Back to Journals » Cancer Management and Research » Volume 14
Anti-PD-1 Therapy is Beneficial for the Survival of Patients with Oral Squamous Cell Carcinoma
Authors Feng L, Yin K, Zhang S, Chen Z, Bao Y, Li T
Received 31 March 2022
Accepted for publication 7 September 2022
Published 14 September 2022 Volume 2022:14 Pages 2723—2731
DOI https://doi.org/10.2147/CMAR.S368738
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Harikrishna Nakshatri
Liang Feng,1 Ke Yin,2 Suxin Zhang,3 Zhong Chen,3 Yang Bao,3 Tianke Li3
1Department of Stomatology, Baoding First Central Hospital, Baoding, Hebei, People’s Republic of China; 2Department of Stomatology, Xingtai People’s Hospital, Xingtai, Hebei, People’s Republic of China; 3Department of Stomatology, The Fourth Hospital of Hebei Medical University, Shijiazhuang, Hebei, People’s Republic of China
Correspondence: Tianke Li, Department of Stomatology, The Fourth Hospital of Hebei Medical University, No. 12 Jiankang Road, Shijiazhuang, Hebei, 050000, People’s Republic of China, Email [email protected]
Background: Oral squamous cell carcinoma (OSCC) is one of the most common malignant tumors of the head and neck. Programmed cell death protein 1 (PD-1), and programmed cell death 1 ligand 1 (PD-L1) are often overexpressed in OSCC patients, and their expression level is closely related to tumor prognosis. The objectives of this study were: 1) to evaluate the impact of anti-PD-1 treatment on the immune system and prognosis of OSCC patients and 2) to find possible associations between T-cell immunity and anti-PD-1 therapy.
Methods: A total of 120 patients (divided into two equal groups: “non-anti-PD1 therapy” and “anti-PD1 therapy”) with pathologically diagnosed OSCC participated in the study. Fresh peripheral blood samples (1 mL) were collected 2 days before and 20 days after the treatment. Heparin was used as an anticoagulant. Kaplan–Meier curves were plotted to compare the non-anti-PD-1 therapy and anti-PD-1 therapy groups.
Results: Based on the Spearman-rho test, we found a significant correlation between anti-PD-1 treatment and survival time (P< 0.001). Univariate/multivariate Cox regression analysis revealed that anti-PD-1 therapy is a significant independent risk factor of 5-year overall survival (OS) in OSCC patients (HR: 0.110, 95% CI: 0.062– 0.195, P< 0.001). One-way ANOVA showed that the mean levels of IFN-γ and IL-2 and numbers of CD4+ T cells were significantly increased in the anti-PD-1 therapy group compared with the non-anti PD-1 therapy group (control). The was no change in the number of CD8+ cells between the two groups. Kaplan–Meier curve results showed that the OS of patients in the anti-PD-1 therapy group was significantly longer than that in the non-anti-PD-1 therapy group.
Conclusion: Anti-PD-1 therapy is beneficial to the survival and prognosis of patients with OSCC, improves T-cell immunity, and enhances tumor regression.
