Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 16
Application of MR Imaging Characteristics in the Differentiation of Renal Changes Between Patients with Stage III Type 2 Diabetic Kidney Disease and Healthy People
Authors Zhang H, Yu B, Yang H, Ying H, Qu X, Zhu L, Wang C, Ding J
Received 7 April 2023
Accepted for publication 8 July 2023
Published 24 July 2023 Volume 2023:16 Pages 2177—2186
DOI https://doi.org/10.2147/DMSO.S413688
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Konstantinos Tziomalos
Hao Zhang,* Baoting Yu,* Hongsheng Yang, Hongfei Ying, Xiaolong Qu, Lilan Zhu, Cong Wang, Jun Ding
Department of Radiology, China-Japan Union Hospital of Jilin University, Changchun, 130021, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jun Ding, Department of Radiology, China-Japan Union Hospital of Jilin University, No. 829 of Xinmin Street, Chaoyang District, Changchun, 130021, People’s Republic of China, Tel/Fax +86 04 70 8499 7637, Email [email protected]
Objective: To explore the value of 1.5T magnetic resonance (MR) fat saturation-T2-weighted imaging (FS-T2WI) and apparent diffusion coefficient (ADC) imaging texture features in distinguishing the renal changes of patients with stage III type 2 diabetic kidney disease (DKD) from healthy people.
Methods: This study collected 55 patients with stage III DKD (39 males and 16 females) and 33 healthy controls (13 males and 20 females) from December 2021 to June 2022 in the China-Japan Union Hospital of Jilin University. All subjects were randomly divided in a ratio of 6:4 to extract and screen the FS-T2WI and ADC texture features of the right kidney of the subjects. The area under the curve (AUC) was used to assess the diagnostic accuracy of each model.
Results: There were significant differences between urea, creatinine and sex (p< 0.05) of the two groups in the training and test set, and no significant difference in age and body mass index (BMI). We extracted 1409 imaging features from the original ADC sequence and selected them by wavelet and Laplace-Gaussian filter and LASSO algorithm, and using the same methods of FS-T2WI. Finally, FS-T2WI and ADC models were selected to construct the united model, including 3 first-order features and 8 texture features. The AUC values of the training set of FS-T2WI, ADC, FS-T2WI+ADC combined logistic regression model were 0.96, 0.91, 0.98; the AUC values of the test set were 0.91, 0.89 and 0.93, and the specificity and accuracy values of the united model were 0.90 and 0.89, respectively.
Conclusion: FS-T2WI and ADC imaging features based on 1.5 T MR had diagnostic value in the early diagnosis of DKD stage III, and the combined model of FS-T2WI and ADC had high diagnostic efficiency.
Keywords: diabetic kidney disease, magnetic resonance imaging, radiomics
Introduction
At present, there were about 425 million diabetes mellitus (DM) patients worldwide, and it was estimated that the number of DM patients may reach 693 million by 2045.1 DM can cause a variety of complications, such as cardiovascular and cerebrovascular diseases, retinopathy, peripheral neuropathy, kidney diseases and other complications. Among them, diabetic kidney disease (DKD) was the most important and common chronic complication and was also the main cause of end-stage renal disease.2,3 The proportion of DKD patients in China in patients with end-stage renal disease was about 15% and had increased in recent years.
Patients with early DKD often had no obvious clinical symptoms. It was difficult to find abnormalities by conventional imaging methods of early DKD, and ultrasound diagnosis was also not sensitive. Some studies had shown that early diagnosis of DKD patients can reduce the risk of progressive kidney disease developing into end-stage kidney disease by 80%.4 Therefore, timely and accurate detection and timely clinical intervention of early diabetes kidney changes are particularly important to curb the occurrence and deterioration of DKD and improve the prognosis, which is of great significance both for individuals and society.
