, vital signs, and electrocardiogram (ECG) changes were recorded.
- Local tolerability of the infusion site via pain, swelling, tenderness, and erythema was recorded.
Patient reported satisfaction with the pain relief was considered a secondary outcome and was assessed by the patient global assessment (PGA) of effectiveness, which is a patient-reported outcome that reflects their perception of the study treatment. The PGA was collected at 24-h post-first dose and at End of Treatment. The question posed was “How would you rate the study medication in terms of its effectiveness in controlling your pain?” (0=poor; 1=fair; 2=good; 3=very good; 4=excellent). If the patient ended treatment prior to Hour 24, the patient global assessment was conducted as part of the End of Treatment visit.
Statistical Methods
The Safety Population was defined as all patients who received at least one dose of study medication, and was the primary analytical population used for analysis. Continuous parameters were summarized by the applicable time point using the descriptive statistics n, mean, standard deviation (SD), median, and range (minimum and maximum). Categorical parameters were summarized by time point (as applicable) using frequency counts and rates of occurrence (%).
Verbatim terms used by Investigators to identify AEs in the eCRFs were mapped to the appropriate preferred term and SOC using a standardized coding dictionary (MedDRA Version 20.1). Severity was assessed using the NCI-CTCAE criteria (Table 1).
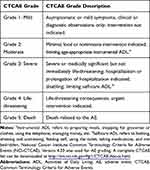 |
Table 1 Common Terminology Criteria for Adverse Events Grade |
Data for the PGA were tabulated as the number (%) of patients answering in each category.
Results
A total of 489 patients were screened; 235 patients failed to meet eligibility, and 3 additional patients were ineligible due to post-surgical eligibility criteria. The most common reasons for eligibility failure were: (1) the patient was unwilling or unable to understand the study procedures, to communicate meaningfully with the study personnel, and to comply with the study protocol; and (2) the patient was not undergoing elective surgery or, in the opinion of the Investigator, was not an appropriate candidate for IV tramadol for pain management postoperatively.
Two hundred fifty-one (251) patients received at least one dose of IV tramadol 50 mg, and thus were included in the Safety Population. Dosing was completed in 95.2% of patients; 11 patients (4.4%) withdrew due to an AE and 1 patient (0.4%) withdrew consent. No patients discontinued due to a lack of efficacy.
The majority of patients were female, non-Hispanic/non-Latino, and White, with median age 48.0 years (ranging from 18 to 75 years). The majority of patients had no prior history of opioid use and were ASA Physical Classification 2. Both orthopedic and soft tissue surgeries are well represented in the study: 89 patients had total joint replacement orthopedic surgeries and 162 patients had various soft tissue procedures. The most common surgery type was breast augmentation (30.7%), followed by total hip replacement (22.7%) and hernia surgeries (19.1%). (Table 2)
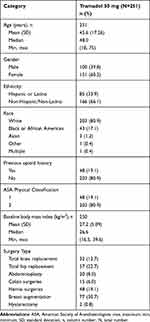 |
Table 2 Demographic and Other Baseline Characteristics |
Patients averaged 12.9 doses of IV tramadol 50 mg during this study. Over 25% of patients received 15 or more doses. The mean duration of exposure to IV tramadol was 47.7 h, and the mean total dose (in mg) was 645 mg. The maximum number of doses in an individual patient was 32, and the maximum dose was therefore 1600 mg. Over 20% of patients were exposed for at least 3 days (72 h), with the maximum duration being 124 h (5.2 days).
Safety Endpoints
Treatment-Emergent AEs (TEAEs)
A total of 59.4% of patients reported at least one TEAE, with 45.8% reporting at least one TEAE that was considered at least possibly related to study medication. The incidences of severe TEAEs (1.2%), SAEs (0.8%), and AEs leading to discontinuation (4.4%) were low, and there were no deaths reported. The generally mild severity of TEAEs in this study was further characterized via the high treatment completion rate (95.2%) and low incidence of premature termination due to AE (4.4%).
Nausea and vomiting, occurring in 28.7% and 19.5% of patients, respectively, were the most frequently reported TEAEs (Table 3). The use of antiemetics/antinauseants or propulsives for AEs was reported in 21.1% of patients. Hypoxia was reported in 6.8% of patients; this TEAE was primarily observed in patients who had undergone hernia surgery (16 of the 17 patients with hypoxia). Of the 17 patients with hypoxia, 12 had a BMI of 29 kg/m2 or higher, 8 had a history of smoking, and 10 experienced hypoxia only during the night. Notably, there were no unexpected TEAEs reported (eg, important cardiac or vascular events).
 |
Table 3 Incidence of Treatment-Emergent Adverse Events Occurring in at Least 2% of Patients |
To assess whether there was an increased risk of TEAEs in older patients, the incidence of TEAEs was compared by age categories of <65 years (non-geriatric) and ≥65 years (geriatric). There were 201 patients <65 years and 50 patients ≥65 years. The overall incidence of patients with at least one TEAE was similar between the age groups: 60.2% for patients <65 years and 56.0% for patients ≥65 years. The profile of TEAE was generally similar between the groups. Overall, there was no increased risk observed of any general type of TEAE in older versus younger patients.
