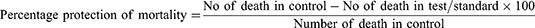Back to Journals » Journal of Experimental Pharmacology » Volume 15
Anti-Convulsant Activity of Soxhlet Leaf Extracts of Ajuga Integrifolia Buch.-Ham. Ex D.Don (Lamiaceae) in Mice
Authors Desalegn T, Engidawork E
Received 6 March 2023
Accepted for publication 23 May 2023
Published 30 May 2023 Volume 2023:15 Pages 241—253
DOI https://doi.org/10.2147/JEP.S409099
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Prof. Dr. Junmin Zhang
Tesfaye Desalegn, Ephrem Engidawork
Department of Pharmacology and Clinical Pharmacy, School of Pharmacy, College of Health Sciences, Addis Ababa University, Addis Ababa, Ethiopia
Correspondence: Ephrem Engidawork, Department of Pharmacology and Clinical Pharmacy, School of Pharmacy, College of Health Sciences, Addis Ababa University, P. O. Box 9086, Addis Ababa, Ethiopia, Email [email protected]
Background: The leaves of Ajuga integrifolia Buch.-Ham. ex D.Don (Lamiaceae) have long been used as an anti-convulsant remedy in Ethiopian traditional medicine. However, the evidence supporting their use is sparse in the literature. This study was conducted to add to the existing body of knowledge about the anti-convulsant activity of the plant.
Methods: The anti-convulsant activity of the extract was investigated in both acute (pentylenetetrazol [PTZ], 80 mg/kg; and maximal electroshock [MES]) and chronic (PTZ, 35 mg/kg) kindling seizure models. For the experimental paradigms, various doses of the extract (100, 200, and 400 mg/kg) were administered. Positive controls received sodium valproate (200 mg/kg) for the PTZ model and phenytoin (25 mg/kg) for the MES model. Parameters including the onset of clonus and duration of hindlimb tonic extension were recorded and compared with controls. Moreover, the total alkaloid, flavonoid, and phenol contents of the extracts were determined.
Results: Ethyl acetate extract produced a superior effect among all solvent extracts in both the PTZ and MES models. At all doses, it significantly delayed the mean onset of clonus (p< 0.01) in the PTZ test compared to controls. It also significantly reduced (p< 0.001) the mean duration of hindlimb tonic extension in the MES model. Treatment of mice with 200 mg/kg (p< 0.01) and 400 mg/kg (p< 0.001) of ethyl acetate extract significantly protected against PTZ-induced kindling compared to controls. The leaf was found to contain 10.002± 0.119 mg atropine equivalent per gram of dry extract of alkaloids, 9.045± 0.8445 mg quercetin equivalent per gram of dry extract of flavonoids, and 21.928± 1.118 mg gallic acid equivalent per gram of dry extract of phenols.
Conclusion: This study indicated that the plant A. integrifolia has anti-convulsant activity in both acute and chronic models of seizure. This plant represents a potential source for the development of a new anti-epileptic drug for pharmacoresistant epilepsy.
Keywords: Ajuga integrifolia, anti-convulsant, epilepsy, kindling, phytoconstituents, seizure
Background
Epilepsy is one of the most common chronic neurological disorders, affecting many people in different parts of the world.1 Despite their differences, the terms epilepsy and seizure are often confused.2 As defined by the International League Against Epilepsy (ILAE), epilepsy is a disease of the brain characterized by any of the following conditions: 1) at least two unprovoked (or reflex) seizures occurring >24 h apart; 2) one unprovoked seizure and a probability of further seizures similar to the general recurrence risk (at least 60%) after two unprovoked seizures, occurring over the next 10 years; and/or 3) the presence of an epilepsy syndrome, even if the risk of subsequent seizure is very low.3 Seizures are characterized by disturbed cerebral function caused by abnormal, excessive, and synchronous electrical discharges in groups of cortical neurons that may produce subclinical or various clinical phenomena.4,5
The genus Ajuga is a medicinal plant in the Lamiaceae family comprising more than 100 species and 50 subspecies distributed around the world.6 It is traditionally used for the treatment of distinctive sicknesses.7–19 Ajuga integrifolia Buch.-Ham. ex D.Don (Lamiaceae) (synonyms: Ajuga remota Benth., Ajuga bracteosa Wall ex Benth.) is a shrub that grows broadly in East Africa, Saudi Arabia, Yemen, Afghanistan, and East Asia.20 In Ethiopia, it grows in different parts of the country,21 and is known by different vernacular names such as Armagusa (Afan Oromo),22 Akorarach or Tut astil (Amharic),23 and Anamuro (Guragegna and Sidamigna).24,25
Ethnobotanical studies conducted in different parts of the world have revealed that A. integrifolia is utilized for the treatment of numerous conditions, including edema, febrile conditions, gout, rheumatism, amenorrhea, malaria, and diabetes.26–29 In Ethiopian folk medicine, the plant is used for the treatment of diabetes, retained placenta, malaria, stomach ache, wounds, amoebiasis, cancer, diarrhea, liver problems, anthrax, hypertension, pneumonia, and epilepsy.30–45 The plant parts used for the specified activities are the leaf, stem, and root.20
Experimental studies carried out to evaluate acute toxicity and the claimed activity of the leaf have demonstrated that it is safe and possesses anti-hypertensive,46 anti-malarial,47 anti-type I and type II HIV,48 and anti-Mycobacterium tuberculosis activity.49 Reports are also available showing anti-epileptic activity of the leaf50 and stem.51 The root is also reported to possess anti-diabetic activity.20,52 The leaf is reported to contain secondary metabolites such as phenols, flavonoids, alkaloids, terpenoids, carbohydrates, and steroids.53 It also contains essential oils such as limonene, α-humulene, β-myrcene, elemol, camphene, β-caryophellene, and α-phellendrene.54 Different diterpenes such as ajugarin I, ajugarin II, ajugarin IV, ajugarin V, and ajugapitin have been isolated using high-performance liquid chromatography from the aerial parts (leaf and stem) of the plant.55 Triterpenes, such as ergosterol-5,8-endoperoxide, with anti-Mycobacterium tuberculosis activity have also been isolated from the aerial parts.49 The bioactive substances found in different parts of the plant may play a predominant role in the effectiveness of the plant in many conditions.56
Many studies have reported that herbal medicines are commonly used for the treatment of epilepsy because anti-epileptic drugs (AEDs) fail to control seizures in 30% of the epileptic patients and because of economic and cultural factors.57 Hence, continuing the search for new therapies of plant origin with fewer side effects and better efficacy is of paramount importance. A recent experimental study reported the anti-epileptic activity of A. integrifolia.50 However, the study was limited to acute models and there is a need to replicate and reproduce earlier studies. Hence, the present study was initiated to assess the efficacy of the plant in both acute and chronic models, and to quantify the major constituents thought to be responsible for the anti-convulsant effect.
