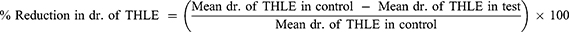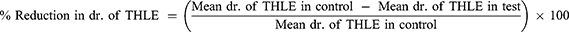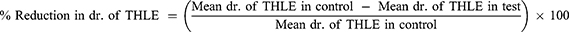Back to Journals » Journal of Experimental Pharmacology » Volume 14
Anticonvulsant Activity of Hydro Alcoholic Extract and Solvent Fractions of Biophytum umbraculum Welw. Syn (Oxalidaceae) Root in Mice
Authors Fisseha N , Hammeso WW , Nureye D
Received 30 May 2022
Accepted for publication 18 October 2022
Published 20 October 2022 Volume 2022:14 Pages 291—299
DOI https://doi.org/10.2147/JEP.S374890
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Manfred Ogris
Nebeyi Fisseha, Workineh Woldeselassie Hammeso, Dejen Nureye
Department of Pharmacy, School of Pharmacy, College of Health Sciences, Mizan-Tepi University, Mizan-Aman, South West, Ethiopia
Correspondence: Nebeyi Fisseha, Tel +251913214835, Email [email protected]
Background: Scientists and researchers continue to focus on medicinal plants as a potential source of lead chemicals in the search for and development of new antiepileptic medicines. Biophytum umbraculum Welw. Syn is used to treat epilepsy in Ethiopian traditional medicine. The anticonvulsant effect of Biophytum umbraculum Welw. Syn hydroalcoholic extract and solvent fractions was evaluated in this study since the claim has not been thoroughly explored.
Methods: The plant’s root was extracted using the maceration procedure, with aqueous, butanol, and chloroform as solvents. The maximum electroshock and pentylenetetrazol model tests were used to assess anticonvulsant activity. Mice were divided into five groups (n = 6) at random. The test groups received 100, 200, and 400 mg/kg of hydroalcoholic extract and solvent fraction, respectively. For the maximum electric shock test, the positive control groups received 25 mg/kg phenytoin and 200 mg/kg valproate for the pentylenetetrazol test. The negative control was given 10 mL/kg of pure water or 2% Tween 80.
Results: There were no signs of toxicity in the hydroalcoholic extract or solvent extraction. When compared to the negative control, the hydro-alcoholic extract had a significant anticonvulsant effect in both the maximum electric shock test and the pentylenetetrazol test. In both cases, the butanol component had a comparable impact. In the pentylenetetrazol test, the chloroform fraction had a significant anticonvulsant effect when compared to the control at dosages of MB200 and MB400. Flavonoids, phenols, tannins, steroids, terpenoids, and saponins were found in both the hydroalcoholic and solvent fractions of the plant extract.
Conclusion: The plant appears to have promising anticonvulsant properties, and it might be used to generate novel anti-epileptic drugs, according to this study.
