Back to Journals » Journal of Inflammation Research » Volume 16
APOL1 Induces Pyroptosis of Fibroblasts Through NLRP3/Caspase-1/GSDMD Signaling Pathway in Ulcerative Colitis
Authors Zhu F, Li S, Gu Q, Xie N, Wu Y
Received 13 October 2023
Accepted for publication 19 December 2023
Published 27 December 2023 Volume 2023:16 Pages 6385—6396
DOI https://doi.org/10.2147/JIR.S437875
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Adam D Bachstetter
Fangqing Zhu,1 Sheng Li,2 Qiuping Gu,1 Ningsheng Xie,1 Yinxia Wu3
1Department of Gastroenterology, Ganzhou People’s Hospital, Ganzhou, Jiangxi, 341000, People’s Republic of China; 2Department of Gastroenterology, Yuebei People’s Hospital, Shantou University Medical College, Shaoguan, Guangdong, 512026, People’s Republic of China; 3Department of Rehabilitation, Ganzhou People’s Hospital, Ganzhou, Jiangxi, 341000, People’s Republic of China
Correspondence: Fangqing Zhu; Yinxia Wu, Email [email protected]; [email protected]
Background: Pyroptosis is a form of proinfammatory gasdermin-mediated programmed cell death. Abnormal infammation in the intestine is a critical risk factor for Ulcerative colitis (UC). However, at present, it is not clear whether pyroptosis of colonic fibroblasts is involved in the pathogenesis and progression of UC.
Methods: In this study, key genes associated with UC were identified by bioinformatics analysis. Datasets were downloaded from the Gene Expression Omnibus (GEO) database (GSE193677). The differentially expressed genes were analyzed, and the hub genes were screened by weighted gene co-expression network analysis (WGCNA) and differentially expressed genes. We also downloaded the dataset from GEO for single-cell RNA sequencing (GSE231993). The expression of key genes was verified by immunohistochemistry, immunofluorescence and Western blot, and the specific pathways of key genes inducing pyroptosis in cell lines were explored.
Results: The results of bioinformatics analysis showed that the expression of APOL1 and CXCL1 in UC tissues was significantly higher than that in normal tissues. The results of single-cell analysis showed that the two genes were co-localized to fibroblasts. These results were consistent with the results of immunohistochemistry and immunofluorescence colocalization in human intestinal mucosa specimens. Furthermore, APOL1 overexpression induced NLRP3-caspase1-GSDMD-mediated pyroptosis of fibroblasts, which was confirmed by Western blot.
Conclusion: APOL1 induces pyroptosis of fibroblasts mediated by NLRP3-Caspase1-GSDMD signaling pathway and promote the release of chemokines CXCL1. Fibroblasts may play a crucial role in the pathogenesis and progression of UC.
Keywords: apolipoprotein L1, pyroptosis, fibroblasts, gasdermin-D, Ulcerative colitis
Introduction
Ulcerative colitis (UC) represents a chronic non-specific bowel disorder characterized by abdominal pain, diarrhea, tenesmus, and bloody mucopurulent stool, characterized by diffuse and persistent inflammation of the colorectal mucosa.1 UC is often recurrent in clinical practice, with a long treatment cycle and persistent ulcer, which is an important risk factor for inducing colorectal cancer.2 Since the 21st century, the incidence of UC has been on the rise, especially in Asia. So far, the etiology of UC is not fully understood, there is no effective cure for UC, and the recurrence rate is high. Therefore, understanding the exact molecular mechanisms of UC is crucial for the development of therapeutic approaches.3–5
Pyroptosis was previously defined as caspase-1-mediated necrosis, triggered primarily in response to bacterial invasion.6 Gasdermin D(GSDMD) and Gasdermin E (GSDME) are cleaved by active caspase-1/4/5/11 and caspase-3, respectively, through the intermediate linker to release their GSDM-N fragments and penetrate the membrane, inducing pyroptosis.7–9 Subsequently, the pore-forming activity of GSDMD cause cytoplasmic swelling and intracellular content release, including immunogenic damage-associated molecular patterns (DAMPs). Consequently, pyroptosis is redefined as Gasdermin-mediated proinflammatory cell death.10 Moreover, fibroblasts are important in epithelial stem cell maintenance and differentiation, immune homeostasis, and endothelial cell function.11 Novel fibroblast-specific mechanisms and unexpected heterogeneity were revealed, which can be attributed to differences in expressions and locations of each subpopulation.12 Meanwhile, a recent study examining the colon at various intervals throughout the stages of acute injury and repair has indicated that fibroblasts play a significant role in influencing other cells and potentially serve as a hub for cellular interactions throughout acute inflammation.13 Currently, intestinal epithelial pyroptosis is associated with colitis and colitis-associated colorectal cancer, but there is no study on the relationship between fibroblast pyroptosis and colitis.
