Back to Journals » Infection and Drug Resistance » Volume 15
Anti-Interferon-γ Autoantibodies Impair T-Lymphocyte Responses in Patients with Talaromyces marneffei Infections
Authors Chen ZM, Yang XY, Li ZT, Guan WJ, Qiu Y, Li SQ, Zhan YQ, Lei ZY, Liu J, Zhang JQ, Wang ZF, Ye F
Received 7 March 2022
Accepted for publication 3 June 2022
Published 28 June 2022 Volume 2022:15 Pages 3381—3393
DOI https://doi.org/10.2147/IDR.S364388
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Zhao-Ming Chen,1,* Xiao-Yun Yang,1,2,* Zheng-Tu Li,1,* Wei-Jie Guan,1,3 Ye Qiu,4 Shao-Qiang Li,1 Yang-Qing Zhan,1 Zi-Ying Lei,5 Jing Liu,4 Jian-Quan Zhang,6 Zhong-Fang Wang,1,2 Feng Ye1
1State Key Laboratory of Respiratory Disease, National Clinical Research Center for Respiratory Disease, Guangzhou Institute of Respiratory Health, the First Affiliated Hospital of Guangzhou Medical University, Guangzhou, Guangdong, People’s Republic of China; 2Guangzhou Laboratory, Bio-Island, Guangzhou, Guangdong, People’s Republic of China; 3Department of Thoracic Surgery, Guangzhou Institute for Respiratory Health, the First Affiliated Hospital of Guangzhou Medical University, Guangzhou, Guangdong, People’s Republic of China; 4Department of Comprehensive Internal Medicine, the Affiliated Tumor Hospital of Guangxi Medical University, Nanning, Guangxi, People’s Republic of China; 5Department of Infectious Diseases, the Third Affiliated Hospital of Sun Yat-Sen University, Guangzhou, Guangdong, People’s Republic of China; 6Department of Infectious Diseases, the Eighth Affiliated Hospital of Sun Yat-Sen University, Shenzhen, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Feng Ye, State Key Laboratory of Respiratory Disease, National Clinical Research Center for Respiratory Disease, Guangzhou Institute of Respiratory Health, the First Affiliated Hospital of Guangzhou Medical University, Guangzhou, Guangdong, 510120, People’s Republic of China, Tel +86-020-83062836, Fax +86-020-83062836, Email [email protected]
Background: Although anti-IFN-γ autoantibodies predispose patients to Talaromyces marneffei infection, whether this is mediated by T cell attenuation remains elusive.
Methods: Total peripheral blood mononuclear cells (PBMCs) from healthy donors or patients with T. marneffei infection were stimulated with M158− 66, and immunodominant influenza H1N1 peptide, or heat-inactivated T. marneffei in the presence of serum from anti-IFN-γ autoantibody-positive patients or healthy controls. The percentages of IFN-γ+TNF+CD8+ T cells and IFN-γ+CD4+ T cells were determined by flow cytometry and cytokines released in the supernatant were detected by Cytometric Bead Array. Furthermore, PBMCs from patients with T. marneffei infection and healthy individuals were stimulated with IFN-γ and anti-CD3/CD28 beads, and the levels of STAT1 and STAT3 phosphorylation were detected by Western blot.
Results: The M1-reactive CD8+ T cells that expressed IFN-γ+ TNF-α+ of healthy controls were clearly reduced in serum with high-titer anti-IFN-γ autoantibodies. In addition, the CD4+ T cell response, designated by the expression of IFN-γ, against T. marneffei in PBMCs of patients were significantly decreased when cultured in high-titer anti-IFN-γ autoantibody serum culture, compared to the healthy compartments. Moreover, the release of the cytokines IFN-γ, TNF-α and IL-2 was significantly decreased, while IL-10 was significantly increased. There was no significant difference in the phosphorylation levels of STAT1 and STAT3 protein between patients and healthy controls after IFN-γ or anti-CD3/CD28 beads stimulation.
Conclusion: Anti-IFN-γ autoantibodies presence in the serum inhibited CD4+ Th1 and CD8+ T cell immune responses. There was no congenital dysfunction of STAT1 and STAT3 in anti-IFN-γ autoantibody-positive patients with T. marneffei infection. These results suggest that the production of anti-IFN-γ autoAbs impair T-lymphocyte responses.