Keywords: OSCC, oral cancer, PD-1, PD-L1, targeted therapy, Squamous cell carcinoma
Introduction
Oral cancer, originating from the oral mucosal epithelium, is one of the most common malignant tumors of the head and neck. Oral squamous cell carcinoma (OSCC) accounts for more than 90% of oral cancers, and a recent rise in its incidence has been observed, particularly among the young. The incidence of OSCC in patients aged <45 years accounts for approximately 1% to 6% of cases.1 OSCC causes oro-facial destruction, with cervical lymph node metastasis, and ultimately blood-borne dissemination.2 At present, the treatment of OSCC is mainly based on surgery, radiotherapy, and chemotherapy and the prognosis of OSCC is poor due to lymph node metastasis and a high rate of recurrence.3 More than 50% of OSCC patients experience tumor recurrence or metastasis within 3 years, and the 5-year survival rate is about 50%.4–6 With rapid developments in biomedicine in recent years and the attainment of a good understanding of the links between immunity and tumors, immunotherapy has emerged as an important mode of cancer therapy. Some studies have found that molecules such as programmed cell death protein 1 (PD-1) and programmed cell death 1 ligand 1 (PD-L1) are often overexpressed in OSCC patients, and the level of expression of these molecules is closely related to tumor prognosis.7–9
PD-1, a member of the CD28 superfamily, belongs to the immunoglobulin superfamily Type I transmembrane glycoproteins and is mainly expressed on the surface of activated T lymphocytes, natural killer (NK) cells, monocytes, and B lymphocytes. Furthermore, as an important immune checkpoint for immunosuppression of tumor-infiltrating T cells, PD-1 is upregulated in various tumor microenvironments, such as in liver, lung, colorectal cancers and hematologic malignancies.10–12 The major ligand of PD-1 binds to negatively regulated T-cell receptor signaling. Tumors may silence the immune system via the PD-1/PD-L1 inhibitory pathway. Thus, blocking this interaction could improve the ability of T cells to attack tumor cells.
T cells can be divided into CD4+ and CD8+ T cells according to differences in their surface antigens, and the balance of these two types of T cells enables the body to maintain a stable immune function. Increasing the CD4+/CD8+ ratio may improve immune function. A study found that the levels of PD-1 and PD-L1 expression on the surface of CD4+ and CD8+ T cells of OSCC patients were significantly higher than those of the control group, and the level of PD-1 in plasma was also significantly higher than that in the control group.13 Interferon-gamma (IFN-γ), a pro-inflammatory cytokine, is an important immune response regulator produced by activated T cells. IFN-γ can modulate the immune system by affecting multiple processes including the regulation of the cell cycle, promotion of cell apoptosis, and inhibition of angiogenesis. Numerous studies have demonstrated that IFN-γ can enhance the regression of oral malignancy via several different mechanisms.16,17 Interleukin-2 (IL-2) can restore the activity of FBXO38 and return PD-1 to normal levels, thus improving the antitumor function of T cells. IL-2 is already a useful drug for melanoma and kidney cancer.14,15
Therefore, the main objective of this study was to evaluate and verify the impact of anti-PD-1 treatment on the immune status and prognosis of patients with OSCC. Another objective was to look for possible associations between IFN-γ and IL-2 levels, numbers of CD4+ T and CD8+ T cells, and anti-PD-1 therapy. This study will provide new ideas for the targeted therapy of OSCC.
Methods
Patients and Informed Consent
A total of 120 OSCC patients with diagnoses confirmed by pathological analysis were enrolled for this prospective study. The patients were seen at the Fourth Hospital of Hebei Medical University, China, between March 2017 and February 2018. Only those patients who had not received antitumor treatment before admission were included, and those with systemic diseases were excluded. Incidence locations included tongue, cheek, gingiva, and mouth floor. The clinical stage of the patients was determined according to the eighth edition of the American Joint Committee on Cancer (AJCC) Staging Manual, Head and Neck Section,18 and only those at clinical stages III and III were included in the study. The patients were divided into two groups: A non-anti-PD-1 therapy group (60 cases) and an anti-PD-1 therapy group (60 cases). The anti-PD-1 drug used was pembrolizumab, which was administered intravenously over a period of 30 minutes under the supervision of a physician experienced in the treatment of cancer. Palivizumab was administered in 200-mg doses every 3 weeks or 400-mg doses every 6 weeks. Patients in the non-anti-PD-1 treatment group were treated with conventional chemotherapy with paclitaxel, administered intravenously at a dose of 135–175mg/ m2 over 3 hours, and repeated every 3 weeks. For the 60 patients who were treated with anti-PD-1 immunotherapy, 1 mL of fresh peripheral blood was taken 2 days before and 20 days after the treatment. Heparin was used as an anticoagulant. Heparin is composed of two polysaccharides alternately linked to form a polymer and exhibit in vivo and in vitro anticoagulant action. The most important anticoagulation effect depends on antithrombin III. After combining with antithrombin III, the configuration of antithrombin III changes, and it can no longer participate in the coagulation pathway. Heparin can also bind with various coagulation factors, inhibiting their action.