In recent years, with the development of artificial intelligence and the inspiration of emerging technologies such as genomics and proteomics, Lambin et al5 and others formally put forward the concept of radiomics in 2012, that is, to extract high-throughput features from medical images, and further use diversified statistical analysis and data mining methods to extract information that plays a real key role in glass from millions of image data, and finally use it for disease diagnosis, forecast or classification.6 At present, radiomics technology has played a huge role in the diagnosis and prediction of colorectal cancer, breast cancer, cervical cancer7 and other diseases.
Due to the rapid development of renal damage in the event of clinical diabetic kidney disease, there is still no effective treatment, and most patients enter end-stage renal disease in a short time. Therefore, targeted prevention and treatment must be given in the early stage, that is, kidney disease is still in stage I–III, in order to effectively control and delay the further development of kidney disease. Through previous studies, our research team found that there were differences in imaging manifestations of stage III diabetic kidney disease, so we conducted radiomics research on stage III diabetic kidney disease. The purpose of this study was to analyze the texture characteristics of early diabetes nephropathy and normal subjects through MRI, obtain the quantitative data of these texture characteristics, establish a diagnostic model, and explore the value of fat saturation T2 weighted imaging (FS-T2WI) and apparent diffusion coefficient (ADC) imaging texture characteristics in distinguishing the renal changes of patients with stage III type 2 DKD from healthy people.
Materials and Methods
Basic Materials of Patients
This study collected 55 patients with stage III DKD (39 males and 16 females) and 33 healthy controls (13 males and 20 females) from December 2021 to June 2022 in the China-Japan Union Hospital of Jilin University. The preoperative MR examinations of DKD confirmed by biopsy were obtained before June 2022. The basic flow chart of the experiment is shown in Figure 1. Diagnostic criteria for stage III diabetic nephropathy: In stage III, there will be continuous microalbuminuria, urinary albumin excretion rate continues at 20 ~ 200μg/min, glomerular filtration is higher than normal or normal.8
 |
Figure 1 Basic flow chart of the experiment. |
The inclusion criteria were pathologically diagnosed as DKD stage III with complete clinical data, no concomitant diseases, can cooperate with MRI examination, a history of DM2, stable comorbidities, treatment regimen, oral hypoglycemics, without change in weight, diet, lifestyle and medication therapy for comorbidity in the last 6 months. The main inclusion criteria for the control group were adult patients of both sexes in the age range of 30–70 years, without DM, hypertension, heart or kidney disease. The exclusion criteria for all groups were pregnancy and lactation, liver disease, infectious diseases, malignant diseases, patients with extremity amputations, urinary tract infections, or some other disease, cardiac insufficiency, patients with and relative contraindications for MR imaging (absolute contraindications: patients with pacemakers or with ferromagnetic foreign bodies; relative contraindications: claustrophobia, restless patients). Due to the presence of artifacts, post-processing MRI quality is not ideal.
Equipment and Scanning Parameters
MR was performed by using a 1.5-T Avanto scanner (Siemens Healthineers, Erlangen, Germany) and an 8-channel phased array abdominal coil. Subjects should be fasting and fasting at least 4 hours before the examination. The subjects were instructed to relax and lie on the examination table, make the coil close to the abdomen of the subjects. The scanning range ranged from the level of xiphoid process to the level of pubic symphysis, and non-contrast axial FS-T2WI and ADC images were obtained under a variety of breath-holding conditions. FS-T2WI scanning parameters: repetition/echo time was 5080/87ms, slice thickness/interstratified space was 4.0/0.4mm, number of layers was 20, matrix was 256×320.
Imaging Acquisition and Data Processing
The imaging data of all DKD patients and normal subjects were exported to the external storage device in DICOM format through PACS (image storage and transmission system), and the radiologist 1 used ITK-SNAP (ITK-SNAP Home (itksnap.org)) to manually sketch the region of interest (ROI) of the images to obtain the mask file (Figure 2), and the image quality was checked by the high-level radiologist 2 with more than 10 years of work experience.