Materials and Methods
Drugs and Chemicals
Pentylenetetrazol (PTZ) (Sigma Aldrich, Germany), gallic acid (Merck, Germany), Folin–Ciocalteu reagent (Loba Chemie, India), NaOH (Loba Chemie, India), AlCl3 (Loba Chemie, India), chloroform (Loba Chemie, India), HCl (BDH Laboratory Supplies, UK), citric acid (Avonchem, UK), Na2HPO4 (BDH Laboratory Supplies, UK), atropine (BDH Chemicals, UK), quercetin dihydrate (Sigma Aldrich, Germany), Tween80 (Loba Chemie, India), BCG (Sisco Chemical Laboratories, India), n-hexane (Loba Chemie, India), ethyl acetate (Trust Chemical Laboratories, UK), methanol (Sisco Research Laboratories, India), normal saline solution (Sansheng Pharmaceuticals, Ethiopia), potassium acetate (Blulux, India), sodium valproate (Sanofi, Spain), and phenytoin (Macleods Pharmaceuticals, India) were obtained from their respective vendors. All drugs and chemicals used were of analytical grade.
Experimental Animals
Healthy male Swiss albino mice (8–10 weeks, 22–28 g) were used for the current study. The animals were obtained from the Ethiopian Public Health Institute and the animal unit of the School of Pharmacy, College of Health Sciences, Addis Ababa University, Addis Ababa, Ethiopia. The animals were housed in groups of six and acclimatized to laboratory conditions for a week before starting the experiment. The mice were kept under standard environmental conditions (12 h light/dark cycle) and provided with commercial food pellets and water ad libitum. All procedures and techniques used in this study were conducted in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.58 The protocol was approved by the institutional review board of the School of Pharmacy, with approval number ERB/SOP/199/2019.
Collection of Plant Material
Leaves of A. integrifolia were collected from the plant’s natural habitat in Girar Jarso district, Oromia Region, located 180 km north of Addis Ababa. Collected plant specimens were identified and authenticated by a taxonomist, Mr Melaku Wondafrash, and a voucher specimen (TD001) was deposited at the National Herbarium, College of Natural and Computational Sciences, Addis Ababa University, for future reference. The collected leaves were washed thoroughly with tap water to remove dirt and soil, and shade-dried at room temperature for 3 weeks.
Preparation of the Extracts
The air-dried leaves were subjected to size reduction using a mechanical grinder to obtain a coarse powder. The powdered plant material (500 g) was extracted by a successive Soxhlet extractor (Pyrex, Quickfit, UK) using 4 L of each of the following solvents: n-hexane (AHE), ethyl acetate (AEE), and methanol (AME). After extraction with the above solvents, the remaining residue was macerated with 1 L of distilled water three times for 72 h, with occasional shaking, to obtain the aqueous extract (AAE). Each time, before extracting with the next solvent, the powdered plant material was air-dried overnight. The resulting solution was first filtered through a cotton gauze for the aqueous extract and later through Whatman filter paper (no 1) for all solvent extracts. The non-aqueous filtrates were concentrated in a rotary evaporator (Buchi, Switzerland) under reduced pressure at 40°C and the water fraction was freeze-dried using a lyophilizer (Korea Vacuum Co, South Korea) to obtain the respective extracts. The yields (w/w) in terms of dry material for n-hexane, ethyl acetate, methanol, and water were 2.95%, 4.4%, 15.45%, and 3.29%, respectively. The dried extracts were kept in a refrigerator at −20°C until use.
Grouping and Dosing of Animals
The animals were randomly assigned into five groups for each solvent extract, each group containing six mice. In the acute model, group I served as a negative control and was treated with the vehicle used for reconstitution (2% Tween 80 for the non-aqueous extracts and distilled water for the aqueous extract). Group II was a positive control and was treated with sodium valproate 200 mg/kg (SV200) for the PTZ model and phenytoin 25 mg/kg (PHY25) for the maximum electric shock (MES) model. Groups III–IV were test groups and were administered with 100, 200, and 400 mg/kg doses of the respective extracts. The most active extract (AEE) was investigated in the chronic model of epilepsy (PTZ kindling) using the same grouping (SV200 was used as standard). All doses were administered orally and the maximum volume used was 10 mL/kg. The doses were determined in a pilot study conducted before the start of the experiment.
Anti-Convulsant Activity Test
The anti-convulsant activity of the plant was evaluated using acute and chronic models of epileptic seizure.
