Back to Journals » Journal of Pain Research » Volume 12
Anti-nociceptive and anti-inflammatory activities of crude root extract and solvent fractions of Cucumis ficifolius in mice model
Authors Demsie DG , Yimer EM , Berhe AH , Altaye BM , Berhe DF
Received 1 November 2018
Accepted for publication 18 February 2019
Published 30 April 2019 Volume 2019:12 Pages 1399—1409
DOI https://doi.org/10.2147/JPR.S193029
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor E Alfonso Romero-Sandoval
Desalegn Getnet Demsie,1,2 Ebrahim M Yimer,1 Abera Hadgu Berhe,1 Birhanetensay Masresha Altaye,3 Derbew Fikadu Berhe1
1Department of Pharmacology and Toxicology, College of Health Sciences, Mekelle University, Mekelle, Ethiopia; 2Department of Pharmacy, College of Medicine and Health Sciences, Adigrat University, Adigrat, Ethiopia; 3Department of Pharmacy, College of Medicine, Debre Berhan University, Debre Berhan, Ethiopia
Background: Societies in developing countries use traditional medicine as alternatives for management of pain and inflammation. The plant Cucumis ficifolius has been used in Ethiopia to treat many ailments including inflammation and pain. The objective of this study was to evaluate the antinociceptive and anti-inflammatory activities of the crude root extract and solvent fractions of C. ficifolius.
Methods: The analgesic activity of crude extract and solvent fractions of C. ficifolius was evaluated with acetic acid-induced writhing, hot plate, and formalin-induced paw licking tests. The anti-inflammatory effect of crude methanolic root extract and solvent fractions of C. ficifolius was evaluated using carrageenan-induced paw edema. The crude extract was given at 200, 400 and 800 mg/kg. Butanol and aqueous fractions were given at 100 and 200 mg/kg doses. The negative control groups were treated with distilled water (10 mL/kg). Standard drugs used wereacetylsalicylic acid(ASA) in acetic acid, formalin tests and carrageenan-induced paw edema and morphine (20 mg/kg) in hot plate test.
Results: The crude extract, at its maximum dose, produced comparable analgesic activity (72.5%) to ASA in acetic acid writhing test. In the hot plate test, both the crude extract and solvent fractions exhibited a significant prolongation of nociception reaction time. Formalin test result indicated a significant reduction of mean lick time with maximal protection of 64% (early phase) and 83% (late phase). Aqueous and butanol fractions showed good analgesic activity in the three models. Inflammation was decreased by 69% with butanol (200 mg/kg); 71% (800 mg/kg) of crude extract and by 41% and 56% with the use of aqueous fraction at 100 and 200 mg/kg, respectively (p<0.001).
Conclusion: The present study indicates that the crude methanolic root extract, as well as butanol and aqueous solvent fractions, showed anti-nociceptive and anti-inflammatory activities.
Keywords: hot plate test, writhing test, paw edema, formalin test, carrageenan, 80% methanol
Introduction
Pain and inflammation impose an enormous economic and health problem globally. They are associated with a number of adverse health consequences including the inability to carry out daily activities, impaired patients’ quality of life and risk of all-cause mortality.1
Currently, a number of drug classes are available to manage inflammation and pain. However, the clinical uses of anti-inflammatory and analgesic agents are limited by their affordability, accessibility, and adverse drug reactions, and many medicines are not effective as expected in all patients.2 Tolerance and dependence is another challenge, particularly with the chronic use of opioids.3
Pharmaceutical development has led to a great number of medicines, however many of them have limmitted efficacy; patients can encounter escalating health-care costs; and adverse drug reactions are reported in some patients.4–6 As a result, there is an increased tendency to use traditional medicine, with an extent not less than 80% worldwide.40
Plants have been used since ancient times to treat diseases and infections. Medicinal plants are cheap, easily available and affordable.7,8 Besides traditional use, scientific study of medicinal plants has found herbal medicines to be a medicinal resource for drug discovery.9
A variety of medicinal plants have been used to treat pain and inflammation. These plants include Malva verticillata, Otostegia integrifolia,10 croton macrostchyus,11 Ocimum suave,5 Cucumis ficifolius,12 Arisaema schimperianum, Euclea racemosa, Malva verticillata,13 Impatiens tinctoria,14 Balanites, Ehretia cymosa,15 and a host of others.