Keywords: epilepsy, seizure, pentylenetetrazol, anticonvulsant, Biophytum umbraculum
Background
According to a World Health Organization study, epilepsy is a major source of disability, accounting for more than seven million disability-adjusted life years of the worldwide burden of illness.1 In Africa, more than 75% of people with epilepsy do not have access to healthcare and are not properly treated.2 People in most poor countries link epilepsy with demonic spirits and supernatural causes; as a result, people with epilepsy are stigmatized, discriminated against, and suffer from psychosocial mental problems.3
For a long time, pharmacological medications have been the primary therapy for epilepsy. Despite this, the majority of AEDs are linked to side effects and medication interactions.4 Drug-resistant epilepsy has become more common in patients taking AEDs in recent years. In the current clinical context, however, there are no viable treatment therapies accessible to control medication-resistant epilepsy. Antiepileptic medicines now available have little effectiveness, and their unfavorable qualities limit their usage and complicate patient care.5 Interictal activity is resistant to AEDs,6 and many (but not all) regularly used AEDs have a rebound effect, which is another reason to look into novel medications.7
Furthermore, commercially available medications have failed to prevent the development of epilepsy and associated co-morbidities by blocking the actions of epileptogenic substances.8 Antiepileptic medicines can give symptomatic relief by suppressing seizures, but they cannot cure epileptogenesis. Antiepileptic medication usage is restricted for lengthy periods of time due to side effects, withdrawal symptoms, drug interactions, and financial load, particularly in poor nations.9
Thus, developing novel AEDs with minimum side effects, anti-epileptogenic impact, and efficacy against drug-resistant epilepsy is desirable.10 Traditional medicines are widely used in poor nations, with up to 80% of the population relying on them or folk treatments for their primary health care. Medicinal plants are thought to be a major source of novel chemical compounds with medicinal promise. Several plants used for the treatment of epilepsy in various systems of traditional medicine have shown action when tested in contemporary bioassays for anticonvulsant activity, and many more are still being studied scientifically.11
Because of their cultural beliefs and limited access to contemporary medical treatment, Ethiopians commonly employ medicinal herbs to treat neurological disorders.12 Biophytum umbraculum Welw. Syn is one such plant that has long been used to treat epilepsy.13
However, no experimental investigations of the supposed anti-epileptic herb Biophytum umbraculum Welw. Syn. have been done in Ethiopia to date. The goal of this work was to assess the anticonvulsant properties of Biophytum umbraculum Welw, Syn. Hydro alcoholic extract and solvent fractions. This work contributed to the synthesis of crude drugs as well as the recording of traditional plant knowledge through solvent fractionation. These also enable us to isolate and identify the active component that might be employed as a medication or a lead chemical.
Materials and Methods
Chemicals and Materials
Among the chemicals and solvents used in this study were distilled water (Ethiopian Pharmaceutical Manufacturing, Ethiopia), absolute methanol (Carlo Erba, France), chloroform (Loba Chemie, India), Phenytoin (Brawn, India), Sodium Valproate (Elfin Drugs, India), n-butanol (Indenta, India), Normal saline (Aculife Health Care, India) and Pentylenetetrazole (Sigma-Aldrich Chemie, USA). All of the chemicals and drugs utilized in this study were analytical grade.
Collection of Plant Material
On January 20, 2021, fresh roots of Biophytum umbraculum Welw. Syn were taken from Tarcha town, Dawuro zone, and South West area, 507 km from Addis Ababa, Ethiopia. Mr. Melaku Wondafrash was in charge of the plant’s identification and authenticity. For future reference, a voucher specimen NF/001/2021 was supplied and placed at Addis Ababa University’s National Herbarium, College of Natural and Computational Sciences.
Experimental Animals
Healthy Swiss albino mice (28±7g) were purchased from the Ethiopian Public Health Institute (EPHI) in Addis Ababa, Ethiopia, and housed in the animal house at Mizan Tepi University’s School of Pharmacy. All of the animals were kept in a room with air conditioning. The animals were housed at room temperature (21–25℃) and given a 12-hour cycle of light and darkness. The animals had unlimited access to pellets and water. For toxicity testing female mice and for anticonvulsant activity investigations male mice were used. The animals were cared for and handled in accordance with international standards for the use of experimental animals.14 The study was approved by the Mizan Tepi University School of Pharmacy’s Ethics Committee.
Extraction of the Plant
First, the roots of the plant were washed gently with tap water to remove soil. Then, it is air-dried under shade for three weeks until the root becomes fully dried. Next, the dried roots were grounded to a coarse powder using a mortar and pestle. Around 350gm of air-dried coarse powdered plant material was subjected to cold maceration extraction with 1050mL of hydro alcoholic for three consecutive days at room temperature.