PTZ-Induced Seizure
For this test, the method by Salem et al,59 with slight modification, was used. After 60 min of oral administration, as described in the Grouping and Dosing of Animals section, freshly prepared PTZ (80 mg/kg) in normal saline was administered to the scruff of the neck of each mouse. The animals were then placed in a transparent cage and observed for convulsive behavior for 30 min using a video recorder. Forelimb or hindlimb clonic seizure was taken as the endpoint. The latency to clonic convulsion and percentage protection against mortality were recorded and compared with negative controls. Percentage protection from mortality was calculated as follows:
MES-Induced Seizure
For this experiment, the method described by Raza et al60 was used. After an hour of oral administration, as described in the Grouping and Dosing of Animals section, seizure was induced by auricular stimulation (50 mA, 150 Hz, 0.2 s) using an electro-convulsometer (Rolex Ambala, India). The ear-clip electrodes were moistened with normal saline before application for better conductance. Each animal was closely followed for 2 min using a video recorder. Upon exposure to the electric current, the animals exhibited various phases of tonic–clonic seizure, including immediate short-lived flexion of the forelimbs followed by extension of the hindlimbs. After the end of the extensor phase, they showed a stupor phase that finally led to recovery or death. The duration of hindlimb tonic extension (HLTE) (ie, outstretching of the animals 180° to the body axis) and protection against mortality were recorded. Percentage protection from mortality was calculated as follows:
In this experiment, reduction in the mean duration of HLTE of MES convulsion was considered as having anti-convulsant activity.61
PTZ-Induced Kindling
One hour after administration, as described in the Grouping and Dosing of Animals section, mice were kindled with repeated (every 48 h) intraperitoneal administration of freshly prepared PTZ (35 mg/kg) for 13 days. On each day, animals were closely observed for 30 min after PTZ injection using a video recorder to measure the intensity of seizures. The following seizure scores were used to identify fully kindled mice: stage 0 (no response); stage 1 (hyperactivity, ear and facial twitching); stage 3 (forelimb clonic seizure); stage 4 (generalized clonic seizure with falling); and stage 5 (generalized tonic–clonic seizures). Animals that showed at least three consecutive stage 4 or stage 5 seizure scores were thought to be kindled. Animals that did not show three consecutive stage 4 or 5 seizures were considered to be protected.62
Quantification of Total Flavonoid Content
The total flavonoid content (TFC) was estimated as described by Chang et al,63 with slight modification. First, 10 mg of AEE was dissolved in methanol to prepare a stock solution of 1 mg/mL. Then, 1 mL of the stock solution was transferred to a test tube and mixed with 0.1 mL of 10% aluminum chloride, 0.1 mL of 1 M potassium acetate, and 2.8 mL of distilled water. The mixture formed was allowed to stand for 30 min at ambient temperature, after which absorbance was recorded at 415 nm using a UV spectrophotometer (Jenway Model 6500, UK). Quercetin, used as a standard, was dissolved in methanol and a serial dilution was used to prepare 1.5625, 3.125, 6.25, 12.5, and 25 µg/mL standard solutions. The same procedure was followed to prepare the standard and blank solution. All experiments were performed in triplicate and the average value was recorded. A linear calibration curve was plotted with a regression coefficient (R2)=0.9964, slope (m)=0.02432 and y-intercept=0.0159 (Figure 1A), and this curve was used to determine TFC, expressed as milligrams of quercetin equivalent per gram (mg QE/g).
 |
Figure 1 Standard curve constructed for the quantification of secondary metabolites: (A) quercetin; (B) gallic acid; and (C) atropine. |
Quantification of Total Phenolic Content
Folin–Ciocalteu reagent was used for this assay. Folin reagent (1 mL of 2 N) was diluted with 20 mL of distilled water. To determine the total phenolic content (TPC), 1 mL of the prepared AEE solution (250 µg/mL) was transferred to a test tube, 0.5 mL of Folin reagent was added, and the tube was allowed to stand for 8 min. Thereafter, 2 mL of 7.5% sodium carbonate in distilled water was mixed with the solution and incubated for 30 min at ambient temperature. The absorbance was later recorded at 765 nm using a UV spectrophotometer. Gallic acid, used as standard, was prepared in different concentrations of 3.125, 6.25, 12.5, and 25 µg/mL by serial dilution to draw a standard curve. The same procedure was followed to prepare gallic acid and the blank solution. All experiments were conducted in triplicate and the average value was taken. A linear calibration curve was constructed with R2=0.9982, slope (m)=0.009323, and y-intercept=0.005289 (Figure 1B) to determine TPC, expressed as milligrams of gallic acid equivalent per gram (mg GAE/g).64
Quantification of Total Alkaloids
The total alkaloid content (TAC) of the plant was determined according to the method described by Tabasum et al,65 with slight modification. The reaction that took place between alkaloid and bromocresol green (BCG) was used to quantify TAC by a spectrophotometric method. Accordingly, 2 mL of AEE in methanol (1 mg/mL) was dissolved in 2 mL of 2 N HCl and filtered. Then, 1 mL of this solution was transferred to a separating funnel and washed with 5 mL of chloroform twice. The pH of the solution was adjusted to neutral by adding 0.1 N NaOH. After pH adjustment, 5 mL of BCG solution (prepared by heating 69.8 mg of BCG with 3 mL of 2 N NaOH and 5 mL of distilled water, and then diluted to 1000 mL with distilled water) along with 5 mL of phosphate buffer (prepared by adjusting the pH of 2 M sodium phosphate [71.6 g of Na2HPO4 in 1 L distilled water] to 4.7 with 0.2 M citric acid [42.02 g] citric acid in 1 L distilled water) was added. The aggregate formed was shaken and the mixture formed was extracted with 5 mL of chloroform by vigorous shaking. The extract was collected in a 10 mL volumetric flask and the volume was made up to 10 mL with chloroform. The absorbance of the mixture in chloroform was measured at 470 nm using a UV spectrophotometer. For the construction of a standard curve (Figure 1C), atropine was dissolved in methanol to prepare different concentrations (0.5, 0.25, 0.125, 0.062, and 0.03125 mg/mL) of the standard solution. Then, 1 mL of this solution was taken and transferred to a separating funnel, 5 mL of phosphate buffer along with 5 mL BCG solution was added, followed by gentle shaking with 5 mL of chloroform twice. The mixture formed was collected in a 10 mL volumetric flask and diluted to volume with chloroform, and the absorbance was measured at 470 nm. The blank was prepared as described, but without atropine. The assay was run in triplicate and the average value was taken. TAC was determined from a calibration curve with R2=0.9938, slope (m)=0.002625, and y-intercept=0.001750 (Figure 1C), and described as milligrams of atropine equivalent per gram (mg ATE/g).
