Back to Journals » International Journal of General Medicine » Volume 15
Antimicrobial and Microbiological Characteristics of Critically Ill Patients with Complicated Intra-Abdominal Infection: Observational Data from Beth Israel Deaconess Medical Center
Authors Tong Y, Li Q, Liu S, Liu T, Qu K, Liu C, Zhang J
Received 22 December 2021
Accepted for publication 10 February 2022
Published 25 February 2022 Volume 2022:15 Pages 2127—2136
DOI https://doi.org/10.2147/IJGM.S354258
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Yingmu Tong,1,* Qinglin Li,1,* Sinan Liu,1,2 Tong Liu,1 Kai Qu,1 Chang Liu,1,2 Jingyao Zhang1,2
1Department of Hepatobiliary Surgery, The First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, Shaanxi, 710061, People’s Republic of China; 2Department of SICU, the First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, Shaanxi, 710061, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Chang Liu; Jingyao Zhang, Department of SICU, The First Affiliated Hospital of Xi’an Jiaotong University, 277 Yanta West Road, Xi`an, 710061, People’s Republic of China, Tel +86-29-85323900, Fax +86-29-85324642, Email [email protected]; [email protected]
Purpose: We describe the clinical characteristics, isolated microorganisms and antibiotics used in patients with cIAIs during ICU stay.
Methods: A retrospective analysis of data from Beth Israel Deaconess Medical Center was performed.
Results: A total of 316 patients with cIAIs were included, 57.0% of them were male and the median age was 63 years. A total of 239 patients did have cultures taken, and 74 patients had a positive microbial result. The main pathogens were Escherichia coli, Staphylococci (coagulase negative), Enterococcus sp. and Bacteroides fragilis. The main antibiotics given were vancomycin, metronidazole, piperacillin tazobactam and ciprofloxacin. Univariable and multivariable Cox regression analyses showed that receiving more antibiotics reduced ICU mortality, but the same results were not obtained in the analysis of hospital mortality.
Conclusion: The main Gram-positive microorganisms for empirical antimicrobial therapy were Staphylococci (coagulase negative), Enterococcus sp. and Staphylococcus aureus, and Gram-negative microorganisms were Escherichia coli, Bacteroides fragilis and Klebsiella pneumoniae. The use of more antibiotics reduced ICU mortality, but the same results were not obtained in the analysis of hospital mortality.
Keywords: intra-abdominal infection, culture, microorganism, antibiotics, intensive care unit, mortality
Introduction
Intra-abdominal infections (IAIs), the second most common cause of sepsis1 and the second most common infectious disease among patients,2 is a series of diseases ranging from unruptured appendicitis to severe peritonitis.3 Complicated intra-abdominal infections (cIAIs) involve IAIs that extend into the sterile area of the abdomen and cause localized (abscesses in the abdominal cavity) or systemic peritonitis.4 cIAI is a common and important illness in the intensive care unit (ICU) for which the mortality rates are typically 9.2% to 42.0%.5–8
The optimal treatment of cIAI patients relies on a multimodal approach, including early diagnosis, judicious use of antimicrobials, and source control.9 Antimicrobial therapy plays a pivotal role in the management of cIAIs. The initial antibacterial treatment is usually empirical because patients need immediate treatment, while the results of culture and drug sensitivity tests take 48–72 hours to guide targeted treatment, especially in critically ill patients.10 Inadequate antimicrobial regimens are one of the factors leading to a poor prognosis.11 However, the details regarding antibiotic agents used in most of the studies are lacking, and there are significant differences in the choice and combination of antibiotics when these details are available.
There are many types of pathogens that could be carried by patients with cIAIs, including Gram-positive and Gram-negative aerobic bacteria, anaerobes and fungi.12 Although the results of microbiological tests may be of great importance for the choice of treatment strategies for each patient, especially for patients with unpredictable physical risks for targeted antimicrobial therapy, the contribution of a microbiological diagnosis is not straightforward as cultures cannot always readily discriminate true pathogens from harmless microorganisms.4,13 Furthermore, there will often be cases in which the culture result is negative.14
The aims of this study were to describe the clinical characteristics of cIAI patients in the MIMIC-III database, analyze the pathogenic characteristics and describe the antibiotic use.