The mixture of powdered root and hydro alcoholic was occasionally swirled at 130 rpm in a mechanical shaker. The resulting hydro-alcoholic extract was separated from the marc with gauze and further filtered by Whatman filter paper No. 1. This procedure was repeated by adding another fresh solvent into the mixture. The filtrates were combined and the solvent was removed by evaporation in an oven at a temperature not exceeding 40 ℃. After that, the extract was dried in the oven. Finally, the 47gm of concentrated and dried extract was placed in vials and stored at 20℃ until use.
Around 45mg of hydro-alcoholic extract was fractionated using solvents with different polarities, such as chloroform, butanol, and water. The extract was then suspended in distilled water using a separatory funnel and agitated before adding chloroform. Separation of the chloroform fraction was attained after the formation of two layers. The procedure was repeated until the solution was obvious. The butanol fraction was obtained by shaking the aqueous layer with butanol. The filtrate of butanol, chloroform, and aqueous fractions was concentrated in a 40℃ oven. Finally, the fractions 14.9% yield from chloroform, 31.9% yield from butanol, and 53.2% yield from water were stored in an amber glass container in a refrigerator 20℃ for use during treatment.
Acute Toxicity Study
A healthy, non-pregnant female Swiss albino mouse weighing (26±4g) and aged (6–9 weeks) was utilized in the acute oral toxicity test. The test followed the OECD 425 Guidelines, with a maximum dosage of 2000 mg/kg.15 There were five mice in all. A mouse was given an oral dosage of 2000 mg/kg on the first day. The mice were then watched for behavioral or physical changes for the first 30 minutes (with specific attention paid to the first four hours), and then for the next 24 hours. Four more mice were given the same amount after 24 hours and examined for signs of harm.
Grouping and Dosing of Animals
Mice were placed into five groups, each with six mice, for the anticonvulsant activity test. For reconstitutions, Group I was given 2 mL/100 g (distilled water for aqueous extract and 2% Tween 80 in water for non-aqueous extract). The second group was used as a positive control and was given standard therapy (valproic acid 200 mg/kg for PTZ-induced seizures and phenytoin 25 mg/kg for MES-induced seizures). The extract doses for the remaining three groups were varied. The dosages were determined based on the results of an acute oral toxicity test, with the middle dose being one tenth of the hazardous dose and the other two doses being half and twice the medium dose.
Anticonvulsant Activity Tests
MES-Induced Seizure Test
Bilateral corneal electrical stimulation causes tonic hind limb seizures, which are hypothesized to predict anticonvulsant treatment effectiveness against generalized tonic-clonic seizures.16 Seizures were generated in mice using an electroconvulsiometer and a pair of ear clip electrodes to administer a 50mA electroshock for 0.2 seconds.17 Before 30 minutes of maximum electroshocks, mice in each group received a different number of extracts, a standard medication (phenytoin 25 mg/kg), and a vehicle (distilled water and 2% Tween 80). Using an electroconvulsometer, the mice were then given maximum electroshocks of 50 mA for 0.2 seconds using ear-clip electrodes. The duration of hind limb tonic extension, total recovery time and the protection against mortality was recorded by using video recorder.18 Then, the percentage protection was calculated as:
Note: Prevention or decrease in hind limb tonic extension was considered as protective action.
PTZ-Induced Seizure Test
The PTZ-induced seizure test is used to determine if a medication has anticonvulsant activity against absence and myoclonic seizures. The approach given by Ya’u et al was employed in this investigation.19 Through the oral route, the mice were given varying doses of hydro alcoholic extracts, solvent fractions, sodium valproate, and vehicle. PTZ at 85 mg/kg in normal saline solution was given subcutaneously to each animal after 60 minutes of these treatments. Each mouse was placed in a glass cage and video recorded for 30 minutes to look for convulsive activity. The forelimb and hind limb clonic seizures were used as the test’s endpoints.
Statistical Analysis
Analyses were performed using the statistical package for the social sciences (SPSS), version 21. The results were expressed as mean SEM (standard error mean) and a statistical significance test was carried out by one-way ANOVA followed by a Tukey’s post hoc test to compare results among groups. Values of p<0.05 were considered statistically significant.