Cucurbit plants (cucurbitaceace) are cultivated widely in the subtropical and tropical countries. They have demonstrated anti-inflammatory, antitumor, hepato-protective, cardiovascular, immunoregulatory, anti-fungal, antibacterial, anti-viral, anti-diabetic, anti-tumor and anti-HIV/AIDS activities.16 The diverse biological actions of the Cucurbitaceae family is believed to be due to the presence of different bioactive constituents such as cucurbitacins, triterpenes, sterols, alkaloid, saponins, tannins, flavonoids, and phenolic compounds.17 Cucumis melo extract demonstrated antioxidant and anti-inflammatory properties.18 Methanolic extract of Cucumis colossus exhibited significant analgesic anti-inflammatory activity.19
In this study, the roots of Cucumis ficifolius (Cucurbitaceace) was collected from Kilteawlaelo district in Ethiopia in 2017. This pre-clinical study was performed 1) to predict the safety and efficacy data from animal models to support further research in human beings; and 2) to check the relationship between traditionally claimed use and scientific research. The plant is known locally in Amharic as “yemdir enbuay”, and in Tigrigna as “Rambo-ambo”. It is found widely distributed in East Africa, especially in Ethiopia and Kenya between altitudes of 1,000 and 2,700 m. It is perennial, usually prostrate herb that stems up to 1 m long, deeply lobed leaves and small, yellow flowers followed by oblong, dark yellow fruits to about 5 cm long that are covered in bristly pustules.20,21
The root of C. ficifolius is chewed with the diseased teeth to treat toothache; crushed, macerated with water, filtered and the fluid is drunk to treat joint pain; mixed with bark of Croton macrostachyus, the dried paste is mixed with butter and drunk or the product is chewed and then the fluid is drunk to treat stomach ache.12 The plant is also used to treat bloody diarrhea, evil eye, lifie (wound) and expel ear-mites.22 An 80% methanolic extract of C. ficifolius showed significant anti-oxidant and hepatoprotective activities.23 Phytochemical screening demonstrated that C. ficifolius constituted secondary metabolites such as, phenols, flavonoids, terpenoids, steroids and saponins,23 which might confer anti-oxidant and hepatoprotective effects.
Thus, the aim of this study is to investigate the anti-inflammatory and anti-nociceptive activities of C. ficifolius.
Materials and methods
Chemicals and drugs
Chemicals and solvents of laboratory and analytical grade were used throughout this project: carrageenan (Tokyo Chemical Industries, Tokyo, Japan), methanol (Alpha Chemika, Mumbai, India), n-butanol (Carlo Erba Reagents SAS, Val de Reuil, France), chloroform (Labort Fine Chem Pvt Ltd, Surat, India), acetylsalicylic acid (ASA) (Addis Pharmaceutical Factory, Adigrat, Ethiopia/APF; Batch no., CA1704016), acetic acid (Thermo Fisher Scientific, Waltham, MA, USA), morphine (Amino Ltd, Neuenhof, Switzerland; Batch no., 6910/10) and formalin (Albert Rose Chemicals IP Ltd, Ahmedabad, India) were used.
Collection, identification, and preparation of plant materials
The roots of C. ficifolius were collected from Kilte Awlaelo district of Tigray Regional State, 47.7 km from Mekelle and 829 km from Addis Ababa. Identification and authentication of the plant were carried out by Dr Getnet Masresha, and sample specimen was deposited at Herbarium unit of Department of Biology, College of Computational and Natural Science, University of Gondar for future reference with voucher number of DG0024/210.
Preparation of extracts and fractions of plant material
The roots were cleaned of dust and debris and washed gently with water. The root was reduced to an appropriate size and air dried under shade for two weeks. The dried root material was then pulverized with a grinder. A total amount of 1.335 kg dried root powder was macerated in methanol (80%) (1:6) with occasional shaking using orbital shaker at 120 rotations per minute for 72 h.24 The mixture was first filtered using muslin cloth followed by Whatman filter paper no.1. To maximize yield, re-maceration was done for another 72 h twice. The filtrates were then evaporated with an oven dryer at 40°C until dried. The 80% methanolic crude extract of C. ficifolius was gummy and yellowish in color with 5.69% (w/w) yield. Among the fractions, the highest yield was found from the aqueous fraction. The dried powder of the methanolic crude extract was kept in a refrigerator at 4ºC in air-tight containers until use.
The liquid–liquid extraction was performed three times starting from chloroform then n-butanol. After collecting the chloroform and n-butanol fractions, the remaining residue was considered as an aqueous fraction. An oven dryer at 40°C was used to concentrate the fractions. The dried matter was stored in a refrigerator at 4ºC until use.
Experimental animals
The experiments were performed on an equal number of male and female in-house bred Swiss albino mice age of 6–8 weeks and weight of 25–35 g. The animals were housed in polypropylene cage and maintained under standard animal housing condition (at ambient temperature, and with a 12 h light-dark cycle) and allowed free access to the standard pellet diet and water ad libitum. The animals were acclimatized to laboratory conditions for seven days prior to initiation of the experiment. All procedures have been undertaken as per the guidelines for the care and use of laboratory animals.25 The protocol was approved by the Health Research Ethics Review Committee HRERC, College of Health Sciences, Mekelle University with protocol number (ERC1206/2018). Animals were sacrificed under anesthesia after completion of the experiments.
Acute oral toxicity test
The acute oral toxicity test was performed on five female Swiss albino mice using the limit test recommendations of the Organization for Economic Co-operation and Development (OECD) 425 Guideline.25 For this test, we used healthy, nulliparous and non-pregnant female Swiss albino mice (age of 8–12 weeks) weighing 20–30 g. On day one, a four-hour fasted mouse was given 2,000 mg/kg (1 mL/100 g) of the extract orally in distilled water. The mouse was observed for 24 h for possible behavioral or physical changes. More emphasis was given to the first 4 h. After 24 h, four other mice were given the same dose and observed for 14 days for manifestations such as tremors, convulsions, salivation, diarrhea, lethargy, sleep and coma.