Methods
The present study is reported in accordance with the REporting of studies Conducted using Observational Routinely collected health Data (RECORD) statement.15
Setting
We performed a secondary analysis of the electronic health records of patients included in the Medical Information Mart for Intensive Care III (MIMIC-III v1.4), which is a large, publicly available database that includes unidentified health-related data from approximately sixty thousand patients who were admitted to critical care units of the Beth Israel Deaconess Medical Center (BIDMC).16 The MIMIC-III contains high-resolution patient data, including information about patient admission, discharge, demographic data, physiologic data (from bedside monitors, ventilators, and other devices), laboratory results, severity scores, procedures, and medications.
Ethical Approval
This study has been approved by the Institutional Review Board (IRB) of the Massachusetts Institute of Technology (MIT). After successfully accomplishing the National Institutes of Health’s (NIH) online training course and the Protection of Human Research Participants Examination, we had the access to extract data from MIMIC database. All data were extracted by the corresponding author. This study was also reviewed by IRB of the First affiliated hospital of Xi’an Jiaotong University and waived the requirement for ethics approval. Given that all patients in this database were de-identified, informed consent was waived.
Study Population
Patients aged >18 years between 2008 and 2012 (the years for which microbial culture information was more comprehensive) were selected. Based on the patient’s International Classification of Diseases (ICD-9) codes, all adult patients suspected complicated intra-abdominal infection were further analyzed. The specific codes and corresponding diagnosis are shown in Tables S1 and S2 (ESM).
Patients with infections in other parts of the body, patients without antibiotic use information, patients who were pregnant and those who had an ICU length of stay less than 24 hours were excluded. For patients who had multiple ICU admissions, only the first admission record was kept.
Data Extraction
The following variables were extracted during ICU stay: age, gender, ethnicity, admission weight, admission height, admission type, marital status, insurance, unit type, Sequential Organ Failure Assessment (SOFA) score, Acute Physiology Score III (APS III), Elixhauser Comorbidity Score, length of stay (both ICU and hospital), results of microbiological isolation, use of antibiotics and other important laboratory tests. The classification method for antimicrobial agents is shown in Table S3 (ESM). SOFA score was calculated within the first 24 h after the ICU admission. If a variable was measured more than once in the first 24 h, the value associated with the greatest severity of illness was used. For example, the lowest value of mean blood pressure and Glasgow Coma Scale (GCS) score reported in the first 24 hours were used in the study.
We extracted all information about antibiotic treatment in each patient during ICU stay, and all information about microbial culture from blood, bile, or peritoneal fluid was also collected at the same time. For the extraction of data and the calculation of disease scores, we applied the corresponding code in the MIMIC Code Repository.17
Outcomes
The primary outcome of the study was to describe the microorganisms isolated and the antibiotics used in patients with cIAIs during ICU stay. Secondary outcomes included description of clinical characteristics and identification of associations between microorganisms isolated or antibiotics use and the prognosis of the patients.
Statistical Analysis
We expressed continuous data as medians with interquartile ranges and categorical data as proportions, the distribution of data was represented by bar charts or tables, and the specific states of different indicators were displayed in a bar chart. We tested the collinearity of the variables included in the statistical analysis and found that the variance inflation factor (VIF) of all variables was <3; hence, there was no statistical collinearity in the included variables. Variables with p <0.10 in univariate analysis were included in the Cox regression model as confounders. Results from the Cox proportional hazards model were represented as hazard ratios including 95% CI. All statistical analyses were performed using IBM SPSS Statistics version 26 (IBM Corp., Armonk, NY), and figures were performed using Prism 8 (GraphPad Software Inc.).