Results
Acute Toxicity Study
Both the hydro alcoholic extract and solvent extraction did not produced any sign of toxicity.
MES-Induced Seizure Test
It was observed in Figure 1 that a dose of MB400 hydro alcoholic extract significantly (p< 0.001) reduced the duration of THLE compared with the negative control. In addition to that, the percentage reduction in the duration of THLE by MB400 was 47.91%, which was greater than that of MB100 and MB200. Moreover, the standard drug, PTN25, provided a percentage reduction in the duration of THLE of 84.38%.
The anticonvulsant activity test of the solvent fraction of Biophytum umbraculum Welw. Syn was further evaluated by using MES test in Figure 2. All doses of BB significantly (p <0.001) reduced the mean duration of THLE and when compared with negative control. In addition, BB400 showed the maximum percentage reduction (65.63%) in the duration of THLE than other fraction, although the reduction was not significant when compared with PTN25. All tested doses of AB and CB exhibited lesser percentage reduction in the duration of THLE compared to BB and PTN25.
Anticonvulsant Activity in PTZ Induced Seizure
When compared to the negative control, the latency time to clonic seizure was considerably enhanced (p<0.001) for the MB400 dosage and (p<0.05) for the MB200 dose, as shown in Figure 3. When compared to the MB100, the MB400 saw a substantial increase in latency time (p<0.01). When compared to CON, MB400 provided the highest percentage protection (66.67%) against death and clonic seizure.
In comparison to the negative control, BB, AB, and CB had considerable anticonvulsant effects against PTZ-induced clonic seizure, as shown in Figure 4. Furthermore, as compared to the negative control, all dosages of BB examined demonstrated a significant (p<0.001) increase in the mean latency time of clonic seizure.
When compared to the negative control, BB400 provided the highest percentage (50.00%) of mortality prevention. In addition to BB, CB400 (p<0.001), CB200 (p<0.001), and CB100 (p<0.05) demonstrated significant effects when compared to the negative control group. BB200, CB200, and CB400, on the other hand, achieved a clonic seizure protection percentage of 33.33%.
Phytochemical Examination of Extracts
Biophytum umbraculum Welw. Syn phytochemical analysis of hydro alcoholic extract in conclusion Table 1 shows that secondary metabolites of alkaloids were not present in the extract. Alkaloids and steroids are not found in the butanol fraction. Saponins, glycosides, and tannins were found in the aqueous fraction. However. phenols, steroids, tannins, and terpenoids were found in the chloroform fraction.
 |
Table 1 Preliminary Phytochemical Screening of the Hydroalcoholic Extract and Solvent Fractions of Biophytum umbraculum Welw. Syn |
Discussion
The purpose of this study was to determine the anticonvulsant activity of the hydro alcoholic root extract and solvent fraction of Biophytum umbraculum Welw. Syn., a plant that has been utilized in Ethiopian traditional medicine to treat epilepsy.13 According to tradition, hydro alcoholic was utilized as a solvent for extracting plant material as a solvent. The polarity divergence of fractional solvents was taken into consideration. To simulate the traditional manner of administration, the extract and fractions were given orally. Female mice were employed in the acute toxicity test because they were more susceptible to poisoning than male mice.
In the acute toxicity investigation, no animal died or exhibited indications of toxicity after being given 2000 mg/kg of the test extracts for 24 hours or 14 days. This might indicate that traditional healers are using plants safely.20 Because they are simple to operate, time and cost-efficient, and demonstrate high repeatability, the MES and PTZ-produced seizure models were employed in this investigation.21
The ability of a drug to lessen the duration of THLE is considered anticonvulsant activity in this MES produced seizure model research. The results show that hydro alcoholic extract significantly shortened the duration of THLE in mice at all dosages examined. In both the MES and PTZ models, the greater dosage of hydro alcoholic extract was demonstrated to be an efficacious dose.