Anti-nociceptive activity
Acetic acid-induced writhing test
As described by Birhane et al, the test was carried out to assess the peripheral analgesic action of C. ficifolius.26 Mice of either sex (26–35 g) were used. Animals fasted overnight (for 12 h) were randomly assigned into five groups for crude extract and six groups for solvent fractions. In each group we used six mice.
The first group of mice was given distilled water (DW) 10 mL/kg as a negative control. Groups II, III and IV were administered the crude methanolic root extract at 200, 400, and 800 mg/kg, respectively, and the fifth group received 150 mg/kg of ASA. In solvent fractions, six groups of animals were used: Group I received DW (10 mL/kg); Group II were challenged with 100 mg/kg and Group III with 200 mg/kg of aqueous fraction. Mice under group IV and V received 100 mg/kg, and 200 mg/kg of butanol fraction, respectively. The positive control group (Group VI) were challenged with ASA 150 mg/kg. Thirty minutes after administration of vehicle, standard drug and test substance; the animals were subjected to intraperitoneal (i.p.) injection of acetic acid solution (0.6%, 10 mL/kg).
Nociception response was quantified after 5 min of latency for 20 min. The observation for the response includes stretching of the hind limbs, contraction of abdominal muscles and arching of the back. Percent protection was calculated by applying the formula:27
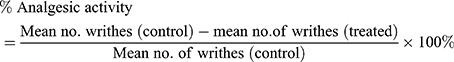
Hot plate test
The hot plate test was used to evaluate the central anti-nociceptive action of the plant material.28 The hot plate apparatus was maintained at 55±0.1°C. Overnight fasted mice were randomly assigned into different groups. The positive control group received morphine 20 mg/kg orally. The animals were treated with the vehicle, standard and test substances as described in the acetic acid-induced writhing test.
The pre-drug nociception latencies were measured twice at 15 min intervals. The first reading was discarded to avoid the effect of stress on the nociception latency values, and the second reading was used as a pre-drug latency.28 Then post-drug latencies were quantified at 30, 60, 90 and 120 min after administering standard drug, test substance or vehicle.29 A cutoff time of 20 s was considered by taking three times the mean pre-drug latency to minimize tissue damage. The nociceptive latency in seconds was quantified by taking the time interval between the animal reaching the hot plate and licking its paw or jumping off the hot plate.
The post-drug latency: T1 was estimated according to the reaction time of each mouse at 30, 60, 90 and 120 min after treatment. T0 represented the mean pre-drug latencies. For each group, the percentage of protection against thermal stimulus was determined by using the formula:30
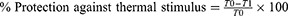
Formalin test
As described by Hunskaar et al, a formalin-induced lick test was performed.31 Mice fasted overnight with the provision of water were used. Then the overnight fasted mice were randomly selected and assigned into groups of five, each group with six mice. Group I receiving DW (10 mL/kg) was assigned as the negative control while mice receiving ASA at a dose of 200 mg/kg served as positive control (group II). The remaining groups (III to V) were given the test extract at a dose of 200, 400 and 800 mg/kg, respectively. To test the anti-nociceptive activity of aqueous and butanol fractions six groups with six mice each were used. Group I received distilled water (10 mL/kg); group II was given ASA 200 mg/kg and the remaining four groups were treated with 100 and 200 mg/kg dose of aqueous and butanol fractions. Before starting the experiment, mice in each group were allowed 20-min acclimatization in a transparent observation cage.32 After acclimatization, formalin solution (0.02 mL of 5%) was administered by intraplantar injection into the mice dorsal surface of the right hind paw.
Two phases of nociception, namely the early and late phase, were observed during the course of the experiment. The first phase was recorded by taking the time of the animals spent licking their paw for 0–5 min after the injection of formalin.33 The second phase was recorded by taking the time the animal spent licking its paw for 15–30 min after formalin injection.34,35 The percentage inhibition of nociception for the two phases was calculated using the following formula:32
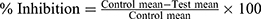
Anti-inflammatory activity
Swiss albino mice fasted overnight were randomly assigned into nine groups each with six mice. Thirty minutes before injection of carrageenan, DW (10 mL/kg), the test substances and ASA (200 mg/kg) were administered orally. The rest of the seven groups were treated with crude extract at doses of 200, 400 and 800 mg/kg, and butanol and aqueous fractions at a dose of 100 and 200 mg/kg. Just before induction of inflammation, the leg of each mouse was marked on the skin over the lateral maleous.36 The basal volume of the right hind paw of individual mice was measured with a digital plethysmometer (PLM 02). Then, a 0.05 mL of 1% carrageenan in normal saline was injected into the dorsal surface of the right hind paw. The volume of injected paw was measured at 1, 2, 3 and 4 h after carrageenan injection.
Paw diameter before carrageenan injection was compared with the same paw diameter after administration of carrageenan by calculating the percentage inhibition applying the following formula:5

Where: Vt = post-carrageenan injection mean paw volume in treated and negative control groups at time t and Vo = pre-carrageenan injection mean paw volume in treated and negative control groups.
In addition, the increase in paw volume, ie, inflammation expressed in percentage was calculated according to the formula given by:5
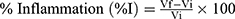
Where:, Vi is the initial mean paw volume before carrageenan injection and Vf is the final volume (after carrageenan injection).