Results
Clinical Characteristics
A total of 316 patients were included in the study (Figure 1). Table 1 shows the baseline characteristics. The median age of the patients was 63 (52–76) years. Male patients (180) were more than females (136). The median SOFA score was 5 (3–8) points, and the median APS III score was 52 (41–69) points. The most common diagnosis relevant to cIAIs was peritonitis or peritoneal abscess (222, 70.3%), next by perforation or fistula of intestine (42, 13.3%). The Elixhauser comorbidity score was 11 (IQR 5–19).
 |
Table 1 Demographic Characteristics |
 |
Table 2 Type of Antimicrobial Agent(s) Used Daily During the ICU Stay |
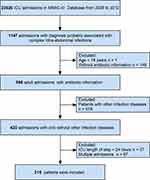 |
Figure 1 Flowchart of the patient selection process in this study. |
The median ICU length of stay was 2.8 (IQR 1.8–5.3) days, and the median hospital length of stay was 10.3 (IQR 6.8–20.1) days. The ICU mortality was 13.3%, and hospital mortality was 19.3%.
Microbiological Findings and Antibiotics Used
77 patients had no culture; of all the 239 patients who had microbiological culture performed in the study, only 74 (31.0%) were positive since 165 patients had a negative culture result (Table S4). 54 patients carried only one microorganism, 11 patients carried two microorganisms, 5 patients carried three microorganisms and one patient carried 8 microorganisms (Figure S1). The top five microorganisms were Escherichia coli, Staphylococci (coagulase negative), Enterococcus sp., Bacteroides fragilis and Staphylococcus aureus (Table S4, Figure S1).
57 patients used only one antibiotic, 91 patients used 2 antibiotics, 76 patients used 3 antibiotics, 47 patients used 4 antibiotics and two patients used 10 antibiotics (Figure S1). The top four antibiotics used were vancomycin, metronidazole, piperacillin tazobactam, ciprofloxacin, and piperacillin (Table S5, Figure S1).
We evaluated the combination use of antibiotics with the first day’s antibiotic information (Figure 2A). Metronidazole was used in 51.06% of patients prescribed vancomycin, piperacillin tazobactam in 45.39% and ciprofloxacin in 26.24%. Among all the patients treated with metronidazole, 51.06% received vancomycin, 51.06% received ciprofloxacin, and 17.73% received piperacillin tazobactam. Of all patients treated with piperacillin tazobactam, 65.98% were concomitantly treated with vancomycin and 25.77% with ciprofloxacin. Among the patients who received ciprofloxacin, 85.71% also received metronidazole.
Types of antibiotics used during ICU stay are shown in Table 2 and Figure 2B. Vancomycin, metronidazole, piperacillin tazobactam and ciprofloxacin were the four antibiotics with the highest usage rate every day during the entire ICU period. With the extension of ICU stay time, the proportion of use of piperacillin tazobactam gradually increased over metronidazole and the proportion of use of ciprofloxacin gradually decreased, while the proportion of use of fluconazole and cefepime gradually increased.
Figure S2 shows that for patients with both positive and negative microorganism isolated, the most commonly used antibiotics were vancomycin, metronidazole, piperacillin tazobactam and ciprofloxacin. For patients with positive microorganism isolated, the use of meropenem, cefepime and micafungin increased. Antifungals were used to varying degrees in patients with positive microorganism isolated, with a higher use rate in patients with fungi isolated.
The Number of Antibiotics Used Was Associated with ICU Mortality
Both univariate analysis and multivariate analysis showed that the number of antibiotics used was associated with ICU mortality but had no effect on hospital mortality (Tables S6 and S7). Multivariate Cox regression analysis showed that the use of five or more antibiotics was associated with lower ICU mortality (HR 0.293, 95% CI 0.097–0.884; p = 0.029) (Figure 3). Sensitivity analysis was performed in patients who had microbiological culture performed, and the result was similar to that observed in the primary analysis (Table S8).
 |
Figure 3 Forest plots showed the results of multivariate Cox regression analysis of ICU mortality. |
Discussion
In this study, we described the basic clinical characteristics, microbial infections and antibiotic use of 316 cIAI patients from Beth Israel Deaconess Medical Center. The results showed that only 74 (23.4%) patients had positive microbial culture results, and of these, 54 patients cultured only one microorganism. The main pathogens were Escherichia coli, Staphylococci (coagulase negative), Enterococcus sp., Bacteroides fragilis and Staphylococcus aureus. Patients using 2 antibiotics were the most common, followed by 3 and 1 antibiotics. The main antibiotics were vancomycin, metronidazole, piperacillin tazobactam and ciprofloxacin. The hospital mortality was 19.3%.