This study complied with the Declaration of Helsinki, and it was approved by the Human Ethics Committee of the Fourth Hospital of Hebei Medical University, and written informed consent was obtained from all patients and their families.
Immunological Detection
The percentages of CD4+ and CD8+ T lymphocytes were measured by flow cytometry, and the ratio of CD4+/CD8+ T cells was calculated. The flow cytometer used was an EPICS XL (Beckman-Coulter Corp., USA) with its supporting data processing system, SZ-1 rapid mixer, and clean bench (BIOLOGICAL Safety Cabinets ClassII, USA). Mouse anti-human CD4-FITC monoclonal antibody and mouse anti-human CD8-PE monoclonal antibody were used for the detection of CD4+ and CD8+ T lymphocytes, respectively.
The excitation source was a 15 mW argon ion laser with an excitation wavelength of 488 nm. Immunofluorescence analysis was performed using Expo32ADC software. Flow-checkTM Flourpheres (10 µm) fluorescent microspheres (REF 6605359 Beckman-Coulter, Inc. Fullerton, CA92835) were used to verify the instrument optical alignment and fluidics; CV was adjusted to less than 2%.
Measurement of IFN-γ and IL-2 Levels
The human IFN-γ ELISA kit (ab174443, abcam) and human IL-2 ELISA kit (PD2050, Bio-Techne China Co. Ltd.) were used according to the manufacturer’s instructions. The OD values of samples were substituted into the curve to calculate the concentrations, and the results were exported as Microsoft Excel files.
Recording of Clinical Indicators
Sex, age, tumor size, lymphatic metastasis, and other basic demographic and clinical characteristics of patients with OSCC were recorded. Tpatients were divided into stage III or IV based on the clinical stage of the tumor. Methods for determining lymphatic metastasis included physical, imaging, and pathological examinations. Lymph node metastasis results in a noticeable increase in lymphoid tissue or lymph node morphogenesis abnormalities. When deemed appropriate, a lymphatic puncture biopsy was performed to clarify the nature of the cancer. Lymphatic puncture biopsy can help in the preliminary assessment of lymph node hyperplasia and enlargement. Lymphatic puncture biopsy is generally the gold standard for the diagnosis of various diseases, including malignant tumors. It has the advantages of being rapid, simple, economical, minimally invasive, and has a low false positive rate. The lymph node biopsies were sent for pathological analysis to obtain the histopathological diagnosis of the tumor. The results of the histopathological examination can be used to determine the treatment plan of the patient. Clinical experience shows that the pathological analysis results are quite accurate and can be used to clarify the nature of the disease and determine the treatment. Two senior doctors confirmed the diagnosis based on the results of the lymphatic puncture biopsy and pathological examination combined with the clinical symptoms and the results of CEA, SCC, Sifra, and other examinations. We analyzed these clinical data to explore the effect of PD-1 immunotherapy on the survival time of patients.
Statistical Analysis
The data were expressed as percentages. Pearson’s chi-squared test was used to determine the associations between the clinical parameters and gene expression levels. Spearman correlation test was used to study the correlation between the clinical data and anti-PD-1 therapy. Univariate and multivariate Cox regression analysis was used to calculate the hazard ratio (HR) of survival time for potential correlation factors. All statistical analyses were performed using SPSS software, version 21.0 (IBM Corp., Armonk, NY, USA). A p-value <0.05 was considered significant.