Bioinformatics contributes to the early diagnosis of key genes in patients, the development of effective treatment strategies, and the prediction of clinical challenges.14 The aforementioned innovative methodology has been extensively employed in studying diverse malignancies and in discovering novel biomarkers for numerous non-neoplastic disorders.15–17 Through bioinformatics analysis, two significant differentially expressed genes (DEGs) associated with UC were identified, Apolipoprotein L1 (APOL1) and C-X-C motif chemokine ligand 1 (CXCL1). Meanwhile, single-cell analysis showed that these two genes were mainly expressed in colonic fibroblasts. As a common chemokine in UC, CXCL1 has been reported in many studies.18 However, the correlation between APOL1 and UC remains unreported. Therefore, this study focuses on exploring the relationship between the APOL1 gene in colon fibroblasts and UC, which may represent an innovative biomarker or potential target for UC patients. APOL1 is a secreted high-density lipoprotein involved in lipid transport and metabolism.19 Recent studies have found that APOL1 variants mediate cell death, and O’Toole et al20 proposed in their study that there may be a positive feedback regulation between APOL1 variants and inflammatory response pathways. APOL1 variant genes are regulated by inflammatory cytokines, but their overexpression can lead to inflammatory cell death.21 Cytokines released after cell death can up-regulate the level of APOL1 variant genes, and this process of cell death includes pyroptosis.22
Through bioinformatics analysis and experimental validation, we found that APOL1 was significantly elevated in UC patient tissues. In addition, APOL1 overexpression can induce pyroptosis in fibroblasts and release a series of inflammatory factors and a series of chemokines, including CXCL1. Bioinformatics analysis showed that APOL1 overexpression exhibited a positive correlation to the NOD-like receptor signaling pathway. We experimentally verified that APOL1 induced pyroptosis by activating NLRP3/Caspase1/GSDMD signaling pathway. Overall, our findings suggest that APOL1 may represent an innovative UC therapeutic target.
Materials and Methods
Dataset Analyses
The Gene expression omnibus (GEO) database serves as a widely utilized functional genomic repository, facilitating the storage and retrieval of high-throughput gene expression data, chips, and microarrays. Its primary objective is to enable qualitative investigations of the expression patterns of genes associated with diseases. We obtained the GSE193677 dataset from the GEO database. Colon biopsy specimens obtained from non-inflammatory controls and from individuals diagnosed with UC within the GSE193677 dataset were selected for data analysis using weighted gene co-expression network analysis(WGCNA). Additionally, we obtained the GSE231993 dataset from the GEO database for single-cell sequencing data analysis.
Tissues and Cell Lines
The Department of Gastroenterology at Ganzhou People’s Hospital obtained Pathological sections of intestinal tissue from individuals with and without UC. According to the guidelines formulated by the Ethics Committee of Ganzhou People’s Hospital, the intestinal tissue pathological sections of patients were collected with informed consent. The CCD-18Co colonic fibroblast cell line was purchased from Zhejiang Meisen Cell Technology Co., LTD. (Meisen, Zhejiang, China). We used 1μg/mL LPS (Sigma, USA) and 5mM ATP (Sigma, USA) to induce pyroptosis in colonic fibroblasts in vitro.
Western Blot Analysis
Antibodies to NLRP3(ab270449), Caspase1(ab207802), GSDMD(ab219800), GSDME(ab215191) were purchased from Abcam. Antibodies to GAPDH were purchased from Abclonal(a19056). Antibodies to α-SMA(67735-1-lg) and APOL-1(11486-1-AP) were purchased from Proteintech(Wuhan, China). RIPA lysis buffer (Fude, China), including protease inhibitors(Yeasen, China), was utilized to extract total proteins and protein quantification was conducted using the BCA kit(Beyotime, Jiangsu, China). Cell-extracted proteins were added to a 5X loading buffer and boiled for 10 min before electrophoresis on SDS-PAGE gels. The proteins were placed onto PVDF membranes(EMD Millipore, Billerica, Massachusetts, USA) and subsequently subjected to incubation with their respective antibodies. The target proteins were observed by chemiluminescence. The protein band density was quantified using ImageJ software.