CIAIs extend beyond the hollow mucosa originating into the peritoneal space and are accompanied by abscess formation or peritonitis. In the MIMIC-III database, the patient’s diagnosis is recorded by the International Classification of Diseases (ICD-9) codes. However, there is no exact ICD-9 code to define cIAIs, so it is difficult to study cIAIs in this database. In this study, for the first time, we used the ICD-9 codes that are generally related to cIAIs and information on antibiotic use and microbial culture to define patients with cIAIs. That makes it possible to study cIAIs in this database.
The positive rate of microbial culture in cIAI patients varied widely in different studies. A multi-center retrospective cohort study of the Premier database showed that of the 321,317 cIAI patients, 4453 (1.4%) were positive,14 while a multicenter study in Colombia7 showed that 70.8% of cIAI patients involved had an isolated microorganism. In this study, only 23.4% of the patients had a positive result, which means that 76.6% of patients had a negative microbial culture result and the choice of antibiotics was completely empirical.
In the last decade, there have been only a few studies on the results of microbial culture in patients with complicated intra-abdominal infections, and limited data suggest that Gram staining, culture, and drug sensitivity data may provide information that may improve the prognosis of patients with cIAIs.4 One of the aims of microbiology research is to understand the most common isolated microorganisms in order to select the most appropriate empirical therapies. In our study, 58.11% of patients with positive microbial isolation had Gram-positive organisms isolated and 50.00% had Gram-negative bacteria isolated, which was different from previous studies that showed that Gram-negative bacteria were isolated almost twice as frequently as Gram-positive bacteria.5,11 The possible reasons for this were differences in the study design and different sources of data. The most common Gram-positive bacteria isolated were Staphylococci (coagulase negative), Enterococcus sp., Staphylococcus aureus and Gram-positive rods, and the most common Gram-negative bacteria isolated were Escherichia coli, Bacteroides fragilis and Klebsiella pneumoniae, similar to a study by Zilberberg et al.14
Early and judicious use of empiric antibiotic therapy is particularly important for patients with cIAIs. In our study, the most commonly used antibiotic was vancomycin, which is generally considered the last resort in the treatment of severe life-threatening infections caused by Gram-positive bacteria.18 Although studies have shown that inclusion of vancomycin as part of broad-spectrum coverage does not improve clinical cure rates or reduce mortality,19 SIS 2017 (Surgical Infection Society, version 2017)10 recommends:
Consider use of vancomycin for empiric therapy of hospital-acquired IAIs in patients at risk for infection from Enterococcus spp. or for empiric therapy of patients known to be colonized or at high risk for infection with MRSA
and WSES 2017 (World Society of Emergency Surgery, version 2017)20 recommends:
Consider use of vancomycin for the treatment of Enterococcus spp. infection, especially ampicillin-resistant Enterococcus infection.
Metronidazole was used in nearly half of the patients in our study. As a major antibiotic against anaerobes, metronidazole was recommended as the preferred anti-anaerobic agent to be used in combination regimens for the empiric treatment of cIAIs.10
The third most commonly used antibiotic in our study was piperacillin tazobactam. In a study by Lodise et al,21 piperacillin tazobactam was the most commonly used antibiotic, used in 52% of patients. The broad spectrum of activity of piperacillin tazobactam, including its anti-P. seudomonas effect and anaerobic coverage, makes it a strong option for the treatment of severe IAI.20 SIS 2017 recommends that piperacillin/tazobactam can be used as an empirical drug for IAIS patients, especially for high-risk patients.10
Carbapenems are often recommended as a broad-spectrum empirical treatment for patients with high-risk cIAIs.20 To some extent, concerns about the prevalence of Enterobacteriaceae and Pseudomonas aeruginosa, which produce extended-spectrum beta-Lactamases (ESBL), have prompted the increased use of carbapenems in cIAIs.14 In our study, the most commonly used carbapenem antibiotic was meropenem, which was used by 12.66% of patients. This proportion was consistent with a study by Zilberberg et al,14 while the most commonly used carbapenem antibiotic in their study was ertapenem. This difference is understandable considering that the patients in this study were mainly treated between 2008 and 2012, while those in the study by Zilberberg et al were treated from 2013 to 2017.