In both models, the BB400 dosage of solvent fraction produced similar results. This indicates that the greater dose might be used as the maximal effective dose and that secondary metabolite concentrations are significant. Drugs that operate on Na+ channels (such as carbamazepine and phenytoin) have been demonstrated to successfully eradicate TLHE caused by the MES model.
Drugs having various mechanisms of action, like valproate, phenobarbital, and felbamate, are also efficacious in both animal models.22,23 This might imply that the anticonvulsant activity of the phytoconstituent(s) found in both extracts is mediated by several mechanisms, with broad-spectrum efficacy against myoclonic, absence, and tonic-clonic seizures. However, the actual processes behind Biophytum umbraculum Welw. Syn anticonvulsant’s efficacy are yet unknown.
The hydro alcoholic extract MB400 dosage exhibited considerable anticonvulsant effectiveness against PTZ-induced clonic seizures in this investigation. This might be attributed to active ingredient localization and the existence of most secondary metabolites. The presence of most secondary metabolites, such as flavonoids, saponins, phenols, terpenoid, and tannins, may have contributed to the butanol fraction’s superior increase in latency time to the start of clonic seizure and percentage protection from death.21 However, the impact was less than that of hydro alcoholic extract because hydro alcoholic extract included more secondary metabolites.
The lack of most secondary metabolites may explain the observed activity decrease in latency time to start of clonic seizure and percentage protection against death in the watery fractions. In the PTZ test, the chloroform fraction was revealed to have the second greatest anticonvulsant activity among the fractions. It is possible that the lack of a secondary metabolite explains why this fraction is less active than a butanol fraction.
Furthermore, drugs that reduce T-type calcium currents and/or boost GABAergic neurotransmission, such as ethosuximide, sodium valproate, and benzodiazepines, stop PTZ-induced seizures.10 As a result, the anticonvulsant effects of the study plant in the PTZ model might be attributed to blockage of T-type calcium currents or an increase in GABAergic neurotransmission. However, neither of these mechanisms was investigated in this work.
Flavonoids, saponins, tannins, steroids, phenols, and terpenoids were found in the hydro alcoholic extract, as shown in Table 1, and it seems likely that one of these active constituents is responsible for the anticonvulsant effect.23 Other phytochemical screening investigations on Biophytum sensitivum (L.) DC. leaves, which belong to the same family, backed up this study’s findings.24
It is impossible to say which phytoconstituents are responsible for Biophytum umbraculum Welw’s anticonvulsant action. In conclusion, phytoconstituents with potential anticonvulsant action should be investigated further. Many studies have shown that plant-derived terpenoids can influence GABAergic functioning.24–26 In several investigations,26 flavonoids extracted from various plants have also been shown to have anticonvulsant properties.
Biophytum umbraculum Welw. Syn., which also contains saponins, are antioxidants and free radical scavengers that may play a role in the prevention of diseases caused by reactive oxygen species or free radicals, such as atherosclerosis, cardiovascular disease, ischemia/reperfusion injury, neurodegenerative diseases, and cancer.27 All of these findings support the idea that the study plant has an anticonvulsant effect, most likely through various mechanisms.
Conclusion
The hydroalcoholic extract as well as the fractions showed varied degrees of anticonvulsant efficacy, supporting the traditional usage of Biophytum umbraculum Welw. Syn. as an anticonvulsant drug. In both the PTZ and MES models, the anticonvulsant efficacy of hydro alcoholic and BB was significant. CB, on the other hand, was shown to be efficacious primarily in PTZ-induced seizures, with the aqueous fraction having reduced anticonvulsant effectiveness in both seizure types. This suggests that the components with a medium polarity may be responsible for the anticonvulsant effect.