Extract Imaging Texture Features from ROI
The texture features were extracted from the original images of FS-T2WI and ADC sequences by using the pythics library based on Python (version 3.8.0). The texture features include GLCM (gray level co-occurrence matrix), GLRLM (gray level run length matrix), GLSZM (gray-level size region matrix) and GLDM (gray-level dependence matrix). In addition to the shape features directly calculated from the original images, the other types of features had been processed by one or more filters. We used different λ parameters of high-pass or low-pass wavelet filter, and Laplace filter were used to preprocess the original images.
Characteristic Consistency Test
The right kidney images of 50 subjects (including 25 FS-T2WI sequences and 25 ADC sequences) were redrawn at random by physician 1 for intraclass correlation coefficient (ICC) test. It is generally believed that ICC>0.75 means that the two sketches have better consistency.9
Feature Screening
Before feature screening, we used the median filling method to process the missing values of features, and then used the Z-Core method to preprocess the data. In order to eliminate redundant features, the least absolute shrinkage and selection operator (LASSO) algorithm of R software was used to further filter and fuse features with significant differences to establish a united radiomics model, and the most important features of modeling and improving model performance with non-zero coefficients were determined.
Model Establishment
The texture features were selected to construct the radiomics model to eliminate the redundant features. Logical regression (LR) classifier was used to establish the model through 10-fold cross-validation method. Finally, all data were used for a training set and a test set. The radiomics labels (ie, RS-T2WI, RS-ADC) based on FS-T2WI and ADC were constructed, and the scores of corresponding radiomics model labels (radscore) were calculated. Radscore was calculated as radscore = intercept + bi·Xi, where Xi represents the final filtered feature value, and bi represents the corresponding weight coefficient of the final filtered feature. The clinical factors of DKD were statistically analyzed by univariate analysis, and the clinical factors related to the onset of DKD (P < 0.05) were selected. Then these selected clinical factors were analyzed by multivariate LR.
Quantitative Analysis
This study uses Scikit learning package (version 2.2.3) to create classification models. We used Matplotlib (version 3.1.0) to draw the ROC curve. For statistical analysis of general data, we used SPSS for Windows version 24.0 (IBM Corp., Armonk, NY, USA). Chi-square test was used to detect the difference in classification variables between groups. Independent sample t-test was used to test the inter-group difference of quantitative variables. P <0.05 was considered statistically significant. ROC was used to analyze the diagnostic efficacy and AUC of imaging characteristic parameters for stage III DKD. The predictive efficacy included sensitivity (SEN), specificity (SPE), accuracy (ACC), positive predictive value (PPV), positive predictive value (PPV), negative predictive value (NPV), AUC and 95% confidence interval (CI) were used.
Results
General Clinical Data of Subjects
Table 1 summarizes the characteristics of 88 participants. There were significant differences between urea, creatinine and sex (p < 0.05) of the two groups in the training and test set, and no significant difference in age and BMI. We extracted 1409 imaging features from the original ADC sequence and selected them by wavelet and Laplace-Gaussian filter and LASSO algorithm. There were 2 first-order features and 2 texture features selected by 10 times cross validation method. Using the same methods of FS-T2WI sequence, 1 first-order feature and 7 texture features were selected. Finally, FS-T2WI and ADC models were selected to construct the united model, including 3 first-order features and 8 texture features. The feature lists and coefficient charts are shown in Table 2. The correlation coefficient within ADC group was 0.79 (Figure 3). The correlation coefficient within the FS-T2WI group was 0.83 (Figure 4), which was greater than 0.75, indicating that the feature extraction had good consistency.