In our study, fungi were isolated in 12.16% of all culture-positive patients, mainly Candida, and the most commonly used antifungal agent was fluconazole, followed by micafungin, which was consistent with a study by Blot et al.5 Candida isolated has been identified as an independent risk factor for mortality.22,23 Although SIS 2017 does not recommend the routine use of empirical antifungal therapy in high-risk patients, fluconazole and echinocandin are the preferred empirical agents in patients with isolated Candida.10
Although our study showed that receiving more antibiotics reduced ICU mortality, the same results were not obtained in the analysis of hospital mortality. Given that the median length of ICU stay for patients in this study was 2.8 days, we speculate that for patients with cIAIs, the use of more antibiotics may be beneficial in the short term but contributes little to the overall patient outcome.
Our study has many limitations. First, as a retrospective cohort study, it is susceptible to various types of bias, most notably selection bias. The concept of cIAIs is imprecise, and we had to use ICD-9 codes to select patients, and it is likely that not all clinical situations are coded. Second, we did not collect accurate information about all microbial subtypes or their patterns of resistance or whether antimicrobial coverage was appropriate. Finally, we did not analyze or predict the prognostic factors in this cohort, which will be the main direction of our next study.
Conclusion
In conclusion, this study describes clinical information, summarizing the common pathogens and antibiotics used in patients with cIAIs in the MIMIC-III database. 23.4% of the patients had isolated microorganisms. The main Gram-positive microorganisms for empirical antimicrobial therapy were Staphylococci (coagulase negative), Enterococcus sp. and Staphylococcus aureus, and Gram-negative microorganisms were Escherichia coli, Bacteroides fragilis and Klebsiella pneumoniae. The use of more antibiotics reduced ICU mortality, but the same results were not obtained in the analysis of hospital mortality.
Data Sharing Statement
Data were fully available at https://mimic.physionet.org/.
Ethics Approval and Consent to Participate
This study has been approved by the Institutional Review Board (IRB) of the Massachusetts Institute of Technology (MIT). After successfully accomplishing the National Institutes of Health’s (NIH) online training course and the Protection of Human Research Participants Examination, we had the access to extract data from MIMIC database. All data were extracted by the corresponding author (Certification ID: 28572693). This study was also reviewed by the IRB of the First Affiliated hospital of Xi’an Jiaotong University and waived the requirement for ethics approval. Given that all patients in this database were de-identified, informed consent was waived.
Acknowledgments
We are indebted to all individuals who participated in or helped with this research project.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agreed to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Sakr Y, Jaschinski U, Wittebole X, et al. Sepsis in intensive care unit patients: worldwide data from the intensive care over nations audit. Open Forum Infect Dis. 2018;5(12). doi:10.1093/ofid/ofy313
2. Hecker A, Reichert M, Reuss CJ, et al. Intra-abdominal sepsis: new definitions and current clinical standards. Langenbecks Arch Surg. 2019;404(3):257–271. doi:10.1007/s00423-019-01752-7
3. Menichetti F, Sganga G. Definition and classification of intra-abdominal infections. J Chemother. 2009;21(Suppl 1):3–4. doi:10.1179/joc.2009.21.Supplement-1.3
4. Solomkin JS, Mazuski JE, Bradley JS, et al. Diagnosis and management of complicated intra-abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Surg Infect. 2010;11(1):79–109. doi:10.1089/sur.2009.9930
5. Blot S, Antonelli M, Arvaniti K, et al. Epidemiology of intra-abdominal infection and sepsis in critically ill patients: “AbSeS”, a multinational observational cohort study and ESICM Trials Group Project. Intensive Care Med. 2019;45(12):1703–1717. doi:10.1007/s00134-019-05819-3