Immunohistochemistry and Immunofluorescence Staining
Immunohistochemistry has been used to determine APOL-1 protein expression levels in tissues embedded in paraffin. Protein expression levels analysis and scoring were performed by expert pathologists using Image-Pro Plus software. Co-localization of α-SMA and APOL-1 in fibroblasts was performed by immunofluorescence staining. Incubation was first performed with primary antibodies targeting α-SMA and APOL-1(1:100, Proteintech). Second, the samples were incubated with biotinylated secondary antibodies against immunoglobulin(eBiosciences, BD, USA), and the nuclei were counterstained with DAPI. Finally, fluorescence microscopy was used for observation and analysis.
Gene Overexpression Plasmid
The APOL-1 overexpression plasmid and its control plasmid were customized and synthesized by Beijing Qingke Biotechnology Company. Transfection was performed using transfection reagents.
LDH Release Assay
Fibroblasts were seeded in 6-well plates, pyroptosis was induced using LPS+ATP, and APOL1 overexpression plasmids were transfected. The culture supernatant was collected when obvious pyroptosis vesicles appeared, and the LDH level in the supernatant was detected by LDH detection kit(BC0685, Solarbio, China).
PI (Propidium Iodine) Staining
PI staining was used to observe the cell death morphology induced by LPS+ATP reagent and APOL1 overexpression plasmids. Appropriately treated fibroblasts were seeded in 6-well plates, stained with 100µL PI(P8080-10mg, Solarbio, China) for 15 min at room temperature in the dark, and then washed twice with PBS. Fluorescence microscopy(BX63; Olympus Corporation; Japan) was used for observation. The amplification was x20, the excitation wavelength was 535 nm, and the emission wavelength was 614 nm.
Quantitative Real-Time PCR
Total mRNA extraction was performed from cells utilizing Invitrogen TRIzol reagent and chloroform. Subsequently, the mRNA was extracted and subjected to reverse transcription while being converted into cDNA using the RevertAid First Strand cDNA Synthesis Kit(Thermo Scientific). The study employed the Applied Biosystems SYBR Green PCR Master Mix to conduct qPCR. The calculation of relative mRNA expression of the genes of interest was performed using the DeltaDeltaCt technique, normalizing the values to the GAPDH expression level. The primers used for the target genes are shown in Table S1.
Statistical Analysis
Statistical analyses were performed using GraphPad Prism 9 software unless otherwise indicated. Further, unless otherwise stated, data are presented as mean ± standard deviation (SD). One-way analysis of variance (ANOVA) was used, as appropriate, to determine significant differences between groups. Statistical significance was defined as *p<0.05, **p<0.01, ***p<0.001, and ****p<0.0001.
Results
WGCNA and Hub Genes Detection
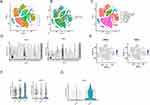
In the study of GSE193677, the soft threshold power value of 6 was utilized to cluster a total of 993 samples (Figure 1A). Furthermore, we used dendrograms and heatmaps to measure the similarity of modules using correlation(Figure 1B and C). The correlations were utilized to establish the connections between the endoscopic and clinical scores and the Brown module. The GO enrichment analysis of the Brown module showed a correlation with functional clusters such as “cell chemotaxis”, “leukocyte migration”, and “leukocyte chemotaxis” (Figure 2A). The Brown module was positively correlated with endoscopic and clinical scores (correlations, 0.48 and 0.51, respectively; P < 0.0001) (Figure 2B and C). Based on the significance of genes in the brown module (P < 0.05), a set of 465 genes exhibiting strong connectivity within the brown module were identified as potential candidate genes, and after screening, we obtained two genes related to our study: APOL1 and CXCL1 (Figure 2D).
Single-Cell Sequencing Data Analysis
First, we analyzed the GSE231993 dataset to evaluate the connection among APOL1, CXCL1 expression, and cells. The cell populations were clustered into 17 populations (Figure 3A and B), which were combined into seven cell populations based on target gene expression, namely B cells, endothelial cells, epithelial cells, fibroblasts, monocytes, NT cells, and T cells (Figure 3C). The study findings indicated that fibroblasts had a predominant high expression of APOL1 and CXCL1 compared to other cell populations (Figure 3D and E). Further analysis revealed that compared to the normal group and UC adjacent tissue group, the UC group had significantly higher expression of APOL1 and CXCL1 (Figure 3F), and CXCL1 expression exhibited a significant positive association with APOL1 expression (Figure 3G).