Statistical analysis
Data were entered and analyzed with the IBM statistical package for social sciences (SPSS) version 21 (IBM Corporation, Armonk, NY, USA). The data obtained in the study were tabulated and expressed as mean ± standard errors of the mean (SEM). The statistical analysis was carried out using one-way analysis of variance (ANOVA) followed by Tukey post-hoc test to compare variations among groups. The result was considered significant when P<0.05.
Results
Oral acute toxicity test
Acute toxicity studies revealed that administration of the crude extract of C. ficifolius (at a dose of 2,000 mg/kg) did not cause manifestations such as drowsiness, salivation, tremor, restlessness, convulsion, piloerection, diarrhea, nor caused mortality in the first 24 h observation as well as during the two-week follow up period.
Analgesic activity
Writhing test
The reduction in the mean number of writhes produced by all dose levels of crude methanolic extract, aqueous and butanol fractions of C. ficifolius were significant compared to negative control. The percentage pain protection produced by the use of the crude at three doses (200, 400 and 800 mg/kg), and 200 mg/kg of ASA was 24.9% (P<0.05), 48.7% (P<0.001), 72.5% (P<0.001) and 80.4% (P<0.001), respectively, as compared to the vehicle received group. As illustrated in Figure 1, 800 mg dose of crude methanolic extract of C. ficifolius revealed a comparable reduction in the mean number of writhes to ASA.
The butanol fraction resulted in greater reduction of the frequency of writhing response with the protection of 35% (P<0.01) and 68% (P<0.001) in the lower and higher dose levels, respectively. And also, a significant reduction in the mean number of writhing was observed at the dose of 100 mg/kg (33%, P<0.01) and 200 mg/kg (62%, P<0.001) of aqueous fraction (Figure 2).
Hot plate test
The crude root extract and solvent fractions of C. ficifolius increased the latency of pain response at all dose levels. Treatment of mice with 200 and 400 mg/kg crude extract significantly increased the latency to the maximum record of 10.12 (46.62%) and 11.16 (52.88%) seconds at 120 min of observation compared to control, respectively. The highest dose level (800 mg/kg) showed greater protection at all times of observation in relation to the vehicle group with maximum pain protection (82%, P<0.001) at 90 min (Table 1). The mean difference between the middle dose and lowest dose levels was not significant throughout the observation. However, 800 mg/kg of the crude root extract showed a significant mean difference compared to post-drug values of the lowest dose while a significant difference was obtained at 90 min (P<0.01) compared to the middle dose.
 | Table 1 Anti-nociceptive effect of Cucumis ficifolius methanolic crude root extract in hot plate test |
With regard to the aqueous and butanol fractions, the delay in nociception reaction was significant with the exception of 100 mg/kg of the aqueous fraction at 30 min, compared to the negative control. Nevertheless, effects attributed to the use were within a considered level of significance in time intervals beyond 30 min (Table 2). The remaining doses of the two fractions significantly prolong the nociception latency in a dose-dependent manner. The aqueous fraction, 100 mg/kg (60%, P<0.01), 200 mg/kg (86.8%, P<0.001) and butanol 100 mg/kg (76%, P<0.001) resulted in greater protection at 90 min, whereas 200 mg/kg butanol fraction provided greater protection at 60 min (89.8%, p<0.001).
 | Table 2 Anti-nociceptive effect of aqueous and butanol fractions of Cucumis ficifolius on hot plate test |
Formalin test
The crude methanolic root extract of C. ficifolius significantly diminished the mean time of animals spent licking the injected paw in the two phases. In the early phase, the 200, 400 and 800 mg/kg doses of the test extract shortened the time of licking by 26%, 53%, and 64%, respectively. In the late phase, percentage protection of 46%, 67%, and 83% were recorded from the lower to the higher dose levels. The 400 (53%) and 800 (64%) mg/kg crude extract exhibited better activity in the early phase of the test as compared to the standard drug, ASA (31%). Although statistically not significant, 800 mg/kg methanolic extract of C. ficifolius exhibited greater pain protection (83%) compared to ASA (72%) in the late phase (Table 3). The analgesic gap between ASA and different doses of the test extract was wider, especially in the initial phase.
 | Table 3 Anti-nociceptive activity of 80% methanolic crude root extract of Cucumis ficifolius in formalin-induced lick test |
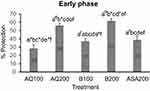
The aqueous fraction at 100 mg/kg and 200 mg/kg decreased the time of licking by 28% and 56%, respectively, in the early phase while butanol fraction showed higher protection action as compared to the aqueous fraction. In the early phase, 37% and 62% of pain protection were attained with the lower and higher doses of butanol fraction (Figure 3). In the late phase, the aqueous fraction decreased the time by 29% and 57% while butanol fraction reduced by 41% and 69% at the respective lower and higher dose levels (Figure 4).