Results
Associations Between Baseline Information and Anti-PD-1 Therapy Based on χ2 Test results
Table 1 summarizes the possible relationship between various clinical and demographic factors and anti-PD-1 therapy, as determined by Pearson’s chi-square test. A higher survival time (P<0.001) was seen in the anti-PD-1 therapy group. No correlation was found between the survival time and sex (P=0.714), age (P=0.144), tumor size (P=0.680), TNM classification (P=0.831), and lymphatic metastasis (P=0.705).
 |
Table 1 Relevant Clinical Characteristics of Oral Squamous Cell Carcinoma |
Effect of Anti-PD1 Therapy on the Expression of IFN-γ, IL-2, and CD4+ and CD8+ Lymphocytes
The data on the expression of IFN-γ, IL-2, and numbers of CD4+T and CD8+ T cells in the two groups are presented in Table 2. The mean levels of IFN-γ and IL-2 and the number of CD4+ T cells were significantly increased in the anti-PD-1 group compared with the control group. However, the mean number of CD8+ T cells was decreased in the anti-PD-1 therapy group (Table 2).
 |
Table 2 Changes in Immune Function of 120 Oral Cancer Patients Before and After Treatment |
Associations Between Patient Characteristics and Anti-PD-1 Therapy by Spearman Correlation Test
Spearman correlation coefficient analysis was also performed to determine the factors that have a significant impact on the survival time in the two groups. The results show that there was a significant correlation between PD-1 and OS (ρ=0.735, P<0.001). However, sex (ρ=0.034, P=0.716), age (ρ=−0.133, P=0.147), tumor size (ρ=−0.038, P=0.683), TNM classification (ρ=0.019, P=0.833), and lymphatic metastasis (ρ=0.035, P=0.708) showed no correlations with the OS in the patients undergoing anti-PD-1 therapy (Table 3).
 |
Table 3 The Relationship Between Characteristics of Patients and PD-1 Therapy |
Univariate Cox Regression for Analysis of Factors Correlated with 5-Year Overall Survival in Patients with OSCC
Table 4 shows the HRs and 95% confidence intervals (95% CI) between the clinically relevant factors and OS in patients with OSCC. The OS of patients who were not treated with anti-PD-1 therapy was significantly lower than those receiving anti-PD-1 therapy, with an HR of 0.114 (95% CI, 0.066–0.196, P<0.001). However, sex (HR=1.159, 95% CI, 0.781–1.721, P=0.464), age (HR=1.475, 95% CI: 0.994–2.190, P=0.054), tumor size (HR=1.283, 95% CI: 0.824–1.995, P=0.270), TNM classification (HR=0.870, 95% CI: 0.553–1.370, P=0.848), and lymphatic metastasis (HR=0.968, 95% CI: 0.647–1.450, P=0.876) showed no significant correlation with the OS in patients with oral cancer.
 |
Table 4 Characteristics and Their Effect on OS of Oral Squamous Cell Carcinoma Patients Based on Univariate Cox Proportional Regression Analysis |
Multivariate Cox Regression for Analysis of Factors Correlated with 5-Year Overall Survival in Patients with OSCC
All risk factors were incorporated into a multivariate Cox regression model to effectively control for the influence of confounding factors, which could also be used to predict independent risk characteristics. Table 5 shows the results of the multivariate Cox proportional regression analysis. The OS of patients with OSCC was significantly associated with anti-PD-1 therapy, with an HR of 0.110 (95% CI: 0.062–0.195; P<0.001) (Table 5).
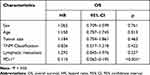 |
Table 5 Characteristics and Their Effect on OS Based on Multivariate Cox Regression Analysis |
Survival Time Analysis
Kaplan–Meier curve analysis showed that the survival time of the anti-PD-1 group was significantly higher than that of the non-anti-PD-1 group (HR=0.114, P<0.001) (Figure 1).