Keywords: anti-interferon-γ autoantibody, T lymphocyte, Talaromyces marneffei, signal transducer and activator of transcription 1, signal transducer and activator of transcription 3
Introduction
Autoantibodies against various cytokines have been reported in various infectious diseases, including those caused by bacteria, viruses and fungi.1 Due to the consequently diminished cytokine function, patients with anti-cytokine autoantibodies may present with similar clinical phenotypes as patients with genetic autoimmune disorders.2 Anti-interferon-γ autoantibodies (anti-IFN-γ autoAbs) are associated with a variety of intracellular infections, and their presence is a symptom of genetic autoimmune disorders related to the interleukin (IL)-12/IFN-γ axis.1–4
Talaromyces marneffei (T. marneffei) is an important pathogenic thermally dimorphic fungus that causes systemic mycosis in Southeast Asia.5 An increasing number of T. marneffei infections among non-human immunodeficiency virus (HIV)-infected patients with impaired cell-mediated immunity have been observed.6,7 A link between anti-IFN-γ autoAbs and severe T. marneffei infection in HIV-negative patients has also been revealed.8–12 The clinical presentation in such cases is often atypical and might be confounded by the manifestations of other infectious diseases. Moreover, recurrent infection can lead to lingering illness and life-threatening complications.10–12
The association between genetic factors and anti-IFN-γ autoAb-associated immunodeficiency has been documented. The HLA class II alleles DRB1*16:02–DQB1*05:02 and HLA-DRB1*15:02–DQB1*05:01 have been strongly associated with anti-IFN-γ autoAb titers in patients from Southeast Asia.11,13,14 Lin et al15 have proposed a molecular mimicry model for anti-IFN-γ autoAb development. Patients display specific human leukocyte antigen (HLA) class II haplotypes, which suggests that a common T cell-dependent and B cell-dependent mechanism might underlie the production of specific anti-IFN-γ autoAbs.
In addition, genes activated by STAT1 (signal transducer activator of transcription 1) execute the biological functions controlled by the IFN/STAT1 signaling pathway.16 Previous studies of anti-IFN-γ autoAbs have typically focused on the genetics of the IFN/STAT1 signaling pathway.9,11,13,14 Anti-IFN-γ autoAbs inhibit STAT1 phosphorylation and IL-12 production and might result in impaired CD4+ T helper type-1 (Th1) cell immune responses. IFN-γ is produced primarily by T lymphocytes (CD3+), which are associated with adaptive immunity, and IFN-γ secreted by CD4+ and CD8+ T cells mediates the elimination of fungal infections.17,18
However, there has been little work demonstrating the effect of anti-IFN-γ autoAbs on CD4+ and CD8+ T cell immune function. Therefore, it is important to understand how T cell immune function in T. marneffei patients is impacted by autoAbs expression. The aim of this study was to explore the impact of anti-IFN-γ autoAbs on T cell-mediated immune responses and the underlying pathogenesis in T. marneffei patients. Our findings will help to identify new diagnostic markers and therapeutic targets for patients with anti-IFN-γ autoAbs.
Materials and Methods
Participants
Non-HIV-infected patients with T. marneffei infections were recruited from four academic centers [The First Affiliated Hospital of Guangzhou Medical University (Guangzhou); The Third Affiliated Hospital of Sun Yat-sen University (Guangzhou); The Affiliated Tumor Hospital of Guangxi Medical University (Nanning); and The Eighth Affiliated Hospital of Sun Yat-Sen University (Shenzhen)] between March 2019 and August 2020. Patients were recruited based on the following criteria: 1) no laboratory evidence of HIV infection; 2) clinical and/or imaging manifestations consistent with T. marneffei infection; 3) microbiological or pathological findings identified from sputum, tracheal aspirate, bronchoalveolar lavage fluid (BALF), lung biopsy sample, pleural effusion, bone marrow smear, skin hydrolipidic film exudate or lymph node smear; and 4) no other serious diseases (such as autoimmune diseases, immunodeficiency, or organ transplantation) that might interfere with the interpretation of the findings of this study. In parallel, 12 healthy controls with normal routine blood test findings and chest radiography were recruited from the health checkup center at The First Affiliated Hospital of Guangzhou Medical University.
A 30 mL blood sample from all participants was collected at enrollment. We then recorded the patients’ clinical characteristics and laboratory findings and measured the levels of anti-IFN-γ autoAbs upon admission. For healthy controls, we documented age, sex, race or ethnicity only. This study was approved by the ethics committee of the First Affiliated Hospital of Guangzhou Medical University (No: 2019026), and patients signed informed consent forms.
Serum and Peripheral Blood Mononuclear Cell Isolation
Peripheral blood specimens were collected for isolation of peripheral blood mononuclear cells (PBMCs) and serum. Serum was separated from 5 mL of peripheral blood by centrifugation at 3000 rpm for 10 minutes. PBMCs were prepared from 10 to 20 mL of peripheral blood by using Ficoll-plaque density gradient centrifugation (General Electric Company, USA).