Anti-inflammatory activity test
As indicated in Table 4, all the tested doses of the crude extract significantly decreased paw volume compared to negative control. The lower dose of methanolic extract achieved a minimum (22%) anti-inflammatory activity at the first hour and maximum value (40%) at the fourth hour while the middle and higher doses inhibit inflammation by 56% (P<0.001) and 71% (P<0.001), respectively, at the fourth hour.
 | Table 4 Anti-inflammatory activity of 80% methanolic extract and solvent fractions of Cucumis ficifolius on carrageenan-induced paw edema |
The aqueous and butanol fractions produced an inhibitory action in all times of observations. At the first hour, the 100 mg/kg of aqueous and butanol fractions inhibited inflammation by 24% and 34%, respectively. On the other hand, maximum activity was recorded with the same doses at the fourth hour with the aqueous fraction (41%, P<0.001) and butanol fraction (53%, P<0.001). The 200 mg/kg of the two fractions attained greater anti-inflammatory action as compared to the lower dose levels and were statistically significant as compared to the negative control group. The aqueous fraction produced 56% (P<0.001) and butanol fraction resulted in 69% (p<0.001) anti-inflammatory activity at the fourth hour (Table 4).
Discussion
In this study, the potential anti-nociceptive and anti-inflammatory effects of crude methanolic extract and solvent fractions of C. ficifolius were investigated using different animal models. In the acetic acid, hot plate and formalin nociception models the test substances significantly provided pain protection. In addition, carrageenan-induced paw edema model was used to evaluate the anti-inflammatory activity of the test substances.
The abdominal constrictions response induced by acetic acid is a sensitive procedure to evaluate peripherally acting analgesics, and a primary tool for screening analgesic potential of test compounds.37 Intraperitoneal injection of acetic acid causes irritation in the peritoneal cavity where various endogenous inflammatory mediators such as histamine, serotonin, bradykinin substance P, and prostaglandins are released. These inflammatory mediators sensitize C fibers in acetic acid-induced visceral pain.38 The stimulation of the nerve endings of the primary afferent nerves produces pain characterized by constriction of abdominal muscle with the extension of the forelimbs and elongation of the body.37,39
The percentage pain protection produced by the use of the crude extract at three doses (200, 400 and 800 mg/kg) was 24.9% (P<0.05), 48.7% (P<0.001) and 72.5% (P<0.001), respectively, as compared to the vehicle group (Figure 1). Butanol and aqueous solvent fractions also exhibited a greater reduction of the frequency of writhing response (Figure 2). Analgesic effect of the crude extract and solvent fractions in this study probably linked to C fibers mediated via inhibition of prostaglandins histamine, serotonin bradykinin, and substance P.
The second pain model used was the hot plate test which is a thermic stimulus involving stimulation of Aδ fibers. In this test two behavioral responses, namely paw licking and jumping, are produced. The two responses are supraspinally integrated, and sensitive to opioid analgesics.38,40 The plate was maintained at 55ºC, and at this temperature only opioid-like agents are active.28 In agreement with this, morphine produced significant analgesia at all times of observation. It was selected as a reference drug by considering its advantages like provision of longer analgesia and less variability of response among animals.41
The highest dose (800 mg/kg) of methanol extract achieved maximum prolongation of reaction time similar to the standard drug (morphine). However, the lowest and middle doses (200 and 400 mg/kg) produced maximum protection later in the course of observation (120 min) (Table 1). The 200 mg/kg butanol fraction exhibited more protection at 60 min. Variation in effect may be attributed to the plasma concentration of the test substances.42 . Overall, methanol extract and solvent fractions elicited a considerable nociception latency comparing to the reaction time values to thermal stimulus in the negative control group. Presumably, the effectiveness of the methanolic extract and solvent fractions of the root of C. ficifolius observed in the present study could be due to an opioid-like action via Aδ and C-fibers.
The third model (formalin test) is known for its validity, reliability, and sensitive for various classes of analgesic drugs as compared to other models of nociception.43 Unlike other pain models, it provides a number of advantages, such as little or no restraint, unhindered observation of the complete range of behavioral responses, and greater resemblance to clinical pain.31,44,45
As the nociception response has a biphasic nature with different pain pathways, two phases of behavioral responses were observed after formalin injection. The early phase (neurogenic phase) involves stimulation of nociceptors directly by a chemical and detected by Aδ fibers of the central nociceptive primary afferent terminals. The late phase is due to direct activation of nociceptors by different chemical mediators that resulted in an increased input from C fibers.33,38,39,45–47
The late phase of formalin-induced paw licking behavior can be inhibited by 1) NSAIDs via their action against prostaglandins; 2) steroids, and 3) centrally acting analgesics.43 The first phase response is mediated by substance P, whereas serotonin, histamine, bradykinin, and prostaglandins are reported to involve in the second phase.32 The crude methanolic root extract of C. ficifolius significantly diminished the mean time animals spent licking the injected paw in the early and late phases formalin-induced pain (Table 3). The aqueous fraction at 100 mg/kg and 200 mg/kg decreased the time of licking by 28% and 56%, respectively, in the early phase while butanol fraction showed higher protection action as compared to the aqueous fraction (Figure 3). In the late phase, the aqueous fraction decreased the time by 29% and 57% while the butanol fraction reduced by 41% and 69% at the respective lower and higher dose levels (Figure 4). In formalin-induced pain the mechanism of test substances may involve inhibition of nociception transmission via central pain pathways in the early phase and inhibitory of inflammatory mediators, such as serotonin, histamine, bradykinin, prostaglandins, in the second phase.