 |
Figure 1 The effects of anti-PD-1 therapy and non-anti-PD-1 therapy on survival time were compared by plotting Kaplan–Meier curves. |
Discussion
Epidemiologic and molecular biological studies have shown that inflammation significantly increases the risk of OSCC.2 The regulation of the inflammatory response is closely related to the activation of T cells. In recent years, immunotherapy has been increasingly used for the treatment of various cancers, with PD-1/PD-L1 inhibitors among the most widely used treatments. PD-1 and PD-L1 are located on the surface of activated T lymphocytes and tumor cells, respectively. Once the PD-1 binds to PD-L1, T lymphocytes treat tumor cells as normal body cells, and do not attack them. Therefore, anti-PD-1 therapy is aimed at changing the inherent relationship between the immune cells and tumor cells, changing the microenvironment of tumor cells, and stimulating T cells to attack tumor cells. PD-L1 is not only overexpressed on the surface of tumor cells but also on the surface of immune cells in the tumor microenvironment, including regulatory T cells (Tregs) and NK cells.19–21 Activated Tregs play an immunosuppressive role and are one of the key factors determining tumor immune escape. Tregs can migrate to inflammatory sites and inhibit a wide range of effector lymphocytes, especially helper T (Th) cell subsets. Foxp3+ is the most specific marker found on Tregs, and Foxp3+ Tregs inhibit the activation, proliferation, and effector functions of many cell types, including CD4+ T cells, CD8+ T cells, dendritic cells (DCs), B cells, and NK cells.
Studies have shown that PD-L1 expression in OSCC is significantly higher than that in the normal oral mucosa. The expression of PD-L1 in peripheral blood of OSCC patients with lymph node metastasis is significantly upregulated compared with that in patients without metastasis, suggesting that the expression of the immune checkpoints of the whole system should be considered in addition to the local tumor.22 Malaspina et al confirmed that PD-1 levels in the peripheral blood of OSCC patients and precancerous actinic cheilitis (AC) patients were significantly higher than those in healthy individuals, while the expression levels of PD-1 on the surface of CD4+ and CD8+T cells in OSCC patients were higher than those in AC patients.23
The results of our study showed that anti-PD-1 therapy was closely associated with survival time. Baseline information and Cox regression analysis showed that the survival time of patients in the anti-PD-1 group was significantly higher than that in the non-anti-PD-1 group, suggesting that PD-1 is an important risk factor for the survival and prognosis of OSCC patients. We also found that anti-PD-1 therapy can improve T-cell immunity and activate T-cell activity.24 Currently, blocking PD-1 and its ligand PD-L1 is one of the most promising ways to activate antitumor immunity, which has achieved great success in a variety of malignant tumors.25 PD-1 is expressed on the surface of activated T cells and inhibits the proliferation, survival, and effector functions of T cells by transmitting immunosuppressive signals, thus significantly inhibiting tumor-specific immune responses and tumor regression.26,27 It was also found that, with the exception of CD8+ T cells, all other indicators of immune system function increased following anti-PD-1 treatment compared with the control group.
CD4+ and CD8+ T are two subsets of T cells, and the balance between these two cell types enables the body to maintain a stable immune function. The normal value of the CD4+/CD8+ ratio is 1.4 to 2.0. If the ratio is greater than 2.0 or less than 1.4, this usually indicates cellular immune dysfunction. Low or high CD4+/CD8+ ratio is more common in immune deficiency diseases, such as AIDS, malignant tumors, systemic lupus erythematosus, and other immune diseases.28,29 It has been shown that the CD4+/CD8+ ratio is a key factor in many immunological diseases. Tsukamoto et al found that in melanoma mice, CD4+ T-cell infiltration in the tumor tissues, which promotes IFN-γ production in the tumor, can play a synergistic antitumor effect during anti-PD-1/PD-L1 therapy.30 Zhu et al observed that survival rates among breast cancer patients were correlated with a high frequency of CD8+ cytotoxic T cells among the lymphocytes, highlighting the role played by CD8+ T cells in controlling tumor growth and prolonging patient survival.31 Thompson et al found that the greater the density of CD8+ T cells in gastric cancer, the higher the expression of PD-L1, suggesting the possibility of an adaptive immune resistance mechanism.32 Lyford-pike et al used quantitative RT-PCR to study the expression of various immune system effector molecules. They found that compared with PD-L1, the expression of CD8 mRNA in PD-L1 (+) in oropharyngeal carcinoma significantly increased. Tokito et al33 reported that PD-L1 immunodeficiency accompanied by an increase in CD8+ T cells was significantly associated with favorable survival in non-small-cell lung cancer (NSCLC). The number of CD8+ cells increased in the OSCC group, and the number of PD-L1 and Foxp3+ cells lacked correlation. The number of CD8+ cells was negatively correlated with the immune expression of PD-L1, suggesting that tumor-infiltrating CD8+ cells may not have an inhibitory function.