Overall, 105 patients (41.8%) experienced an ORAE. The most common of these were nausea (28.7%) and vomiting (19.5%). Constipation was reported in 5.6% of patients.
Nausea and vomiting tended to occur during the early part of the treatment period (from 0 to 4 h) compared to later time periods, and constipation was only reported after 8 h of treatment (Table 4). New-onset use (ie, initiation of medication after the first dose of study medication) of antiemetics and antinauseants was reported in 8.0% of patients, new onset use of propulsives was reported in 15.5%, and new-onset use of drugs for constipation was reported in 39.0% (which were used prophylactically in many cases, as there was a low incidence of constipation).
 |
Table 4 Opioid-Related Adverse Events by Time to Onset Post-First Dose |
There were 2 patients with an SAE of post-procedural hematoma, each reported in a female patient who had undergone breast augmentation surgery. Neither SAE was considered to be at least possibly related to study drug.
Clinical Laboratory, Vital Sign, and Electrocardiogram (ECG) Changes Were Recorded
Mild increases in CPK were observed for some patients, the majority occurring in patients who had undergone hip surgery [after which, increases in CPK are not uncommon] (Berglund 1979, Laurence 2000, Mouzopoulos 2007; Mjaaland 2015). There were no other clinically meaningful changes in clinical laboratory parameters for chemistry, hematology, and urinalysis testing. Mean systolic and diastolic blood pressures and mean heart rate were generally unchanged through the observation period. Overall, the average ECG parameters tended to change very little over the course of 24 h following the first dose. Median heart rate increased slightly from baseline (the last value prior to dosing) as patients recovered from their surgical procedure.
TEAEs related to local tolerability at the infusion site were reported in 7.6% of patients.
Efficacy Endpoint
Although assessment of efficacy was not a primary objective of this study, the patients rated study drug in terms of effectiveness in controlling pain at 24-h post-initiation of treatment and at the End of Treatment. At 24 h, the majority of patients (92.5%) reported that study medication was good, very good, or excellent for controlling pain. At the End of Treatment, 94.8% reported that study medication was good, very good, or excellent for controlling pain (Figure 1).
 |
Figure 1 Patient global assessment of treatment at 24 and end of study treatment. Abbreviations: IV, intravenous; PGA, patient global assessment; mg, milligram. |
Discussion and Conclusions
This study was a Phase 3, multicenter, single-arm, open-label, uncontrolled, repeat-dose trial to assess the safety of IV tramadol 50 mg in the management of postoperative pain in patients who had undergone elective surgery. The primary objective of the study was to evaluate safety and tolerability, with the primary endpoints including AEs (including local tolerability), clinical laboratory tests, vital sign assessments, and ECGs, and local tolerability.
Both orthopedic and soft tissue surgeries are well represented in the study. Patients underwent various types of surgeries that included breast augmentation, total hip replacement, hernia repair, total knee replacement, abdominoplasty, colon surgery, and hysterectomy. The mean duration of exposure to IV tramadol was 48 h, and over 20% of patients were exposed for at least 72 h (3 days), with the maximum duration being 5 days (124 h).
IV tramadol was found to be well tolerated in this real-world study, with no unexpected safety findings reported. The AEs reported were consistent with known pharmacology of tramadol. In addition, tramadol was shown to be safe and well tolerated in combination with non-opioid analgesics, reflecting the effectiveness of tramadol in this multimodal treatment setting. Importantly, 94.8% of the patients reported that study medication was good, very good, or excellent for controlling pain, and no patient discontinued the study due to a lack of efficacy.
In the context of the ongoing US opioid epidemic, physicians and hospitals are under pressure to minimize the use of conventional, ie, Schedule II, opioids without sacrificing the quality of care. However, clinicians are currently limited in their choices of intravenous analgesics. The approved intravenous analgesics in the US for post-surgical pain generally include three pharmacological classes: acetaminophen, NSAIDs, and conventional opioids. The lack of options contributes to the fact that IV conventional opioids are still heavily used in the acute pain setting. Following administration of intravenous Schedule II conventional opioids, physicians tend to transition patients to oral Schedule II conventional opioids as part of their outpatient pain management. Many of these (including hydromorphone and oxycodone) have been shown to have a significant association with opioid misuse.8 In the context of a multimodal analgesic treatment paradigm, IV tramadol may fill the gap between non-opioid medicines and conventional opioids and thus may reduce the reliance on conventional opioids in the post-surgical setting.