Data Analysis
All experimental data are expressed as mean ± standard error of the mean (SEM) and were subjected to statistical analysis using SPSS for Windows, version 25, statistical packages. Statistical analysis of the difference among groups was performed with one-way analysis of variance (ANOVA) followed by Tukey’s post-hoc test. For PTZ kindling, two-way analysis of variance and Bonferroni’s post-hoc test were used for multiple comparisons of the mean difference. The analyses were performed with 95% confidence intervals and the level of significance was set at p<0.05.
Results
Anti-Convulsant Activity in PTZ-Induced Seizure
All of the extracts except for AAE possessed anti-convulsant activity against the PTZ model of seizure, as evidenced by an increased mean latency to clonic convulsion (Table 1). AEE was the most effective extract, as it prolonged the mean onset of clonus and decreased the percent mortality better than the other extracts. The mean latency to clonic seizure was significantly increased (p<0.01) with all doses of AEE compared to controls, the maximum effect (13.17 min) being achieved with 400 mg/kg (AEE400). The increment in mean onset of clonus occurred in a dose-dependent manner, as AEE400 significantly delayed the onset of clonus compared to AEE 200 mg/kg (AEE200) (p<0.01) and AEE 100 mg/kg (AEE100) (p<0.001). AEE also decreased the percent mortality in a dose-dependent manner, with maximum protection (66.67%) conferred by AEE400 (Table 1).
 |
Table 1 Anti-Convulsant Activity of Soxhlet Leaf Extracts of Ajuga Integrifolia in Pentylenetetrazol-Induced Seizure |
AHE also significantly increased the mean latency to clonic seizure compared to controls, with a maximum increase (12.67 min, p<0.001) displayed by AHE 400 mg/kg (AHE400). The effect produced by AHE400 was significantly greater than the effects produced by AHE 100 mg/kg (AHE100) (p<0.001) and AHE 200 mg/kg (AHE200) (p<0.01). With regard to mortality, while AHE200 and AHE400 were able to decrease death by 33.33% and 50%, respectively, AHE100 was devoid of any effect (Table 1).
AME at 100 mg/kg (AME100) was not effective on either parameter of this experimental paradigm. However, AME at doses of 200 mg/kg (AME200) and 400 mg/kg (AME400) significantly increased (p<0.001) mean latency and provided fairly good protection against mortality compared to controls. By contrast, AAE was devoid of any effects at any dose (Table 1).
The standard drug used (SV) was superior in both measures, as it significantly increased latency (p<0.01) and decreased mortality (83.33%) compared to all doses of the extracts used in this experiment.
Anti-Convulsant Activity in MES-Induced Seizure
As depicted in Table 2, all leaf solvent extracts except for AAE produced variable results in reducing the mean duration of HLTE and percent mortality in the MES test. AEE was the most effective extract, as it reduced the duration of HLTE and percent mortality better than the other extracts. The mean duration of HLTE was significantly decreased (p<0.001) with all doses of AEE compared to controls, with the maximum reduction (6.33 s) being conferred by AEE400. The reduction in the mean duration of HLTE occurred in a dose-dependent manner, as AEE400 significantly decreased the duration of HLTE compared to AEE200 (p<0.01) and AEE100 (p<0.001). AEE also decreased the percent mortality, with the maximum protection (50%) being achieved by AEE200 and AEE400 (Table 2).
 |
Table 2 Anti-Convulsant Activity of Soxhlet Leaf Extracts of Ajuga Integrifolia in maximal electroshock-Induced Seizure |
AHE also significantly decreased the mean duration of HLTE compared to controls, with the maximum reduction (8.17 s, p<0.001) being obtained by AHE400. The effect displayed by AHE400 was significantly greater than the effects produced by AHE100 (p<0.05) and AHA200 (p<0.001). Concerning mortality, AHE200 and AHE400 decreased death by 16.67% and 33.33%, respectively, while AHE100 was devoid of any effect.
AME100 was not effective on either parameter used to evaluate the anti-convulsant activity of the plant. However, AME200 and AME400 significantly reduced (p<0.001) the mean duration of HLTE and resulted in a percent protection against mortality to the same extent (16.67%) compared to controls. By contrast, AAE was devoid of any effect at any dose (Table 2).
The standard drug used (PHY) was superior in both measures as it significantly reduced the occurrence of HLTE (p<0.001) and decreased mortality (83.33%) compared to all doses of the extracts used in this experiment.