Co-Expressed Networks and Potential Function of APOL1 and CXCL1 in Fibroblasts
To explore the potential roles and underlying mechanisms of APOL1 and CXCL1 within fibroblasts, we analyzed the co-expression network of APOL1 and CXCL1 in fibroblasts. The study analyzed APOL1 and CXCL1 co-expressed genes (P < 0.05) by conducting Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analyses. The GO results showed that APOL1 and CXCL1 co-expressed genes were enriched in B cell-mediated immune responses, B-cell receptor signaling pathways, antigen receptor-mediated signaling pathways, and positive modulation of B cell activation (Figure 4A). Unsurprisingly, UC was associated with co-expressed genes in fibroblasts, and we judged that APOL1 and CXCL1 were involved in the progression of UC. Furthermore, the KEGG results showed that the intestinal immune network, NOD receptor signaling, and other related pathways were involved (Figure 4B).
 |
Figure 4 GO and KEGG pathway analysis of APOL1 and CXCL1 co-expressed genes. (A) GO analysis of APOL1 and CXCL1 co-expressed genes. (B) KEGG pathway analysis of APOL1 and CXCL1 co-expressed genes. |
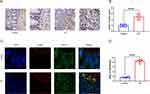
Validation of APOL1 Expression in Human Samples
First, we confirmed that APOL1 exhibited elevated expression levels within the intestinal mucosa of UC individuals, and we selected the intestinal mucosa of healthy subjects and UC individuals for immunohistochemical detection (Figure 5A and B). The study findings indicated a significant upregulation of APOL1 expression in the intestinal mucosa of individuals diagnosed with UC compared to a control group. Second, the results of bioinformatics analysis showed that APOL1 was mainly expressed in fibroblasts. Immunofluorescence co-localization was conducted on the intestinal mucosa samples obtained from individuals diagnosed with UC as well as healthy individuals serving as controls to detect α-SMA, a marker protein of fibroblasts, and APOL1, a target protein, and the findings revealed that APOL1 exhibited significant high expression within fibroblasts in UC individuals (Figure 5C and D).
APOL1 Overexpression Can Induce Pyroptosis in Fibroblasts
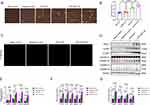
To elucidate the association between fibroblasts and cell pyroptosis, LPS+ATP was used to induce fibroblasts cell pyroptosis for in vitro experiments. The group was as follows:(1) Blank control group;(2) Negative control(empty plasmid) group;(3) LPS+ATP group; (4) LPS+ATP+OE(overexpression plasmid) group. Pyroptosis vesicles were observed in both LPS+ATP group and LPS+ATP+OE group, but not in blank control group and negative control group. Compared with LPS+ATP group, pyroptosis vesicles were significantly increased in LPS+ATP+OE group (Figure 6A). Simultaneously, we performed LDH assay and PI staining to verify pyroptosis. The results showed that compared with LPS+ATP group, LDH level and PI staining positive rate were significantly increased in LPS+ATP+OE group (Figure 6B and C), indicating that APOL1 gene overexpression was involved in pyroptosis.
Gsdmd-Mediated Pyroptosis Performs a Crucial Function Within the Release of Proinflammatory Cytokines and Chemokines from Fibroblasts
We performed Western blot to verify the important pyroptosis protein GSDMD and GSDME, and the results indicated that GSDME was not expressed in fibroblasts, while the activation band of GSDMD exhibited a significant elevation in LPS+ATP group and LPS+ATP+OE group, and there was a statistically significant difference between the two groups. Thus confirming the hypothesis that GSDMD is involved in the fibroblast pyroptosis. Bioinformatics analysis showed that the high expression of APOL1 was positively correlated with NOD-like receptor signaling pathway, so we further explored the upstream signal molecules of NLRP3. Western blot results showed that NLRP3 and Caspase1 proteins were significantly activated. Therefore, we suggest that APOL1 overexpression in fibroblast cell lines activates NLRP3-Caspase1-GSDMD signaling pathway mediated pyroptosis (Figure 6D and E). Next, we continued to explore the changes of downstream in related inflammatory factors and chemokines after pyroptosis. We identified the inflammatory factors and chemokines commonly released after pyroptosis, such as TNF-α, HMGB1, IL-1β, IL-18, and IL-6, Chemokines CXCL1, CXCL2, CXCL9, and CXCL10. These factors were detected by real-time PCR, The findings suggested that compared with LPS+ATP group, the expression of inflammatory factors and chemokines in LPS+ATP+OE group were significantly increased (Figure 6F and G). This result also confirmed the above results of bioinformatics analysis.