 |
Table 1 Patient Characteristics in the Training and Test Set |
 |
Table 2 The Imaging Features Based on FS-T2WI, ADC and United Model |
 |
Figure 3 The ICC of ADC. |
 |
Figure 4 The ICC of FS-T2WI. |
Results of Logical Regression Model Based on Imaging Features
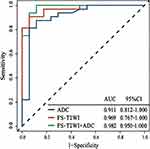
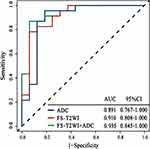
To evaluate the performance of the prediction model in the training set and test set, we focus on evaluating the ACC, SEN and SPE of the prediction model. The results are presented in Table 3. We verified the prediction and discrimination ability of the combined model through ROC curve analysis. The results showed that the diagnostic accuracy of stage III type 2 diabetic kidney disease and normal people was as high as 0.98 (all AUC > 0.89). In the 10-fold cross-validation of the training set, we found that the AUC of the LR model was 0.98, tending to the upper left corner and away from the diagonal (Figure 5). The combined model had the advantages of high positive predictive value (96.7%) and specificity (94.7%). In addition to the above training data set, we need preprocessing or class distribution equalization to test the performance of the united model. In the test set, the machine learning algorithm used the same machine learning algorithm as the training set. In the aspect of the classifier performance in the test set, ROC curve could show that the model had high classification accuracy, AUC value was 0.93 (Figures 6).
 |
Table 3 Performance of Each Model in Training Set and Test Set |
Discussion
The diagnosis of DKD could be divided into pathological diagnosis and clinical diagnosis. In the past, renal pathology was considered as the gold standard for diagnosis, but the operation of puncture biopsy was difficult and invasive, and it was difficult for a considerable number of primary medical institutions to implement it. As we had discussed before, although urinary microprotein was the main indicator for diagnosis of diabetes, its reliability was not absolute, and some end-stage renal diseases may also be normal. Similarly, indicators such as creatinine and urea were also affected by gender and age. Based on this background, it is particularly important to carry out non-invasive early screening and diagnosis of early DKD patients with normal laboratory indicators but with kidney changes. In this study, we aimed to evaluate the value of 1.5T MR FS-T2WI and ADC imaging texture features in distinguishing the kidney changes of patients stage III type 2 DKD from healthy people.
As a new non-invasive examination technology, radiomics provides more biological explanations for the subtle texture changes of images. At present, many studies had shown that functional magnetic resonance imaging (fMRI), including diffusion weighted imaging (DWI), diffusion tensor imaging (DTI) and blood oxygen level-dependent MRI (BOLD), was a safe and non-invasive imaging method that could provide quantitative parameters to evaluate the changes in renal microstructure and function.10–16 In this study, ADC and FS-T2WI sequences were selected, because T2WI sequence had good spatial resolution,17 good image contrast, and could better display the signal difference between different tissues. The model based on T2WI had better fitting.18 In the research of Wang Siyuan et al,19 the AUC values of the training set and test set of the early DKD diagnosis model based on T2WI imaging histology diagnosis were 0.89 and 0.76. Respectively, the AUC value of FS-T2WI training set and test set selected in this study was slightly higher than that of conventional T2WI, suggesting that FS-T2WI may have higher spatial resolution compared with conventional T2WI by using fat suppression technology. ADC was an artificial parameter for the dispersion and micro-perfusion of reactive water molecules, which was calculated by DWI. It makes up for the low resolution of DWI and the inaccurate evaluation of water molecular diffusion caused by the penetration effect of T2WI. Relevant literature studies had pointed out that, except for early DKD (stage I), the renal ADC value in most chronic kidney disease stages is significantly lower than the normal level, and the renal ADC value was negatively correlated with the patient’s serum creatinine level.20 The increase of ADC value also suggested that renal tubulointerstitial fibrosis and water molecule dispersion were limited. In addition, the study of Xu et al also showed that the ADC value of renal cortex and medulla in DKD was significantly different,21 generally low in patients with renal insufficiency, which can be used as a good non-invasive indicator for evaluating renal function.