Anti-Convulsant Activity in PTZ Kindling Model
The most active extract (AEE) in the acute seizure model also showed anti-convulsant activity in the chronic seizure model (Figure 2). For this test, 13 injections of PTZ (35 mg/kg ip) on alternate days produced full kindling starting from the 11th to the 13th injections in controls, while treatment of mice with different doses of AEE produced variable effects. AEE400 significantly protected (p<0.001) the animals from developing consecutive stage 4 and/or 5 seizures on the last three injections compared to controls, which indicated protection of the animals. Likewise, AEE200 also produced a significant effect (p<0.01) in protection from kindling compared to controls. On the other hand, AEE100 was ineffective in the parameter used in this experimental model. The standard drug used (SV) was superior in the parameter used as it significantly prevented (p<0.001) the occurrence of kindling compared to controls.
As can be observed from Figure 2, AEE reduced the mean seizure stage in a dose-dependent manner. Statistical analysis revealed that all doses of the extract and the standard drug (SV) significantly reduced (p<0.05) the seizure stage on the first injection compared to controls. On the second injection, the seizure score observed was significant for all doses of the extract (p<0.01) and the standard (p<0.001) compared to the negative control, but no significant difference was seen between the extract- and SV-pretreated groups. A difference in the stage of seizure after PTZ injection between treatment groups was observed from the third injection onwards.
As the number of injections increased, significant differences in the mean seizure stage between AEE100 and the negative control group started to disappear. Indeed, no detectable difference in mean seizure stage was noted between the two groups from the 9th to the 13th injections. This indicated the failure of AEE100 to protect the animals from PTZ-induced kindling. The AEE400-pretreated group started to respond to the chemoconvulsant PTZ from the sixth injection, but no significant effect was seen between AEE400 and SV200 until the ninth injection.
Quantification of Secondary Metabolites
Quantitative phytochemical analysis revealed that AEE contained 9.045±0.8445 mg QE/g, 21.928±1.118 mg GAE/g, and 10.002±0.119 mg ATE/g of total flavonoids, phenols, and alkaloids, respectively.
Discussion
In the current study, solvent leaf extracts of A. integrifolia were found to possess anti-convulsant activity in both acute and chronic seizure models, which appeared to vary with dose and the nature of the extract. AEE produced the highest effect among all solvent extracts in both acute models, the rank order being AEE>AHE>AME≫AAE. Despite having anti-convulsant activity in both models, not all doses of AEE showed an equally appreciable effect in delaying the mean onset of attack and protecting the animals from death. The effect was produced in a dose-dependent manner and AEE400 considerably increased the onset and percent protection from mortality compared to the remaining doses of the extract. The anti-convulsant activity demonstrated by AEE could be attributed to the presence of secondary metabolites, such as flavonoids, phenols, alkaloids, terpenoids, and steroids, which were reported in earlier research,53 as well as in the present study.
In line with the test result, the anti-convulsant activity displayed by AHE, in terms of the parameters used, was lower than that displayed by AEE. This may be related to the absence of major phytoconstituents such as phenols and flavonoids in the leaf hexane fraction of the plant, as reported elsewhere.66 Likewise, the absence of steroids in the methanol extract49 might have contributed towards the reduced anti-convulsant activity in the present study. At the doses used, AAE did not produce a considerable effect in either the PTZ or the MES model. This may indicate that semi-polar and non-polar constituents are responsible for the anti-convulsant activity of the plant.
A previous similar study on the leaf crude extract and solvent fractions of the plant collected from different geographical locations reported the anti-convulsant activity of A. integrifolia in PTZ and MES models.50 The most active fraction in this study (butanol 400 mg/kg) tended to produce a better effect than AEE400 in the PTZ model (mean latency to clonic seizure 15.51 vs 13.17 min), but the reverse was true in the MES model (reduction in mean duration of HLTE 8.33 vs 6.33 s). The present study upholds the earlier observation, and the findings of both studies strongly indicate the potential anti-convulsant activity of the plant. The subtle difference observed between the studies may be due to the variation in the geographical location as well as the method of extraction.
The MES and PTZ models for acute seizure do not simulate the chronic dysfunction of the brain often seen in epilepsy and thus cannot be used for the discovery of potential AEDs for pharmacoresistant epilepsy. Therefore, kindling is widely employed to study the process of epileptogenesis and to promote AED discovery.67 Thus, the present study also investigated the effect of the most active extract (AEE) in a chronic model of epilepsy. AEE dose dependently prevented the occurrence of consecutive stage 4 and/or stage 5 seizures, although the lowest dose failed to do so. During the initial phases of the kindling process, the lower dose (AEE100) prevented the animals from developing the maximum stages of seizure, which only lasted for a short time. This may be due to the accumulation of PTZ in the brain following repeated administration, which could result in prolonged antagonism of GABA, making the lowest dose ineffective to overcome this antagonism. The anti-convulsant effect demonstrated by the AEE in PTZ-induced kindling might be attributed to the presence of secondary metabolites, as the leaf is reported to possess a wide range of secondary metabolites.53 Indeed, although terpenoids were not determined in the present study, repeated administration of a triterpenoid such as oleanic acid is reported to protect animals against PTZ-induced seizures.68
Although the exact mechanism of the anti-convulsant activity of the study plant remains to be elucidated, it can be generalized that the active solvent extracts can act through multiple mechanisms similar to the conventional medicines. Indeed, the secondary metabolites determined in the present study have been demonstrated to produce anti-convulsant activity through a host of mechanisms, including antioxidant,69,70 Na+ channel blocking, and modulation of GABAA receptors.71 The TPC and TFC determined in the present study were found to be four- to five-fold greater than those reported for the methanol extract of the aerial part of the same plant from Pakistan,8 suggesting that the geographical location, plant part, and solvent used for extraction could contribute toward variation in the quantity of active constituents, even in the same plants.