Abbreviations
AEDs, Anti-Epileptic Drugs; AB, Aqueous Fraction of Biophytum umbraculum; ANOVA, Analysis of Variance; BB, Butanol fraction of Biophytum umbraculum; CF, Chloroform Fraction of Biophytum umbraculum; CS, clonic seizure; MB, Hydro alcoholic extract of Biophytum umbraculum; MES, Maximal Electrical Shock Min-Minute; Mg, Milligram; NF, Nebeyi Fisseha; OECD, Organization of Economic Cooperation and Development; PTZ, Pentylenetetrazol; S, Second; SEM, Standard Error of the Mean; THLE, Tonic Hind Limb Extension.
Data Sharing Statement
All data pertaining to this study are within the manuscript.
Ethical Approval
The proposal was reviewed and approved with approval number of (SOP3/15/14) by the ethical review committee of Mizan-Tepi University’s School of Pharmacy in Mizan Teferi, Ethiopia. The experimental animals were handled according to the guidelines for care and use of laboratory animals and OECD-guidelines14.
Acknowledgments
The financial support of Mizan-Tepi University was gratefully acknowledged.
Author Contributions
All authors contributed significantly to the work that was published, whether it be in the ideation, study design, implementation, data collection, analysis, and interpretation, or in all of these areas. They also all participated in writing, revising, or critically evaluating the article; gave their final approval for the version that would be published; agreed on the journal to which the article would be submitted; and agreed to be responsible for all aspects of the work.
Disclosure
The authors declare that there is no conflict of interest regarding the publication of this article.
References
1. Boer HM, Mula M, Sander JW. The global burden and stigma of epilepsy. Epilepsy Behav. 2008;12(4):540–546. doi:10.1016/j.yebeh.2007.12.019
2. Singh A, Trevick S. The epidemiology of global epilepsy. Neurol Clin. 2016;34(4):837–847. doi:10.1016/j.ncl.2016.06.015
3. Mbelesso P, Luna J, Yangatimbi E, Mboukou C, Preux PM. Sociocultural representations of epilepsy in the Central African Republic: a door-to-door survey. Seizure. 2019;67(67):23–26. doi:10.1016/j.seizure.2019.02.018
4. Perucca E, Tomson T. The pharmacological treatment of epilepsy in adults. Lancet Neurol. 2011;10(5):446–456. doi:10.1016/S1474-4422(11)70047-3
5. Dalic L, Cook MJ. Managing drug-resistant epilepsy: challenges and solutions. Neuropsychiatr Dis Treat. 2016;12:2605. doi:10.2147/NDT.S84852
6. D’Antuono M, Köhling R, Ricalzone S, Gotman J, Biagini G, Avoli M. Antiepileptic drugs abolish ictal but not interictal epileptiform discharges in vitro. Epilepsia. 2010;51(3):423–431. doi:10.1111/j.1528-1167.2009.02273.x
7. Costa AM, Lucchi C, Malkoç A, Rustichelli C, Biagini G. Relationship between Delta rhythm, seizure occurrence and allopregnanolone hippocampal levels in epileptic rats exposed to the rebound effect. Pharmaceuticals. 2021;14(2):127. doi:10.3390/ph14020127
8. Franco V, French JA, Perucca E. Challenges in the clinical development of new antiepileptic drugs. Pharmacol Res. 2016;103:95–104. doi:10.1016/j.phrs.2015.11.007
9. Wahab A. Difficulties in treatment and management of epilepsy and challenges in new drug development. Pharmaceuticals. 2010;3(7):2090–2110. doi:10.3390/ph3072090
10. Löscher W. Critical review of current animal models of seizures and epilepsy used in the discovery and development of new antiepileptic drugs. Seizure. 2011;20(5):359–368. doi:10.1016/j.seizure.2011.01.003
11. Hegde K, Thakker SP, Joshi AB, Shastry CS, Chandrashekhar KS. Anticonvulsant activity of Carissa carandas Linn. root extract in experimental mice. Trop J Pharm Res. 2009;8(2). doi:10.4314/tjpr.v8i2.44519
12. Kassaye KD, Amberbir A, Getachew B, Mussema Y. A historical overview of traditional medicine practices and policy in Ethiopia. Ethiop J Health Dev. 2006;20(2):127–134.