The presence of edema is one of the prime signs of inflammation. Carrageenan-induced paw edema is a well-defined model of acute inflammation and a variety of inflammatory mediators participate in its development.48 The evolution of carrageenan-induced acute inflammation indicates the presence of two phases, namely initial and late phases.49
Following injection of carrageenan, inflammatory mediators such as bradykinin, serotonin and histamine contribute the initial phase occurring from 0 to 2.5 h.5,49–51 As indicated in Table 4, the maximum peak of edema was observed at 180 min, which is thought to be due to the release of kinin-like substances, particularly of bradykinin.49 The second phase of edema is a result of overproduction of prostaglandins in tissues and may occur from 2.5 to 6 h post-carrageenan injection.5
The treatments achieved maximum anti-inflammatory activity at the fourth hour. This is supported by reports that the second phase is proven to be sensitive to the commonly used anti-inflammatory drugs.49 COX mediated production of different prostaglandins accounted for the emergence of the late phase of induced inflammation30 but not to lipoxygenase inhibitors.5
Based on the result of the present study, a methanolic crude extract, aqueous and butanol fractions of C. ficifolius significantly decreased paw edema in both phases of carrageenan-induced acute inflammation. This suggests that bioactive constituents in the crude extract and solvent fractions may suppress both phases of acute inflammation by interfering with the release and/or activity of the chemical mediators, such as histamine, bradykinin, and serotonin in the first phase. In the late phase, a reduction in edema may be attributed to COX inhibitory action of 80% methanol extract and solvent fractions.
Previous phytochemical screening on methanol extract has revealed the presence of phenols, tannins, saponins, terpenoids, and flavonoids.23 The butanolic extract of Cucumis sativus demonstrated the presence of flavonoids, saponins, and steroids.52 In addition, carbohydrates, tannins, alkaloids, saponins, flavonoids, glycosides steroids were found in aqueous extract of Cucumis melo.53 The anti-nociceptive and anti-inflammatory effect of many plants has been attributed to their flavonoid, terpenoid, steroid, tannin, phenol, alkaloid and saponin constituents.26 Flavonoids exhibited anti-inflammatory through their free radical scavenging activity, for example, reactive oxygen species (ROS), and interfere with the action of pro-inflammatory cytokines, such as interleukin −6 (IL-6), TNF-α, IL-1β, and nuclear factor-kappa B.27,49 Hence the analgesic and anti-inflammatory activities of C. ficifolius might be due to the presence of the aforementioned phytoconstituents.
It can be concluded from this study that the crude methanolic extract, aqueous and butanol fractions of C. ficifolius proved to have analgesic properties against thermal and chemical noxious stimuli pain models; and anti-inflammatory activity in carrageenan-induced paw edema. The study justified the local use of C. ficifolius roots in the management of inflammatory and pain conditions.
Conclusion and recommendation
In this study crude methanolic extract, aqueous and butanol fractions of C. ficifolius proved to have analgesic properties against thermal and chemical noxious stimuli pain models; and anti-inflammatory activity in carrageenan-induced paw edema. The crude extract and solvent fractions possess peripheral and central analgesic activity. The mechanism of anti-inflammatory actions may involve a multitude of inflammatory mediators which needs further investigation.
Further constituent isolation, binding studies, and electrophysiological procedures may be useful to fully elucidate the anti-nociceptive and anti-inflammatory effects and mechanism of C. ficifolius.
Acknowledgments
We are most grateful to Gondar University National Herbarium for authenticating the plant species. We would also like to acknowledge Mr Khalid Beshir, School of Pharmacy, Mekelle University, Mekelle, Ethiopia, for contributing carrageenan.
Author contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Bertin P, Fagnani F, Duburcq A, et al. Impact of rheumatoid arthritis on career progression, productivity, and employability: the PRET study. Joint Bone Spine. 2016;83(1):47–52. doi:10.1016/j.jbspin.2015.05.001
2. Croff LJ. Use of NSAIDs in treating patients with arthritis. Arthritis Res Ther. 2013;15(3):S2.
3. Martini L, Whistler JL. The role of mu opioid receptor desensitization and endocytosis in morphine tolerance and dependence. Curr Opin Neurobiol. 2007;17(5):556–564. doi:10.1016/j.conb.2007.10.004
4. Nasri H, Shirzad H. Toxicity and safety of medicinal plants. J HerbMed Pharmacol. 2013;2:21–22.
5. Masresha B, Makonnen E, Debella A. In vivo anti-inflammatory activities of Ocimum suave in mice. J Ethnopharmacol. 2012;142(1):201–205. doi:10.1016/j.jep.2012.04.041
6. Fallis A. The contribution of complementary and alternative medicine to sustainable healthcare in Europe. J Chem Inf Model. 2013;53:1689–1699.
7. Mahomoodally MF. Traditional medicines in Africa: an appraisal of ten potent African medicinal plants. Evid Based Complement Alternat Med. 2013;2013:1–14. doi:10.1155/2013/617459
8.