As this was an open-label study, there are limitations to what conclusions can be drawn. Most notably, the absence of a control arm, placebo or active, precludes drawing definitive conclusion regarding effectiveness. However, this study was performed as part of a broad development program in which multiple controlled studies were performed; including a study with placebo control and active-control arms that was designed specifically with hypothesis testing in mind.9 Controlled studies with strict limitations on procedures are the gold standard for proving effectiveness, but oftentimes do not reflect how a drug might be used in the “real world”. The purpose of this current study was to provide that “real-world” data, allowing physicians flexibility in their management of patient pain, only limiting (precluding) the concomitant use of Schedule II opioids. Further, there were eligibility criteria for this study that may not necessarily be relevant in a broader population. We included limitations on surgical procedure types in this study, in that all procedures were to have been elective (and thus outcomes may not be generalized to a broader ‘all-surgery types’ population, eg, emergency or trauma surgery). The study enrolled multiple surgical types with few restrictions on concomitant therapy, including surgical anesthetics, and thus entry into this study was not as narrowly defined as in many other clinical trials for assessment of new pain relief medications.
This study demonstrated that IV tramadol alone or co-administered with non-opioid medication (when needed) as a multimodal combination analgesic approach, resulted in no unexpected safety outcomes and patients reporting high patient satisfaction with their pain relief. This study has thus shown IV tramadol, a dual-mechanism atypical opioid with lower abuse potential than conventional opioids, could be a useful alternative for pain relief that may help reduce the exposure to conventional opioids in the hospital setting.
Data Sharing Statement
Individual deidentified participant data and other study documents cannot be provided.
Acknowledgments
This study was funded by Avenue Therapeutics. Special thanks to Robert Criscola and Amy Landry Wheeler for support and efforts during the performance of this clinical study.
Disclosure
Dr Harold Minkowitz reports grants, personal fees from Avenue Therapeutics, AcelRX, Durect, and Heron; grants from Trevena and Recro, during the conduct of the study. Dr David Leiman reports grants from Avenue, during the conduct of the study; grants from Acelrx, outside the submitted work. Dr Lucy Lu, Mr Michael Ryan, Mr Mark Harnett are employees of Avenue Therapeutics. Dr Scott Reines is the Chief Medical Officer for Avenue Therapeutics and has a patent use of IV tramadol issued to Avenue Therapeutics; report personal fees from Avenue Therapeutics, during the conduct of the study. Dr Neil Singla is the founder and Chief Scientific Officer of Lotus Clinical Research, which received grants from Avenue Therapeutics in connection with this study; reports grants and other financial compensation from multiple pharmaceutical companies and institutions in connection with clinical trial services, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Grond S, Sablotzki A. Clinical pharmacology of tramadol. Clin Pharmacokinet. 2004;43(13):879–923. doi:10.2165/00003088-200443130-00004
2. Dunn KE, Bergeria CL, Huhn AS, Strain EC. A systematic review of laboratory evidence for the abuse potential of tramadol in humans. Front Psychiatry. 2019;10:704. doi:10.3389/fpsyt.2019.00704
3. World Health Organization (WHO). Expert committee on Drug Dependence. Tramadol Update Review Report. 2014. Available from: https://www.who.int/medicines/areas/quality_safety/6_1_Update.pdf.
4. US Food and Drug Administration (FDA). Assessment of abuse potential of drugs. 2017. Available from: https://www.fda.gov/media/116739/download.
5. World Health Organization 2017. Grünenthal GmBH Application for inclusion of tramadol into the WHO Model List of Essential medicines (EML). 2017. Available from: https://www.who.int/selection_medicines/committees/expert/21/applications/Grunethal_tramadol.pdf.
6. Lu L, Ryan M, Harnett M, Atiee GJ, Reines SA. Comparing the pharmacokinetics of 2 novel intravenous tramadol dosing regimens to oral tramadol: a randomized 3-arm crossover study. Clin Pharmacol Drug Dev. 2019.
7. Chou R, Gordon DB, de Leon-Casasola OA, et al. Management of postoperative pain: a clinical practice guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ committee on regional anesthesia, executive committee, and administrative council. J Pain. 2016;17(2):131–157.
8. Brat GA, Agniel D, Beam A, et al. Postsurgical prescriptions for opioid naïve patients and association with overdose and misuse: retrospective cohort study. BMJ. 2018;360:j5790. doi:10.1136/bmj.j5790
9. Minkowitz H, Salazar H, Leiman D, et al. Intravenous Tramadol is Effective in the Management of Postoperative Pain Following Abdominoplasty: A Three-Arm Randomized Placebo- and Active-Controlled Trial. Drugs R D. 2020. doi:10.1007/s40268-020-00309-0
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