Discussion
UC is a prevalent inflammatory bowel disorder with multiple causal relationships, and the etiology is still unclear.4 Fibroblasts are the most common cells in loose connective tissue, which can coordinate inflammatory responses, regulate tissue homeostasis, and mediate tissue damage. They are also a key cell source of inflammatory cytokines and chemokines and are considered to be the key to the persistence of chronic inflammation.23 In a variety of chronic inflammatory diseases, fibroblasts can play a role as inflammatory cells themselves, recruit white blood cells, and drive angiogenesis and tissue generation.24–26 The dysfunction of fibroblasts leads to an inappropriate and continuous accumulation of inflammatory cytokines, resulting in a prolonged inflammatory response. Regulating fibroblasts to control inflammation has become a new target for treating various chronic inflammatory diseases.
Through bioinformatics data analysis, we identified the genes APOL1 and CXCL1 in colonic fibroblasts as two key genes associated with the progression of UC. Further enrichment analysis showed that the functions of APOL1 and CXCL1 co-expressed genes were enriched in B cell receptor signaling, B cell-mediated immune response, positive regulation of B cell activation, and antigen receptor mediation. More importantly, pathway enrichment analysis suggested that it was related to a NOD-like receptor signaling pathway. The above enrichment results suggested that it was closely related to the pathogenesis of UC. Therefore, two key genes, APOL1 and CXCL1, were followed up.
Existing studies have shown that the APOL protein molecule coding gene is located on human chromosome 22, and six types of APOL protein molecules (APOL i-vi) have been found.27 APOL1 is the only secreted protein in the APOL family, which is unique to humans and some higher primates.28 Since the discovery of APOL1, a new member of the high-density lipoprotein family, by Duchateau et al29 in 1997, the research on APOL has been in the ascendant. Lipoproteins can combine with lipids to form lipoproteins that shuttle among lipids in the systemic circulation. Apolipoproteins are correlated to inflammatory and immune responses and tumor progression.30–32 Serious kidney disease affects blacks more than other ethnic groups, which can be attributed to genetic variants in the APOL1 gene. APOL1 variants are correlated to various kidney diseases, including HIV-associated nephropathy, focal segmental glomerulosclerosis, sickle cell nephropathy, lupus nephritis, and hypertensive nephropathy. However, there is still a lack of consensus on the specific molecular mechanisms driving APOL1 kidney disease or even on the cell types of APOL1 injury.33,34 APOL1 nephropathy in hyperalbuminuric form suggests that podocytes may be the injury site, and podocyte-specific APOL1 overexpression in mice triggers renal dysfunction, unlike the tubular cell APOL1 overexpression.20 APOL1-related glomerulopathy is closely related to podocyte-specific expression. The results of Wu et al22 demonstrate the role of STING and NLRP3 inflammasome activation in APOL1-mediated kidney disease, suggesting the significance of NLRP3 in mediating podocyte toxicity through pyroptosis. APOL1 encodes a secreted high-density lipoprotein and has been identified as a gene that is aberrantly expressed in a variety of cancers. Lin et al35 showed that APOL1 acts as an oncogene in pancreatic cancer, promoting proliferation and inhibiting apoptosis of pancreatic cancer cells by activating the NOTCH1 signaling pathway. Upon conducting a thorough analysis of the existing literature, it was observed that no correlation study has been identified between APOL1 and UC.