9. Asadbeigi M, Mohammadi T, Rafieian-Kopaei M, Saki K, Bahmani M, Delfan M. Traditional effects of medicinal plants in the treatment of respiratory diseases and disorders: an ethnobotanical study in the Urmia. Asian Pac J Trop Med. 2014;7:S364–8. doi:10.1016/S1995-7645(14)60259-5
10. Getnet Z, Chandrodyam S, Masresha G. Studies on traditional medicinal plants in Ambagiorgis area of Wogera district, Amhara Regional state, Ethiopia. Int J Pure Appl Biosci. 2016;4:38–45. doi:10.18782/2320-7051.2240
11. Kamanyi A, Mbiantcha M, Nguelefack TB, et al. Anti-nociceptive and anti-inflammatory activities of extracts from the stem bark of Croton macrostachyus (Euphorbiaceae) in mice and rats. J Complementary Integr Med. 2009;6:1. doi:10.2202/1553-3840.1255
12. Teklay A, Abera B, Giday M. An ethnobotanical study of medicinal plants used in Kilte Awulaelo district, Tigray region of Ethiopia. J Ethnobiol Ethnomed. 2013;9(1):65. doi:10.1186/1746-4269-9-4
13. Enyew A, Asfaw Z, Kelbessa E, Nagappan R. Ethnobotanical study of traditional medicinal plants in and around Fiche district, central Ethiopia. Current Res J Biol Sci. 2014;6(4):154–167.
14. Limenih Y, Umer S, Wolde-Mariam M. Ethnobotanical study on traditional medicinal plants in Dega Damot woreda, Amhara region, north Ethiopia. Int J Res Pharm Chem. 2015;5:258–273.
15. Alemayehu G, Asfaw Z, Kelbessa E. Ethnobotanical study of medicinal plants used by local communities of Minjar-Shenkora district, north Shewa Zone of Amhara region, Ethiopia. J Med Plant Studies. 2015;3(6):01–11.
16. Rajasree RS, Sibi PI, Francis F, William H. Phytochemicals of Cucurbitaceae family—A review. IJPPR. 2016;8(1):113–123.
17. Dhiman K, Gupta A, Sharma DK, Gill NS, Goyal A. A review on the medicinally important plants of the family Cucurbitaceae. Asian J Clin Nutr. 2012;4(1):16–26. doi:10.3923/ajcn.2012.16.26
18. Vouldoukis I, Lacan D, Kamate C, et al. Antioxidant and anti-inflammatory properties of a Cucumis melo LC. extract rich in superoxide dismutase activity. J Ethnopharmacol. 2004;94(1):67–75. doi:10.1016/j.jep.2004.04.023
19. Panda SP, Sarkar N, Das S, Bala A, Haldar PK. Evaluation of Analgesic and Anti-inflammatory activity of Methanol extract of Cucumis callosus roots in animal models. Pharmacologia. 2016;7(5):283–289. doi:10.5567/pharmacologia.2016.283.289
20. Jeffrey C. A review of the Cucurbitaceae. Bot J Linn Soc. 1980;81(3):233–247. doi:10.1111/boj.1980.81.issue-3
21.
22. Chekole G, Asfaw Z, Kelbessa E. Ethnobotanical study of medicinal plants in the environs of Tara-gedam and Amba remnant forests of Libo Kemkem district, northwest Ethiopia. J Ethnobiol Ethnomed. 2015;11(1):4. doi:10.1186/1746-4269-11-4
23. Mebrahtu E, Gebrelibanos M, Gomathi P. Invivo hepatoprotective and invitro radical scavenging activity C.ficifolius A. Rich root extract. [Master’s Thesis]. Mekelle: Mekelle University; 2017
24. Bantie L, Assefa S, Teklehaimanot T, Engidawork E. In vivo antimalarial activity of the crude leaf extract and solvent fractions of Croton macrostachyus Hocsht. (Euphorbiaceae) against Plasmodium berghei in mice. BMC Complement Altern Med. 2014;14(1):79. doi:10.1186/1472-6882-14-79
25. Ocde O. Acute oral Toxicity: up and down procedure. OECD Guideline for Test Chem. 2008;425:1–27.
26. Birhane SW, Asres K. Evaluation of analgesic and Antiinflammatory activities of the root extracts of Indigofera spicata F. in mice. Ethiopian Pharm J. 2014;30:65–76.
27. Tadiwos Y, Nedi T, Engidawork E. Analgesic and anti-inflammatory activities of 80% methanol root extract of Jasminum abyssinicum Hochst. ex. Dc. (Oleaceae) in mice. J Ethnopharmacol. 2017;202:281–289. doi:10.1016/j.jep.2017.02.036
28. Debebe E, Makonnen E, Debella A. Antinociceptive effect of the methanolic extract of roots of Andrachne aspera in three models of nociception. Pharmacologyonline. 2007;1:41–48.
29. Neto AG, Costa JM, Belati CC, et al. Analgesic and anti-inflammatory activity of a crude root extract of Pfaffia glomerata (Spreng) Pedersen. J Ethnopharmacol. 2005;96(1–2):87–91.
30. Yonathan M, Asres K, Assefa A, Bucar F. In vivo anti-inflammatory and anti-nociceptive activities of Cheilanthes farinosa. J Ethnopharmacol. 2006;108(3):462–470. doi:10.1016/j.jep.2006.06.006
31. Hunskaar S, Berge OG, Hole K. Dissociation between antinociceptive and anti-inflammatory effects of acetylsalicylic acid and indomethacin in the formalin test. Pain. 1986;25(1):125–132.