This study compared the diagnosis and analysis of DKD patients using ADC and FS-T2WI sequence models, and found that both had high diagnostic efficiency, and the combined sequence diagnostic model had higher accuracy and specificity, which was similar to the research results of Yu et al22 on stage III DKD patients. Nevertheless, the clinical model we have established may still be unsatisfactory, because the kidney may have undergone obvious pathological changes when the patients with early DKD have slight proteinuria or even in the compensatory period without clinical characteristics.23 Whether we can detect the abnormalities of the renal microstructure by non-invasive means before the patients have proteinuria will be the focus of our future research, we also hope to make new breakthroughs in relevant animal experiments.
Though our research overcome shows the latent value of distinguishing the radiation characteristics of early renal damage and healthy kidney in diabetic patients, our research still has localizations. Firstly, this study is a single-center study and lack of external validation. The stability and generalization ability of the model need to be further strengthened. Secondly, this study is a preliminary study, and the amount of data in this study is small and uneven, which could have certain selective deviation. It is necessary to further strengthen the generalization of the model through multi-center validation. Thirdly, only the right kidney of the subject was used in this study. Although the left kidney was not included because of intestinal gas interference, whether there was any difference between the two kidneys needs further study. Lastly, this study included the intact cortex and medulla of the right kidney, and the texture differences between the cortex and medulla also need to be further explored. The texture feature extraction and the establishment of the diagnostic model in this study are only for scientific research at this stage, and the transformation into clinical application still needs further demonstration.
Conclusions
Our research results suggested that renal changes detected by ADC and FS-T2WI based on 1.5T MR may serve as indicators for early DKD. We analyzed the texture features of patients with stage III type 2 DKD and normal images to obtain quantitative data of texture features. Research had found that FS-T2WI and ADC imaging features based on 1.5T MR had diagnostic value for early diagnosis of DKD stage III, and the joint model of FS-T2WI and ADC had high diagnostic efficiency.
Data Sharing Statement
The original contributions presented in the study were included in the article. Further inquiries can be directed to the corresponding authors.
Ethics Approval and Consent to Participate
This study was conducted in accordance with the Declaration of Helsinki and reviewed and approved by the China–Japan Union Hospital of Jilin University. The participants provided their written informed consent to participate in this study.
Funding
This work was supported by the Industrial Technology Research and Opening Up Project of Jilin Development and Reform Commission (2020C036-6).
Disclosure
All of the authors had no any personal, financial, commercial, or academic conflicts of interest separately.
References
1. Zimmet PZ, Magliano DJ, Herman WH, et al. Diabetes: a 21st century challenge. Lancet Diabetes Endocrinol. 2014;2(1):56–64. doi:10.1016/S2213-8587(13)70112-8
2. Alicic RZ, Rooney MT, Tuttle KR. Diabetic kidney disease: challenges, progress, and possibilities. Clin J Am Soc Nephrol. 2017;12(12):2032–2045. doi:10.2215/CJN.11491116
3. Wild S, Roglic G, Green A, et al. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27(5):1047–1053. doi:10.2337/diacare.27.5.1047
4. Molitch ME, Adler AI, Flyvbjerg A, et al. Diabetic kidney disease: a clinical update from kidney disease: improving global outcomes. Kidney Int. 2015;87(1):20–30. doi:10.1038/ki.2014.128