Through bioinformatics analysis, our data obtained from UC patients showed that APOL1 protein expression increased significantly in the colonic mucosa compared to healthy controls. To further clarify the specific cell types of APOL1 expression, a single-cell analysis was performed, and the results showed that both APOL1 and CXCL1 were mainly highly expressed in fibroblasts in UC patients. Furthermore, to confirm the results of bioinformatics analysis, the intestinal mucosae of UC patients and healthy controls were collected for immunofluorescence co-localization of α-SMA, a marker of fibroblasts, and APOL1, a target protein. The results showed that APOL1 was significantly highly expressed in fibroblasts in UC patients. CXCL1 is a downstream chemokine of APOL1. Intestinal injury is caused by a series of programmed and coordinated events. Various immune cells participate in the inflammatory network and interact with each other through cytokines or chemokines, or both in the inflammatory process.36 Chemokines bind to their receptors and contribute to cell growth, proliferation, differentiation, and apoptosis, among others, which are important mediators involved in the inflammatory response.37 CXCL1 is a common subgroup in the chemokine family. Studies have confirmed that CXCL1 governs intestinal inflammation and is an important neutrophil chemotactic factor.38 Moreover, CXCL1 is involved in inflammation, neuron growth and development, oligodendrocyte proliferation, and migration, as well as intestinal and brain development and injury.39,40
The core of pyroptosis is activating the NLRP3 inflammasome and mediating GSDMD, which rapidly ruptures the cell membrane and releases cell contents, eventually leading to an inflammatory response.41 Colonic pyroptosis can lead to cell death, colonic inflammation, and colonic injury, while inhibition of pyroptosis can reduce pathological damage.7,42 Wu et al22 found that the cytosolic nucleotide sensing pathway (sting), the NLRP3, Caspase1, and GSDMD inflammasome proteins were activated in the glomeruli with variants of the APOL1 gene. Additionally, the cytosolic nucleotide sensing pathway and the inflammasome activation were observed in podocytes with variants of the APOL1 gene in their cultures. Wakashin et al21 found that APOL1-b3 modulates proinflammatory signaling and interacts with NLRP12, a toll-like receptor signaling regulator, to promote inflammatory signaling in podocytes and tubular cells, leading to glomerular injury.
Based on the reports presented above and the results from our bioinformatics analysis, we used a colon fibroblast cell line for experimental verification. First, an APOL1 overexpression plasmid was constructed, and pyroptosis vesicles were observed in some fibroblasts. The presence of pyroptosis has been confirmed through additional LDH detection and PI staining outcomes. Second, in a further mechanistic study, GSDMD protein electrophoresis, an important executive protein of pyroptosis, was performed to demonstrate that the N-terminus of GSDMD(GSDMD-NT) protein was activated by pyroptosis. Finally, the upstream and downstream proteins involved in GSDMD-mediated pyroptosis were investigated. The basic mechanism behind pyroptosis involves the initiation of the inflammasome and the subsequent involvement of GSDMD-NT, resulting in the prompt disruption of the cellular membrane and the subsequent release of intracellular components, ultimately culminating in an inflammatory reaction. Western blot analysis showed that the Caspase1 and NLRP3 upstream proteins inflammasome proteins were significantly activated. The quantitative real-time fluorescence PCR results showed a significant up-regulation of cytokines, including IL-18/1β/6, TNF-α, and HMGB1, and chemokines, including CXCL1/2/9/10. Therefore, we demonstrated that APOL1 overexpression could trigger NLRP3-Caspase1-GSDMD-mediated pyroptosis in fibroblasts. After pyroptosis, plasma membrane pore formation releases a large number of downstream cytokines and chemokines. Among the chemokines we examined, the release of CXCL1 increased significantly in the bioinformatics analysis, which is consistent with the results of the bioinformatics analysis above. Of course, we detected common cytokines, and several other common chemokines were also significantly increased, which is consistent with our clinical indicators in patients with UC.
In summary, the results show that APOL1 overexpression mediates the NLRP3-Caspase1-GSDMD signaling pyroptosis pathway and up-regulates inflammatory factors and chemokines, CXCL1, aggravating colitis. Therefore, APOL1 and CXCL1 contribute significantly to UC, and our study helps to understand UC and provides targeted therapeutic strategies and predictions for UC. However, APOL1 is unique to humans and some higher primates, so we could not verify it in animal experiments. Our study simply revealed that APOL1 could mediate the pyroptosis pathway of GSDMD cells in colonic fibroblasts, up-regulating inflammatory cytokines and chemokines and aggravating colitis in patients with UC. However, its direct role in diagnosing and treating UC patients remains unclarified and needs further research.