IFN-γ is primarily produced by activated T cells and NK cells, and it interferes with the differentiation of some helper T-cell subtypes, including Th2, Th17, and in particular, Tregs, which are thought to be a key factor in the immune escape of tumor cells. Abnormal IFN-γ promoter methylation may be involved in the development of oral cancer.34 Interestingly, IFN-γ downregulates DSPP and MMP-20 and causes disturbance in endoplasmic reticulum (ER) homeostasis, leading to decreased viability, increased migration, and apoptosis of OSCC cells.
IL-2 can enhance the killing activity of T cells, induce the production of cytotoxic T cells (Tc) and, together with IL-4, IL-5, and IL-6, greatly enhance their killing activity, as well as promote the proliferation and maintenance of the long-term growth of NK cells. One study evaluated the therapeutic effect and mechanism of an engineered IL-2 cytokine prodrug, NKTR-214, combined with anti-PD-1 and anti-CTLA-4 checkpoint-blocking therapy or peptide-based immunization in mice. It was found that NKTR-214 had better antitumor activity than IL-2, could expand antitumor CD8+ T cells throughout the body, and induce Treg consumption in the tumor tissues.35 In summary, IL-2 and IFN-γ do not have a direct anticancer effect but are closely associated with T-cell activity and PD-1 mediated signaling and stimulate the antitumor activity of T cells by regulating related immune mechanisms.
The novelty of the present study lies in the fact that it found anti-PD-1 therapy to be safe, feasible, controllable, and well tolerated. Anti-PD-1 therapy is thus a novel treatment option with high efficiency and low toxicity. The toxicity of anti-PD-1 therapy is generally low. Many patients receiving immunotherapy experience fatigue, itching, rash, and other common drug-related adverse reactions. It can also lead to inflammation, rheumatoid arthritis, and colitis. However, toxicity from anti-PD1 therapy rarely reaches grade 3 to 4 toxicity.36 Patients with a low incidence of severe adverse reactions have a longer period of survival and could benefit considerably.
In conclusion, anti-PD-1 therapy is beneficial to the survival and prognosis of patients with OSCC, improves T-cell immunity, and enhances tumor regression.
Abbreviations
OSCC, Oral squamous cell carcinoma; PD-1, programmed cell death protein 1; PD-L1, programmed cell death 1 ligand 1; OS, overall survival; HR, hazard ratio, 95% CI, 95% confidence interval; NK, natural killer; IL-2, interleukin-2; AJCC, American Joint Committee on Cancer; DC, dendritic cell; AC, actinic cheilitis; ER, endoplasmic reticulum; NSCLC, non-small-cell lung cancer.
Data Sharing Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Consent for Publication
All the authors agreed to be published.
Disclosure
The authors declare that there is no conflict of interest in this work.