Conclusion
The results of the present study provide support for the use of A. integrifolia as an anti-convulsant medicinal plant, as solvent extracts from the leaves of the plant displayed anti-convulsant activity in both acute and chronic seizure models. AEE is demonstrated to be the most effective extract in altering the parameters used in both the acute and chronic models of this experimental paradigm. The results of this study suggest that semi-polar to non-polar components are responsible for the anti-convulsant activity of the plant, while polar components are found to be devoid of anti-convulsant activity.
Abbreviations
AED, anti-epileptic drug; GABA, gamma-aminobutyric acid; HLTE, hindlimb tonic extension; MES, maximal electroshock; PTZ, pentylenetetrazol.
Data Sharing Statement
The data sets used and/or analyzed during the current work are available and included in this article.
Ethics Approval and Informed Consent
The protocol was approved by the institutional review board of the School of Pharmacy, Addis Ababa University (reference no ERB/SOP/199/2019).
Acknowledgments
TD is grateful to Madda Walabu University and Addis Ababa University for granting him support during his studies. This paper was uploaded to the Addis Ababa University repository as a thesis in November 2021: http://etd.aau.edu.et/handle/123456789/32068.72 We express our heartfelt thanks to Addis Ababa University for providing financial support and all the necessary materials for the study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
Financial support was provided by Addis Ababa University as part of an MSc research project.
Disclosure
The authors declare that there are no conflicts of interest regarding the publication of this paper.
References
1. Thijs RD, Surges R, O’brien TJ, Sander JW. Epilepsy in adults. Lancet. 2019;393(10172):689–701.
2. Scharfman HE. The neurobiology of epilepsy. Curr Neurol Neurosci Rep. 2007;7(4):348–354.
3. Fisher RS, Acevedo C. official report: a practical clinical definition of epilepsy. Epilepsia. 2014;55(4):475–482.
4. Fisher RS, Cross JH, French JA, et al. Operational classification of seizure types by the International League Against Epilepsy: position Paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017;58(4):522–530.
5. Mosewich RK, So EL. A clinical approach to the classification of seizures and epileptic syndromes. Mayo Clin Proc. 1996;71(4):404–414.
6. Topçu G, Kökdil G, Türkmen Z, Voelter W, Adou E, Kingston DG. A new clerodane diterpene and other constituents from Ajuga chamaepitys ssp. laevigata. J Med. 2004;59B:584–588
7. Pal A, Toppo FA, Chaurasiya PK, Singour PK, Pawar RS. In-vitro cytotoxicity study of methanolic fraction from Ajuga Bracteosa wall ex. benth on MCF-7 breast adenocarcinoma and hep-2 larynx carcinoma cell lines. Res j Pharmacogn. 2014;6(1):1–6.
8. Kayani WK, Dilshad E, Ahmed T, Ismail H, Mirza B. Evaluation of Ajuga bracteosa for antioxidant, anti-inflammatory, analgesic, antidepressant and anticoagulant activities. BMC Complement Altern Med. 2016;16(1):1–13.
9. Gautam R, Jachak SM, Saklani A. Anti-inflammatory effect of Ajuga bracteosa Wall Ex Benth. mediated through cyclooxygenase (COX) inhibition. J Ethnopharmacol. 2011;133(2):928–930.
10. Boudjelal A, Siracusa L, Henchiri C, et al. Antidiabetic effects of aqueous infusions of Artemisia herba-alba and Ajuga iva in alloxan-induced diabetic rats. Planta Med. 2015;81(9):696–704.
11. EL-Hilaly J, Amarouch M-Y, Morel N, Lyoussi B, Quetin-Leclercq J. Ajuga iva water extract antihypertensive effect on stroke-prone spontaneously hypertensive rats, vasorelaxant effects ex vivo and in vitro activity of fractions. J Ethnopharmacol. 2021;270(3):113791.
12. Tahraoui A, El-Hilaly J, Israili Z, Lyoussi B. Ethnopharmacological survey of plants used in the traditional treatment of hypertension and diabetes in south-eastern Morocco (Errachidia province). J Ethnopharmacol. 2007;110(1):105–117.
13. Movahhedin N. Ajuga chamaecistus subsp. scoparia (Boiss.) Rech. f.: a new source of phytochemicals for antidiabetic, skin-care, and neuroprotective uses. Ind Crops and Prod. 2016;94:89–96.
14. Khalil EA, Afifi FU, Al-Hussaini M. Evaluation of the wound healing effect of some Jordanian traditional medicinal plants formulated in Pluronic F127 using mice (Mus musculus). J Ethnopharmacol. 2007;109(1):104–112.
15. Khanavi M, Davoodipoor AM, Sadati SN, Ardekani MRS, Sharifzadeh M. Antinociceptive effect of some extracts from Ajuga chamaecistus Ging. ssp. tomentella (Boiss.) Rech. f. aerial parts. DARU J Pharm Sci. 2014;22:1–6.
16. Rahman N, Ahmad M, Riaz M, Mehjabeen JN, Ahmad R. Phytochemical, antimicrobial, insecticidal and brine shrimp lethality bioassay of the crude methanolic extract of Ajuga parviflora Benth. Pak J Pharm Sci. 2013;26(4):751–756.
17. Setif A. Antibacterial activity of extract of Ajuga iva and Teucrium polium. Adv Environ Biol. 2011;5(2):491–495.
18. Agarwal G, Pant A, Hore S. In vitro evaluation of anthelmintic efficacy of Trichilia and Ajuga species on Ascaridia galli. Hygeia J D Med. 2012;2(2):43–53.
19. Rahman IU, Ijaz F, Iqbal Z, Afzal A. A novel survey of the ethno medicinal knowledge of dental problems in Manoor Valley (Northern Himalaya), Pakistan. J Ethnopharmacol. 2016;194:877–894.