Ethics Approval and Consent to Participate
Our study complies with the Declaration of Helsinki, and that our study was approved by the Ethics Committee of Ganzhou People’s Hospital (TY-ZKY2023-023-1).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Kaser A, Zeissig S, Blumberg RS. Inflammatory bowel disease. Annu Rev Immunol. 2010;28(1):573–621. doi:10.1146/annurev-immunol-030409-101225
2. Shah SC, Itzkowitz SH. Colorectal cancer in inflammatory bowel disease: mechanisms and management. Gastroenterology. 2022;162(3):715–730. doi:10.1053/j.gastro.2021.10.035
3. Du L, Ha C. Epidemiology and pathogenesis of Ulcerative colitis. Gastroenterol Clin North Am. 2020;49(4):643–654. doi:10.1016/j.gtc.2020.07.005
4. Feuerstein JD, Moss AC, Farraye FA. Ulcerative Colitis. Mayo Clin Proc. 2019;94(7):1357–1373. doi:10.1016/j.mayocp.2019.01.018
5. Segal JP, LeBlanc JF, Hart AL. Ulcerative colitis: an update. Clin Med. 2021;21(2):135–139. doi:10.7861/clinmed.2021-0080
6. Cookson BT, Brennan MA. Pro-inflammatory programmed cell death. Trends Microbiol. 2001;9(3):113–114. doi:10.1016/S0966-842X(00)01936-3
7. Tan G, Huang C, Chen J, et al. Gasdermin-E-mediated pyroptosis participates in the pathogenesis of Crohn’s disease by promoting intestinal inflammation. Cell Rep. 2021;35(11):109265. doi:10.1016/j.celrep.2021.109265
8. Shi J, Gao W, Shao F. Pyroptosis: gasdermin-mediated programmed necrotic cell death. Trends Biochem Sci. 2017;42(4):245–254. doi:10.1016/j.tibs.2016.10.004
9. Broz P, Pelegrin P, Shao F. The gasdermins, a protein family executing cell death and inflammation. Nat Rev Immunol. 2020;20(3):143–157. doi:10.1038/s41577-019-0228-2
10. Burdette BE, Esparza AN, Zhu H, et al. Gasdermin D in pyroptosis. Acta Pharm Sin B. 2021;11(9):2768–2782. doi:10.1016/j.apsb.2021.02.006
11. McCarthy N, Kraiczy J, Shivdasani RA. Cellular and molecular architecture of the intestinal stem cell niche. Nat Cell Biol. 2020;22(9):1033–1041. doi:10.1038/s41556-020-0567-z
12. Koliaraki V, Prados A, Armaka M, et al. The mesenchymal context in inflammation, immunity and cancer. Nat Immunol. 2020;21(9):974–982. doi:10.1038/s41590-020-0741-2
13. Davidson S, Coles M, Thomas T, et al. Fibroblasts as immune regulators in infection, inflammation and cancer. Nat Rev Immunol. 2021;21(11):704–717. doi:10.1038/s41577-021-00540-z
14. Wang X, Liotta L. Clinical bioinformatics: a new emerging science. J Clin Bioinforma. 2011;1(1):1. doi:10.1186/2043-9113-1-1
15. Xie K, Kong S, Li F, et al. Bioinformatics-based study to investigate potential differentially expressed genes and miRNAs in pediatric sepsis. Med Sci Monit. 2020;26:e923881. doi:10.12659/MSM.923881
16. Chen R, Lai LA, Brentnall TA, et al. Biomarkers for colitis-associated colorectal cancer. World J Gastroenterol. 2016;22(35):7882–7891. doi:10.3748/wjg.v22.i35.7882
17. Anashkina AA, Leberfarb EY, Orlov YL. Recent trends in cancer genomics and bioinformatics tools development. Int J Mol Sci. 2021;22(22):12146. doi:10.3390/ijms222212146
18. Korbecki J, Maruszewska A, Bosiacki M, et al. The potential importance of CXCL1 in the physiological state and in noncancer diseases of the cardiovascular system, respiratory system and skin. Int J Mol Sci. 2022;24(1):205. doi:10.3390/ijms24010205
19. Tzukerman M, Shamai Y, Abramovich I, et al. Comparative analysis of the APOL1 variants in the genetic landscape of renal carcinoma cells. Cancers. 2022;14(3):733. doi:10.3390/cancers14030733
20. O’Toole JF, Schilling W, Kunze D, et al. ApoL1 overexpression drives variant-independent cytotoxicity. J Am Soc Nephrol. 2018;29(3):869–879. doi:10.1681/ASN.2016121322
21. Wakashin H, Heymann J, Roshanravan H, et al. APOL1 renal risk variants exacerbate podocyte injury by increasing inflammatory stress. BMC Nephrol. 2020;21(1):371. doi:10.1186/s12882-020-01995-3
22. Wu J, Raman A, Coffey NJ, et al. The key role of NLRP3 and STING in APOL1-associated podocytopathy. J Clin Invest. 2021;131(20). doi:10.1172/JCI136329