References
1. Majchrzak E, Szybiak B, Wegner A, et al. Oral cavity and oropharyngeal squamous cell carcinoma in young adults: a review of the literature. Radiol Oncol. 2014;48(1):1–10. doi:10.2478/raon-2013-0057
2. Inaba H, Sugita H, Kuboniwa M, et al. Porphyromonas gingivalis promotes invasion of oral squamous cell carcinoma through induction of proMMP9 and its activation. Cell Microbiol. 2014;16(1):131–145. doi:10.1111/cmi.12211
3. Thomson PJ. Perspectives on oral squamous cell carcinoma prevention-proliferation, position, progression and prediction. J Oral Pathol Med. 2018;47(9):803–807. doi:10.1111/jop.12733
4. Sieviläinen M, Almahmoudi R, Al-Samadi A, Salo T, Pirinen M, Almangush A. The prognostic value of immune checkpoints in oral squamous cell carcinoma. Oral Dis. 2019;25(6):1435–1445. doi:10.1111/odi.12991
5. Chen Y, Li Q, Li X, et al. Blockade of PD-1 effectively inhibits in vivo malignant transformation of oral mucosa. Oncoimmunology. 2018;7(2):e1388484. doi:10.1080/2162402X.2017.1388484
6. Jiang C, Yuan F, Wang J, Wu L. Oral squamous cell carcinoma suppressed antitumor immunity through induction of PD-L1 expression on tumor-associated macrophages. Immunobiology. 2017;222(4):651–657. doi:10.1016/j.imbio.2016.12.002
7. Wu X, Gu Z, Chen Y, et al. Application of PD-1 blockade in cancer immunotherapy. Comput Struct Biotechnol J. 2019;17:661–674. doi:10.1016/j.csbj.2019.03.006
8. Lenouvel D, González-Moles MÁ, Ruiz-ávila I, Gonzalez-Ruiz L, Gonzalez-Ruiz I, Ramos-García P. Prognostic and clinicopathological significance of PD-L1 overexpression in oral squamous cell carcinoma: a systematic review and comprehensive meta-analysis. Oral Oncol. 2020;106:104722. doi:10.1016/j.oraloncology.2020.104722
9. Troiano G, Caponio V, Zhurakivska K, et al. High PD-L1 expression in the tumour cells did not correlate with poor prognosis of patients suffering for oral squamous cells carcinoma: a meta-analysis of the literature. Cell Prolif. 2019;52(2):e12537. doi:10.1111/cpr.12537
10. Yao H, Wang H, Li C, Fang JY, Xu J. Cancer cell-intrinsic PD-1 and implications in combinatorial immunotherapy. Front Immunol. 2018;9:1774. doi:10.3389/fimmu.2018.01774
11. Sui H, Ma N, Wang Y, et al. Anti-PD-1/PD-L1 therapy for non-small-cell lung cancer: toward personalized medicine and combination strategies. J Immunol Res. 2018;2018:6984948. doi:10.1155/2018/6984948
12. Gordon SR, Maute RL, Dulken BW, et al. PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature. 2017;545(7655):495–499. doi:10.1038/nature22396
13. Zhang P, Ouyang S, Wang J, Huang Z, Wang J, Liao L. 程序性死亡分子1及其配体在口腔鳞状细胞癌患者外周血中的表达及临床意义. [Levels of programmed death-1 and programmed death ligand-1 in the peripheral blood of patients with oral squamous cell carcinoma and its clinical implications]. 华西口腔医学杂志 [Hua Xi Kou Qiang Yi Xue Za Zhi]. 2015;33(5):529–533. Chinese.
14. Buchbinder EI, Dutcher JP, Daniels GA, et al. Therapy with high-dose Interleukin-2 (HD IL-2) in metastatic melanoma and renal cell carcinoma following PD1 or PDL1 inhibition. J Immunother Cancer. 2019;7(1):49. doi:10.1186/s40425-019-0522-3
15. Tegos T, Tegos K, Dimitriadou A, Dimitriadis G. Current and emerging first-line systemic therapies in metastatic clear-cell renal cell carcinoma. J Buon. 2019;24(4):1340–1353.