20. Tafesse TB, Hymete A, Mekonnen Y, Tadesse M. Antidiabetic activity and phytochemical screening of extracts of the leaves of Ajuga remota Benth on alloxan-induced diabetic mice. BMC Complement Altern Med. 2017;17(1):1–9.
21. Seifu A. Bioprospecting Potential of Ajuga Integrifolia for Access and Benefit Sharing. Rome, Italy: FAO; 2017.
22. Kefalew A, Asfaw Z, Kelbessa E. Ethnobotany of medicinal plants in Ada’a District, East Shewa Zone of Oromia regional state, Ethiopia. J Ethnobiol Ethnomed. 2015;11(15):1–28.
23. Degu S, Berihun A, Muluye R. Medicinal plants that used as repellent, insecticide and larvicide in Ethiopia. J.Pharm.Pharmacol. 2020;8(5):274–283.
24. Tefera BN, Kim Y-D. Ethnobotanical study of medicinal plants in the Hawassa Zuria District, Sidama zone, Southern Ethiopia. J Ethnobiol Ethnomed. 2019;15(1):1–21.
25. Teka A, Asfaw Z, Demissew S, Van Damme P. Medicinal plant use practice in four ethnic communities (Gurage, Mareqo, Qebena, and Silti), south central Ethiopia. J Ethnobiol Ethnome. 2020a;16(1):1–12.
26. Singh KJ, Thakur AK. Medicinal plants of the Shimla hills, Himachal Pradesh: a survey. J Herb Med. 2014;2(2):118–127.
27. Wangpan T, Chetry LB, Tsering J, Tapi T, Tangjang S. Anti-Malarial Plants of Jonai, India: an Ethnobotanical Approach. Not Sci Biol. 2016;8(1):27–32.
28. Keter LK, Mutiso PC. Ethnobotanical studies of medicinal plants used by Traditional Health Practitioners in the management of diabetes in Lower Eastern Province, Kenya. J Ethnopharmacol. 2012;139(1):74–80.
29. Njoroge GN, Bussmann RW. Diversity and utilization of antimalarial ethnophytotherapeutic remedies among the Kikuyus (Central Kenya). J Ethnobiol Ethnomed. 2006;2(1):1–7.
30. Meresa A, Gemechu W, Basha H, et al. Herbal medicines for the management of diabetic mellitus in Ethiopia and Eretria including their phytochemical constituents. Am J Adv Drug Deliv. 2017;5(1):40–58.
31. Giday M, Asfaw Z, Woldu Z. Medicinal plants of the Meinit ethnic group of Ethiopia: an ethnobotanical study. J Ethnopharmacol. 2009;124(3):513–521.
32. Asnake S, Teklehaymanot T, Hymete A, Erko B, Giday M. Survey of medicinal plants used to treat malaria by Sidama People of Boricha District, Sidama Zone, South Region of Ethiopia. Evid Based Complementary Altern Med. 2016;2016(1):1–9.
33. Gedif T, Hahn H-J. The use of medicinal plants in self-care in rural central Ethiopia. J Ethnopharmacol. 2003;87(2–3):155–161.
34. Regassa R. Assessment of indigenous knowledge of medicinal plant practice and mode of service delivery in Hawassa city, southern Ethiopia. J Med Plants Res. 2013;7(9):517–535.
35. Tilahun Y. Ethnobotanical study of traditional medicinal plants used in and around Adigrat town, eastern Tigray, Ethiopia. JMPS. 2018;6(4):11–19.
36. Giday M, Asfaw Z, Woldu Z. Ethnomedicinal study of plants used by Sheko ethnic group of Ethiopia. J Ethnopharmacol. 2010;132(1):75–85.
37. Tesfaye S, Belete A, Engidawork E, Gedif T, Asres K. Ethnobotanical study of medicinal plants used by traditional healers to treat cancer-like symptoms in eleven districts, Ethiopia. Evid Based Complementary Altern Med. 2020;1(6):1–23.
38. Parvez N, Yadav S. Ethnopharmacology of single herbal preparations of medicinal plants in Asendabo district, Jimma, Ethiopia. Indian J Tradit Knowl. 2010;9(4):724–729.
39. Teka A, Asfaw Z, Demissew S, Van Damme P. Traditional uses of medicinal plants practiced by the indigenous communities in Gurage Zone, south central Ethiopia. Ethnobot Res Appl. 2020b;19(2020):1–31.
40. Mesfin F, Seta T, Assefa A. An ethnobotanical study of medicinal plants in Amaro Woreda, Ethiopia. Ethnobot Res Appl. 2014;12(2014):341–354.
41. Getahun A. Some common medicinal and poisonous plants used in Ethiopian folk medicine. 1997.
42. Teshome D. Concomitant use of medicinal plants and conventional medicines among hypertensive patients in five hospitals in Ethiopia. Ethiop J Health Dev. 2019;33(4):241–249.
43. Regassa R, Bekele T, Megersa M. Ethnobotanical study of traditional medicinal plants used to treat human ailments by Halaba people, southern Ethiopia. JMPS. 2017;5(4):36–47.
44. Abera B. Medicinal plants used in traditional medicine by Oromo people, Ghimbi District, Southwest Ethiopia. J Ethnobiol Ethnomed. 2014;10(1):1–15.
45. Atnafu H, Awas T, Alemu S, Wube S. Ethnobotanical study of medicinal plants in selale mountain ridges, North Shoa, Ethiopia. IJBC. 2018;2(6):567–577.
46. Hailu W, Engidawork E. Evaluation of the diuretic activity of the aqueous and 80% methanol extracts of Ajuga remota Benth (Lamiaceae) leaves in mice. BMC Complement Altern Med. 2014;14(1):1–8.