5. Lambin P, Rios-Velazquez E, Leijenaar R, et al. Radiomics: extracting more information from medical images using advanced feature analysis. Eur J Cancer. 2012;48(4):441–446. doi:10.1016/j.ejca.2011.11.036
6. Georgiev A, Chervenkov L, Doykov M, Doykova K, Uchikov P, Tsvetkova S. Surveillance value of apparent diffusion coefficient maps: multiparametric MRI in active surveillance of prostate cancer. Cancers. 2023;15(4):1128. PMID: 36831471; PMCID: PMC9953850. doi:10.3390/cancers15041128
7. Wang S, Jiang T, Hu X, et al. Can the combination of DWI and T2WI radiomics improve the diagnostic efficiency of cervical squamous cell carcinoma? Magn Reson Imaging. 2022;92:197–202. doi:10.1016/j.mri.2022.07.005
8. Mogensen CE, Schmitz A, Christensen CK. Comparative renal pathophysiology relevant to IDDM and NIDDM patients. Diabetes Metab Rev. 1988;4(5):453–483. PMID: 3061756. doi:10.1002/dmr.5610040504
9. Koo TK, Li MY. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med. 2016;15(2):155–163. doi:10.1016/j.jcm.2016.02.012
10. Notohamiprodjo M, Reiser MF, Sourbron SP. Diffusion and perfusion of the kidney. Eur J Radiol. 2010;76(3):337–347. doi:10.1016/j.ejrad.2010.05.033
11. Rossi C, Artunc F, Martirosian P, et al. Histogram analysis of renal arterial spin labeling perfusion data reveals differences between volunteers and patients with mild chronic kidney disease. Invest Radiol. 2012;47(8):490–496. doi:10.1097/RLI.0b013e318257063a
12. Artz NS, Wentland AL, Sadowski EA, et al. Comparing kidney perfusion using noncontrast arterial spin labeling MRI and microsphere methods in an interventional swine model. Invest Radiol. 2011;46(2):124–131. doi:10.1097/RLI.0b013e3181f5e101
13. Warner L, Glockner JF, Woollard J, et al. Determinations of renal cortical and medullary oxygenation using blood oxygen level-dependent magnetic resonance imaging and selective diuretics. Invest Radiol. 2011;46(1):41–47. doi:10.1097/RLI.0b013e3181f0213f
14. Ebrahimi B, Gloviczki M, Woollard JR, et al. Compartmental analysis of renal BOLD MRI data: introduction and validation. Invest Radiol. 2012;47(3):175–182.
15. Prasad PV. Functional MRI of the kidney: tools for translational studies of pathophysiology of renal disease. Am J Physiol Renal Physiol. 2006;290(5):F958–74. doi:10.1152/ajprenal.00114.2005
16. Chandarana H, Lee VS. Renal functional MRI: are we ready for clinical application? AJR Am J Roentgenol. 2009;192(6):1550–1557. doi:10.2214/AJR.09.2390
17. Zhong Y, Utriainen D, Wang Y, et al. Automated white matter hyperintensity detection in multiple sclerosis using 3D T2 FLAIR. Int J Biomed Imaging. 2014;2014:239123. doi:10.1155/2014/239123
18. Song G, Li P, Wu R, et al. Development and validation of a high-resolution T2WI-based radiomic signature for the diagnosis of lymph node status within the mesorectum in rectal cancer. Front Oncol. 2022;12:945559. doi:10.3389/fonc.2022.945559
19. Wang S, Shi D, Zhang H, et al. Diagnosis of early diabetes nephropathy based on MR T2WI imaging histology. Chinese J Interv Imaging Ther. 2022;19(03):169–172.
20. Xu X, Fang W, Ling H, et al. Diffusion-weighted MR imaging of kidneys in patients with chronic kidney disease: initial study. Eur Radiol. 2010;20(4):978–983. doi:10.1007/s00330-009-1619-8
21. Xu Y, Wang X, Jiang X. Relationship between the renal apparent diffusion coefficient and glomerular filtration rate: preliminary experience. J Magn Reson Imaging. 2007;26(3):678–681. doi:10.1002/jmri.20979
22. Yu B, Huang C, Fan X, et al. Application of MR imaging features in differentiation of renal changes in patients with stage III type 2 diabetic nephropathy and normal subjects. Front Endocrinol. 2022;13:846407. doi:10.3389/fendo.2022.846407
23. Schrijvers BF, De Vriese AS, Flyvbjerg A. From hyperglycemia to diabetic kidney disease: the role of metabolic, hemodynamic, intracellular factors and growth factors/cytokines. Endocr Rev. 2004;25(6):971–1010. doi:10.1210/er.2003-0018
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.