13. Andarge E, Shonga A, Agize M, Tora A. Utilization and conservation of medicinal plants and their associated indigenous knowledge (IK) in Dawuro Zone: an ethnobotanical approach. Int J Med Plant Res. 2015;4:330–337.
14. Institute of Laboratory Animal Resources (US). Committee on care, use of laboratory animals. Guide for the care and use of laboratory animals. In: National Institutes of Health. US Department of Health and Human Services, Public Health Service; 1986.
15. Toxicity–Up AO. OECD guideline for testing of chemicals; 2001.
16. Löscher W, Schmidt D. Which animal models should be used in the search for new antiepileptic drugs? A proposal based on experimental and clinical considerations. Epilepsy Res. 1988;2(3):145–181. doi:10.1016/0920-1211(88)90054-X
17. Kumar S, Madaan R, Sharma A. Pharmacological evaluation of bioactive principle of Turnera aphrodisiaca. Indian J Pharm Sci. 2008;70(6):64. doi:10.4103/0253-7613.41040
18. Kar DM, Rout SK, Moharana L, Majumdar S. Evaluation of anticonvulsant activity of hydroalcoholic extract of Mussaenda philippica on animals. J Acute Dis. 2014;3(1):46–50. doi:10.1016/S2221-6189(14)60010-X
19. Ya’u J, Yaro AH, Malami S, et al. Anticonvulsant activity of aqueous fraction of Carissa edulis root bark. Pharm Biol. 2015;53(9):1329–1338. doi:10.3109/13880209.2014.981280
20. Aragaw TJ, Afework DT, Getahun KA. Assessment of knowledge, attitude, and utilization of traditional medicine among the communities of Debre Tabor Town, Amhara Regional State, North Central Ethiopia: a cross-sectional study. Evid Based Complement Altern Med. 2020;2020:1–10. doi:10.1155/2020/6565131
21. Fisseha N, Shibeshi W, Bisrat D. Evaluation of anticonvulsant activity of 80% methanolic root bark extract and solvent fractions of pentas schimperiana (A. Rich.) Vatke (Rubiaceae) in Swiss Albino Mice. Adv Pharmacol Pharm Sci. 2021;2021:1–7. doi:10.1155/2021/6689879
22. Porter RJ, Meldrum BS. Antiseizure drugs. Basic Clin Pharmacol. 2001;11:403–405.
23. Brodie MJ, Covanis A, Gil-Nagel A, et al. Antiepileptic drug therapy: does mechanism of action matter? Epilepsy Behav. 2011;21(4):331–341. doi:10.1016/j.yebeh.2011.05.025
24. Kumar KS, Kota K, Tahashildar J, Tahashildar J, Ragavendhra P. Antiepileptic activity of ethanolic extract of Biophytum sensitivum (L.) DC. in Animal models. Int J Curr Res Acad Rev. 2015;2015:23–30.
25. Citraro R, Navarra M, Leo A, et al. The anticonvulsant activity of a flavonoid-rich extract from Orange juice 40 | p a g e involves both NMDA and GABA-benzodiazepine receptor complexes. Molecules. 2016;21(9):1261. doi:10.3390/molecules21091261
26. Zhu HL, Wan JB, Wang YT, et al. Medicinal compounds with antiepileptic/anticonvulsant activities. Epilepsia. 2014;55(1):3–16. doi:10.1111/epi.12463
27. Malterud KE. Ethnopharmacology, chemistry and biological properties of four Malian medicinal plants. Plants. 2017;6(1):11. doi:10.3390/plants6010011
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.