16. Kondoh N, Mizuno-Kamiya M, Umemura N, et al. Immunomodulatory aspects in the progression and treatment of oral malignancy. Jpn Dent Sci Rev. 2019;55(1):113–120. doi:10.1016/j.jdsr.2019.09.001
17. Sun Y, Liu N, Guan X, Wu H, Sun Z, Zeng H. Immunosuppression induced by chronic inflammation and the progression to oral squamous cell carcinoma. Mediators Inflamm. 2016;2016:5715719. doi:10.1155/2016/5715719
18. Lydiatt WM, Patel SG, O’Sullivan B, et al. Head and neck cancers-major changes in the American Joint Committee on cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67(2):122–137. doi:10.3322/caac.21389
19. Mandal R, Şenbabaoğlu Y, Desrichard A, et al. The head and neck cancer immune landscape and its immunotherapeutic implications. JCI Insight. 2016;1(17):e89829. doi:10.1172/jci.insight.89829
20. Kansy BA, Concha-Benavente F, Srivastava RM, et al. PD-1 status in CD8(+) T cells associates with survival and Anti-PD-1 therapeutic outcomes in head and neck cancer. Cancer Res. 2017;77(22):6353–6364. doi:10.1158/0008-5472.CAN-16-3167
21. Mattox AK, Lee J, Westra WH, et al. PD-1 expression in head and neck squamous cell carcinomas derives primarily from functionally anergic CD4(+) TILs in the presence of PD-L1(+) TAMs. Cancer Res. 2017;77(22):6365–6374. doi:10.1158/0008-5472.CAN-16-3453
22. Weber M, Wehrhan F, Baran C, et al. PD-L1 expression in tumor tissue and peripheral blood of patients with oral squamous cell carcinoma. Oncotarget. 2017;8(68):112584–112597. doi:10.18632/oncotarget.22576
23. Malaspina TS, Gasparoto TH, Costa MR, et al. Enhanced programmed death 1 (PD-1) and PD-1 ligand (PD-L1) expression in patients with actinic cheilitis and oral squamous cell carcinoma. Cancer Immunol Immunother. 2011;60(7):965–974. doi:10.1007/s00262-011-1007-5
24. Jiang Y, Chen M, Nie H, Yuan Y. PD-1 and PD-L1 in cancer immunotherapy: clinical implications and future considerations. Hum Vaccin Immunother. 2019;15(5):1111–1122. doi:10.1080/21645515.2019.1571892
25. Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–2454. doi:10.1056/NEJMoa1200690
26. Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi:10.1146/annurev.immunol.26.021607.090331
27. Nishimura H, Agata Y, Kawasaki A, et al. Developmentally regulated expression of the PD-1 protein on the surface of double-negative (CD4-CD8-) thymocytes. Int Immunol. 1996;8(5):773–780. doi:10.1093/intimm/8.5.773
28. Sigel K, Dubrow R. CD4/CD8 ratio and lung cancer risk - Authors’ reply. Lancet HIV. 2017;4(3):e103–103e104. doi:10.1016/S2352-3018(17)30026-7
29. Yin Y, Qin J, Dai Y, Zeng F, Pei H, Wang J. The CD4+/CD8+ ratio in pulmonary tuberculosis: systematic and meta-analysis article. Iran J Public Health. 2015;44(2):185–193.
30. Tsukamoto H, Fujieda K, Miyashita A, et al. Combined blockade of IL6 and PD-1/PD-L1 signaling abrogates mutual regulation of their immunosuppressive effects in the tumor microenvironment. Cancer Res. 2018;78(17):5011–5022. doi:10.1158/0008-5472.CAN-18-0118
31. Zhu J, Liao M, Yao Z, et al. Breast cancer in postmenopausal women is associated with an altered gut metagenome. Microbiome. 2018;6(1):136. doi:10.1186/s40168-018-0515-3
32. Thompson ED, Zahurak M, Murphy A, et al. Patterns of PD-L1 expression and CD8 T cell infiltration in gastric adenocarcinomas and associated immune stroma. Gut. 2017;66(5):794–801. doi:10.1136/gutjnl-2015-310839
33. Tokito T, Azuma K, Kawahara A, et al. Predictive relevance of PD-L1 expression combined with CD8+ TIL density in stage III non-small cell lung cancer patients receiving concurrent chemoradiotherapy. Eur J Cancer. 2016;55:7–14. doi:10.1016/j.ejca.2015.11.020
34. Tian S, Jiang C, Liu X, et al. Hypermethylation of IFN-γ in oral cancer tissues. Clin Oral Investig. 2017;21(8):2535–2542. doi:10.1007/s00784-017-2052-z
35. Sharma M, Khong H, Fa’ak F, et al. Bempegaldesleukin selectively depletes intratumoral Tregs and potentiates T cell-mediated cancer therapy. Nat Commun. 2020;11(1):661. doi:10.1038/s41467-020-14471-1
36. Naidoo J, Page DB, Li BT, et al. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann Oncol. 2015;26(12):2375–2391. doi:10.1093/annonc/mdv383
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