23. Wei K, Nguyen HN, Brenner MB. Fibroblast pathology in inflammatory diseases. J Clin Invest. 2021;131(20). doi:10.1172/JCI149538
24. Sinha S, Sparks HD, Labit E, et al. Fibroblast inflammatory priming determines regenerative versus fibrotic skin repair in reindeer. Cell. 2022;185(25):4717–4736. doi:10.1016/j.cell.2022.11.004
25. Komatsu N, Takayanagi H. Mechanisms of joint destruction in rheumatoid arthritis - immune cell-fibroblast-bone interactions. Nat Rev Rheumatol. 2022;18(7):415–429. doi:10.1038/s41584-022-00793-5
26. Dokoshi T, Seidman JS, Cavagnero KJ, et al. Skin inflammation activates intestinal stromal fibroblasts and promotes colitis. J Clin Invest. 2021;131(21). doi:10.1172/JCI147614
27. Friedman DJ, Pollak MR. APOL1 and kidney disease: from genetics to Biology. Annu Rev Physiol. 2020;82(1):323–342. doi:10.1146/annurev-physiol-021119-034345
28. Friedman DJ, Pollak MR. APOL1 nephropathy: from genetics to clinical applications. Clin J Am Soc Nephrol. 2021;16(2):294–303. doi:10.2215/CJN.15161219
29. Duchateau PN, Pullinger CR, Orellana RE, et al. Apolipoprotein L, a new human high density lipoprotein apolipoprotein expressed by the pancreas. Identification, cloning, characterization, and plasma distribution of apolipoprotein L. J Biol Chem. 1997;272(41):25576–25582. doi:10.1074/jbc.272.41.25576
30. Marais AD. Apolipoprotein E in lipoprotein metabolism, health and cardiovascular disease. Pathology. 2019;51(2):165–176. doi:10.1016/j.pathol.2018.11.002
31. Noels H, Lehrke M, Vanholder R, et al. Lipoproteins and fatty acids in chronic kidney disease: molecular and metabolic alterations. Nat Rev Nephrol. 2021;17(8):528–542. doi:10.1038/s41581-021-00423-5
32. Bang-Rudenstam A, Cerezo-Magana M, Belting M. Pro-metastatic functions of lipoproteins and extracellular vesicles in the acidic tumor microenvironment. Cancer Metastasis Rev. 2019;38(1–2):79–92. doi:10.1007/s10555-019-09786-5
33. Chen TK, Surapaneni AL, Arking DE, et al. APOL1 kidney risk variants and proteomics. Clin J Am Soc Nephrol. 2022;17(5):684–692. doi:10.2215/CJN.14701121
34. Parsa A, Kao WH, Xie D, et al. APOL1 risk variants, race, and progression of chronic kidney disease. N Engl J Med. 2013;369(23):2183–2196. doi:10.1056/NEJMoa1310345
35. Lin J, Xu Z, Xie J, et al. Oncogene APOL1 promotes proliferation and inhibits apoptosis via activating NOTCH1 signaling pathway in pancreatic cancer. Cell Death Dis. 2021;12(8):760. doi:10.1038/s41419-021-03985-1
36. Camba-Gomez M, Arosa L, Gualillo O, et al. Chemokines and chemokine receptors in inflammatory bowel disease: recent findings and future perspectives. Drug Discov Today. 2022;27(4):1167–1175. doi:10.1016/j.drudis.2021.12.004
37. Charo IF, Ransohoff RM. The many roles of chemokines and chemokine receptors in inflammation. N Engl J Med. 2006;354(6):610–621. doi:10.1056/NEJMra052723
38. Liu ZY, Zheng M, Li YM, et al. RIP3 promotes colitis-associated colorectal cancer by controlling tumor cell proliferation and CXCL1-induced immune suppression. Theranostics. 2019;9(12):3659–3673. doi:10.7150/thno.32126
39. Korbecki J, Szatkowska I, Kupnicka P, et al. The importance of CXCL1 in the physiological state and in noncancer diseases of the oral cavity and abdominal organs. Int J Mol Sci. 2022;23(13):7151. doi:10.3390/ijms23137151
40. Jiang S, Liang J, Li W, et al. The role of CXCL1/CXCR2 axis in neurological diseases. Int Immunopharmacol. 2023;120:110330. doi:10.1016/j.intimp.2023.110330
41. Wang L, Hauenstein AV. The NLRP3 inflammasome: mechanism of action, role in disease and therapies. Mol Aspects Med. 2020;76:100889. doi:10.1016/j.mam.2020.100889
42. Bulek K, Zhao J, Liao Y, et al. Epithelial-derived gasdermin D mediates nonlytic IL-1beta release during experimental colitis. J Clin Invest. 2020;130(8):4218–4234. doi:10.1172/JCI138103
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.