47. Nardos A, Makonnen E. In vivo antiplasmodial activity and toxicological assessment of hydroethanolic crude extract of Ajuga remota. Malar J. 2017;16(1):1–8.
48. Asres K, Bucar F, Kartnig T, Witvrouw M, Pannecouque C. Antiviral activity against human immunodeficiency virus type 1 (HIV‐1) and type 2 (HIV‐2) of ethnobotanically selected Ethiopian medicinal plants. Phytotherapy Res. 2001;15(1):62–69
49. Cantrell CL, Rajab MS, Franzblau SG, Fronczek FR, Fischer NH. Antimycobacterial ergosterol-5, 8-endoperoxide from Ajuga remota. Planta Med. 1999;65(8):732–734.
50. Getaneh Y. Anti-Convulsant Activity of 80% Methanol Extract and Solvent Fractions of Ajuga Integrifolia Buch.- Ham(Lamiaceae) Leaves in Mice. Thesis. Addis Ababa University; 2020.
51. Qasim S, Uttra AM, Hasan UH, Batool A. Evaluation of anticonvulsant potential of aqueous meth-anolic extract and various fractions of Ajuga bracteosa wall. J Appl Anim. 2017;2(2):137–146.
52. Alene M. Evaluation of Antidiabetic Activity of Ajuga integrifolia (Lamiaceae) Root Extract and Solvent Fractions in Mice. Evid Based Complementary Altern Med. 2020;2020:1–11.
53. Tebeje BA. Phytochemical Screening of Secondary Metabolites of Extracts of the Plant Ajuga Integrifolia Leaves. chem.Mater. 2019;11(50):24281–24283.
54. Vohra A, Kaur H. Chemical investigation of medicinal plant Ajuga bracteosa. J Nat Prod. 2011;1(1):37–45.
55. Coll J, Tandrón Y. Isolation and identification of neo‐clerodane diterpenes from Ajuga remota by high‐performance liquid chromatography. Phytochem Anal. 2005;16(1):61–67.
56. Bekeri D, Adane L, Mamo F. Phytochemical Investigation and Isolation of Compounds From Ajuga integrifolia Root Extract. WJC. 2018;13(1):1–13.
57. Liu W, Ge T, Pan Z, Leng Y, Lv J, Li B. The effects of herbal medicine on epilepsy. Oncotarget. 2017;8(29):48385.
58. Council NR. Guide for the Care and Use of Laboratory Animals.
59. Salem GA, Alamyel FB, Abushaala FA, Hussain MS, Abusheba H, Sahu RP. Evaluation of the hepatoprotective, anti-inflammatory, antinociceptive and antiepileptic activities of Chrysanthemum trifurcatum. Biomed Pharmacother. 2019;117:1–7.
60. Raza M, Shaheen F, Choudhary M, et al. Anticonvulsant activities of ethanolic extract and aqueous fraction isolated from Delphinium denudatum. J Ethnopharmacol. 2001;78(1):73–78.
61. Mahendran S, Thippeswamy B, Veerapur V, Badami S. Anticonvulsant activity of embelin isolated from Embelia ribes. Phytomedicine. 2011;18(2–3):186–188.
62. Abdel-Zaher AO, Farghaly HS, Farrag MM, Abdel-Rahman MS, Abdel-Wahab BA. A potential mechanism for the ameliorative effect of thymoquinone on pentylenetetrazole-induced kindling and cognitive impairments in mice. Biomed Pharmacother. 2017;88:553–561.
63. Chang -C-C, Yang M-H, Wen H-M, Chern J-C. Estimation of total flavonoid content in propolis by two complementary colometric methods. J Food Drug Anal. 2002;10(3):178–182.
64. Maria R, Shirley M, Xavier C, et al. Preliminary phytochemical screening, total phenolic content and antibacterial activity of thirteen native species from Guayas province Ecuador. J King Saud Univ Sci. 2018;30(4):500–505.
65. Tabasum S, Khare S, Jain K. Spectrophotometric quantification of total phenolic, flavonoid, and alkaloid contents of Abrus precatorius L. seeds. Asian J Pharm Clin Res. 2016;9(2):371–374.
66. Sayyah M, Mandgary A, Kamalinejad M. Evaluation of the anticonvulsant activity of the seed acetone extract of Ferula gummosa Boiss. against seizures induced by pentylenetetrazole and electroconvulsive shock in mice. J Ethnopharmacol. 2002;82(2–3):105–109.
67. Kuria KA, Chepkwony H, Govaerts C, et al. The Antiplasmodial Activity of Isolates from Ajuga r emota. J Nat Prod. 2002;65(5):789–793.
68. Akunal Turel C, Yunusoglu O. Oleanolic acid suppresses pentylenetetrazole-induced seizure in vivo. Int J Environ Health Res. 2023;33(5):529–540.
69. Aliyu MM, Musa AII, Kamal MJA, Mohammed MG. Phytochemical screening and anticonvulsant studies of ethyl acetate fraction of Globimetula braunii on laboratory animals. Asian Pac J Trop Biomed. 2014;4(4):285–289.
70. Citraro R, Navarra M, Leo A. The anticonvulsant activity of a flavonoid-rich extract from Orange juice involves both NMDA and GABA-benzodiazepine receptor complexes. Molecules. 2016;21(19):1261.
71. Samokhina E, Samokhin A. Neuropathological profile of the pentylenetetrazol (PTZ) kindling model. Int J Neurosci. 2018;128(11):1086–1096.
72. Dessalegn T. Anticonvulsant Activity of Soxhlet Leaf Extracts of Ajuga Integrifolia Buch.Ham Ex D. Don (Lamiaceae) in Mice. Thesis. Addis Ababa University; 2021.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.