6. Sartelli M, Abu-Zidan FM, Catena F, et al. Global validation of the WSES Sepsis Severity Score for patients with complicated intra-abdominal infections: a prospective multicentre study (WISS Study). World J Emerg Surg. 2015;10:61. doi:10.1186/s13017-015-0055-0
7. Vallejo M, Cuesta DP, Florez LE, et al. Clinical and microbiological characteristics of complicated intra-abdominal infection in Colombia: a multicenter study. Rev Chilena Infectol. 2016;33(3):261–267. doi:10.4067/s0716-10182016000300002
8. van de Groep K, Verhoeff TL, Verboom DM, et al. Epidemiology and outcomes of source control procedures in critically ill patients with intra-abdominal infection. J Crit Care. 2019;52:258–264. doi:10.1016/j.jcrc.2019.02.029
9. Khatri R, Sawyer R. Global perspectives in controversies related to the management of intra-abdominal infections. Surg Infect. 2020;21(7):626–633. doi:10.1089/sur.2020.174
10. Mazuski JE, Tessier JM, May AK, et al. The surgical infection society revised guidelines on the management of intra-abdominal infection. Surg Infect. 2017;18(1):1–76. doi:10.1089/sur.2016.261
11. De Waele J, Lipman J, Sakr Y, et al. Abdominal infections in the intensive care unit: characteristics, treatment and determinants of outcome. BMC Infect Dis. 2014;14. doi:10.1186/1471-2334-14-420
12. Blot S, De Waele JJ. Critical issues in the clinical management of complicated intra-abdominal infections. Drugs. 2005;65(12):1611–1620. doi:10.2165/00003495-200565120-00002
13. Montravers P, Blot S, Dimopoulos G, et al. Therapeutic management of peritonitis: a comprehensive guide for intensivists. Intensive Care Med. 2016;42(8):1234–1247. doi:10.1007/s00134-016-4307-6
14. Zilberberg MD, Nathanson BH, Ditch K, Lawrence K, Olesky M, Shorr AF. Carbapenem treatment and outcomes among patients with culture-positive complicated intra-abdominal infections in US hospitals: a retrospective cohort study. Open Forum Infect Dis. 2019;6(12). doi:10.1093/ofid/ofz504
15. Benchimol EI, Smeeth L, Guttmann A, et al. The REporting of studies Conducted using Observational Routinely-collected health Data (RECORD) statement. PLoS Med. 2015;12(10):e1001885. doi:10.1371/journal.pmed.1001885
16. Johnson AEW, Pollard TJ, Shen L, et al. MIMIC-III, a freely accessible critical care database. Sci Data. 2016;3. doi:10.1038/sdata.2016.35
17. Johnson AEW, Stone DJ, Celi LA, Pollard TJ. The MIMIC code repository: enabling reproducibility in critical care research. J Am Med Inform Assoc. 2018;25(1):32–39. doi:10.1093/jamia/ocx084
18. Al-Tatari H, Abdel-Haq N, Chearskul P, Asmar B. Antibiotics for treatment of resistant gram-positive coccal infections. Indian J Pediatr. 2006;73(4):323–334. doi:10.1007/bf02825827
19. Sanders JM, Tessier JM, Sawyer RG, et al. Inclusion of vancomycin as part of broad-spectrum coverage does not improve outcomes in patients with intra-abdominal infections: a post hoc analysis. Surg Infect. 2016;17(6):694–699. doi:10.1089/sur.2016.095
20. Sartelli M, Chichom-Mefire A, Labricciosa FM, et al. The management of intra-abdominal infections from a global perspective: 2017 WSES guidelines for management of intra-abdominal infections. World J Emerg Surg. 2017;12. doi:10.1186/s13017-017-0141-6
21. Lodise TP, Izmailyan S, Olesky M, Lawrence K. An evaluation of treatment patterns and associated outcomes among adult hospitalized patients with lower-risk community-acquired complicated intra-abdominal infections: how often are expert guidelines followed? Open Forum Infect Dis. 2020;7(7):ofaa237. doi:10.1093/ofid/ofaa237
22. Carneiro HA, Mavrakis A, Mylonakis E. Candida peritonitis: an update on the latest research and treatments. World J Surg. 2011;35(12):2650–2659. doi:10.1007/s00268-011-1305-2
23. Montravers P, Dupont H, Gauzit R, et al. Candida as a risk factor for mortality in peritonitis. Crit Care Med. 2006;34(3):646–652. doi:10.1097/01.Ccm.0000201889.39443.D2
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