32. Chung KM, Song DK, Huh SO, Kim YH, Choi MR, Suh HW. Supraspinal NMDA and non-NMDA receptors are differentially involved in the production of antinociception by morphine andβ-endorphin administered intracerebroventricularly in the formalin pain model. Neuropeptides. 2000;34(3):158–166. doi:10.1054/npep.2000.0805
33. Hunskaar S, Fasmer OB, Hole K. Formalin test in mice, a useful technique for evaluating mild analgesics. J Neurosci Methods. 1985;14(1):69–76.
34. Santos AR, Vedana EM, De Freitas GA. Antinociceptive effect of meloxicam, in neurogenic and inflammatory nociceptive models in mice. Inflammation Res. 1998;47(7):302–307. doi:10.1007/s000110050333
35. Adedapo AA, Aremu OJ, Oyagbemi AA. Anti-oxidant, anti-inflammatory and antinociceptive properties of the acetone leaf extract of Vernonia amygdalina in some laboratory animals. Adv Pharm Bull. 2014;4(Suppl 2):591. doi:10.5681/apb.2014.047
36. Winter CA, Risley EA, Nuss GW. Carrageenin-induced edema in hind paw of the rat as an assay for antiinflammatory drugs. Proc Soc Exp Biol Med, 1962;111(3):544–547.
37. Connor J, Makonnen E, Rostom AM. Comparison of analgesic effects of khat (Catha edulis Forsk) extract, D‐amphetamine and ibuprofen in mice. J Pharm Pharmacol. 2000;52(1):107–110.
38. Afify EA, Alkreathy HM, Ali AS, Alfaifi HA, Khan LM. Characterization of the antinociceptive mechanisms of khat extract (Catha edulis) in mice. Front Neurol. 2017;8:69. doi:10.3389/fneur.2017.00069
39. Adebayo HA, John-Africa LB, Agbafor AG, Omotosho OE, Mosaku TO. Anti-nociceptive and anti-inflammatory activities of extract of Anchomanes difformis in rats. Pak J Biol Sci. 2014;27(2):265–270.
40. Le Bars D, Gozariu M, Cadden SW. Animal models of nociception. Pharmacol Rev. 2001;53(4):597–652.
41. Lighthall GK, Jamieson MA, Katolik J, Brock-Utne JG. A comparison of the onset and clinical duration of high doses of cisatracurium and rocuronium. J Clin Anesth. 1999;11(3):220–225.
42. Hunskaar S, Hole K. The formalin test in mice: dissociation between inflammatory and non-inflammatory pain. Pain. 1987;30(1):103–114.
43. Dubuisson D, Dennis SG. The formalin test: a quantitative study of the analgesic effects of morphine, meperidine, and brain stem stimulation in rats and cats. Pain. 1977;4:161–174.
44. Hasanein P, Parviz M, Keshavarz M, Javanmardi K. CB1 receptor activation in the basolateral amygdala produces antinociception in animal models of acute and tonic nociception. Clin Exp Pharmacol Physiol. 2007;34(5‐6):439–449. doi:10.1111/j.1440-1681.2007.04592.x
45. Kamei J, Kashiwazaki T, Hitosugi H, Nagase H. The role of spinal δ1-opioid receptors in inhibiting the formalin-induced nociceptive response in diabetic mice. Eur J Pharmacol. 1997;326(1):31–36.
46. Meunier CJ, Burton J, Cumps J, Verbeeck RK. Evaluation of the formalin test to assess the analgesic activity of diflunisal in the rat. Eur J Pharm Sci. 1998;6(4):307–312. doi:10.1016/S0928-0987(97)10020-3
47. Sadeghi H, Hajhashemi V, Minaiyan M, Movahedian A, Talebi A. A study on the mechanisms involving the anti-inflammatory effect of amitriptyline in carrageenan-induced paw edema in rats. Eur J Pharmacol. 2011;667(1–3):396–401. doi:10.1016/j.ejphar.2011.05.053
48. Umamaheswari S, Sangeetha KS. Anti-inflammatory effect of selected Dihydroxyflavones. J Clin Diagn Res. 2015;9(5):FF05. doi:10.7860/JCDR/2015/13028.5790
49. Samad TA. Interleukin-1β-mediated induction of Cox-2 in the CNS contributes to inflammatory pain hypersensitivity. Nature. 2001;410:471–475. doi:10.1038/35068566
50. Rauf A, Uddin G, Siddiqui BS, et al. In-vivo antinociceptive, anti-inflammatory and antipyretic activity of pistagremic acid isolated from Pistacia integerrima. Phytomedicine. 2014;21:1509–1515. doi:10.1016/j.phymed.2014.07.015
51. Mallam D, Joseph A, Abdulkadir U, Ben A. ‘Analgesic and anti-inflammatory activities of Rothmannia Longiflora Salisb In Mice And Rats. IOSR J Pharm. 2016;6(8):1–7. doi:10.9790/3013-068010107
52. Minaiyan M, Zolfaghari B, Kamal A. Effect of hydroalcoholic and buthanolic extract of Cucumis sativus seeds on blood glucose level of normal and streptozotocin-induced diabetic rats. Iran J Basic Med Sci. 2011;14(5):436.
53. Babulreddy N, Sahoo SP, Ramachandran S, Dhanaraju MD. Anti-hyperglycemic activity of cucumis Melo Leaf extracts in Streptozotocin induced Hyperglycemia in Rats. Inter J. 2013;2(4):22–27.
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.