Determination of Autoantibody Titers Against IFN-γ
The serum anti-IFN-γ autoAb titers were determined by using an enzyme-linked immunosorbent assay kit (USCN Life Science, Inc., Wuhan, China) according to the manufacturer instructions. According to our previous study,12 based on the 99th percentile of the anti-IFN-γ autoAb titers in 40 healthy controls, the cutoff for anti-IFN-γ autoAb positivity was 594.49 ng/mL, with higher values suggestive of higher levels of anti-IFN-γ autoAbs.
PBMC Stimulation and in vitro Expansion
CD8+ T cells could recognize and respond to relatively conserved viral peptides from the internal components of influenza virus (NP, M1 and PB1, PB2). M158−66 is the best characterized human influenza CD8+ T cell epitope that binds to the HLA-A*02:01 molecule.19,20 To determine the effect of anti-IFN-γ autoantibodies in memory T cell activation, PBMCs of three healthy donors (named A, B and C), who had been proved to possess T cell memory against the M158-66 peptide (personal observations), were treated with peptide in culture medium supplemented with heat-inactivated serum of 12 healthy donors, or heat-inactivated serum of T. marneffei patients with different levels of anti-IFN-γ autoantibodies. Cells cultured in the absence of M158-66 were used as negative controls. Briefly, 5×105 PBMCs were treated with the M158-66 peptide (2μM) in medium containing 10% of serum from healthy donors or anti-IFN-γ autoAb-positive patients, and were incubated for 10 days. During the culture, half of the medium was changed twice per week with fresh PRMI 1640 (Gibco, USA) containing 1% penicillin/streptomycin (Sigma, USA), and supplemented with the same serum indicated above and 10 U/mL rIL-2 (RD, USA). The cells were then re-stimulated at day 10 with medium containing M158-66 peptide and the same serum for overnight and were then proceed with FACS analysis.
To explore the effect of anti-IFN-γ autoantibodies on CD4+ T cells, we randomly selected, regardless of IFN-γ concentrations, 3 anti-IFN-γ autoAb-positive patients (TM2, TM5 and TM11) and 2 anti-IFN-γ autoAb-negative patients (TM12 and TM13). A total of 5×105 PBMCs were stimulated with heat-inactivated T. marneffei (MOI=10) in RPMI-1640 medium containing 1% penicillin/streptomycin, 10 U/mL rIL-2, and 20% of heat-inactivated serum of three different patients with high IFN-γ autoantibodies (TM1, TM2, TM3) and one healthy donor. After 10-day expansion and restimulation for overnight, the cells were performed ICS staining for FACS analysis, and the supernatants were collected for cytokine detection.
Multiparameter Flow Cytometry
Cells harvested from the 10-day stimulation cultures were washed and stained with dead cell discrimination markers (Live Dead Aqua, Life Technologies, USA) in PBS for 15 minutes at room temperature, followed by incubation with a panel of mAbs against surface markers, including FITC-CD3 (BD 555339, 1:50, clone HIT3a), APC-Cy7-CD4 (BD 557871, 1:200, clone RPA-T4), and PerCP/Cyanine 5.5-CD8 (Biolegend 344,709, 1:200, clone SK1), for 30 minutes on ice. Subsequently, cells were fixed with BD Fix/Perm buffer on ice for 20 minutes and stained with a panel of intracellular markers in BD Perm/Wash buffer for 30 minutes on ice. The mAbs used were APC-IFN-γ (BD 554702, 1:200, clone B27) and PE-Cy7-TNF-a (BD 557647, 1:200, clone MAb11). The stained cells were resuspended in FACS buffer for flow cytometry (BD FACS Aria III). Flow cytometry data were analyzed using FlowJo (version 10.6.0). For flow cytometry gating strategy, see Supplementary Figure 1.
Cytokine Measurements
A cytometric bead array (CBA) was used to measure multiple cytokines from supernatant samples (BD 560484). The analyzed cytokines included IL-2, IL-4, IL-6, IL-10, IL-17, IFN-γ and tumor necrosis factor (TNF)-α. Following flow cytometry (BD FACS Verse), cytokine concentrations were calculated based on the standard curves using BD CBA Analysis Software.
Detection of STAT1/STAT3 Protein Phosphorylation Expression
Three anti-IFN-γ autoAb-positive patients, three anti-IFN-γ autoAb-negative patients and three healthy donors were randomly included in further analysis. The blank control group contained only the cells and RPMI-1640 medium with 10% fetal bovine serum (FBS) without stimulants. The experimental groups were treated with recombinant IFN-γ (RD 285-IF-100) or anti-CD3/CD28 microbeads (Invitrogen 11161D). PBMCs (5×107) were cultured in RPMI-1640 medium with 10% fetal bovine serum in the presence of IFN-γ (20 ng/mL) for 15 minutes or anti-CD3/CD28 microbeads (4 beads per cell) for 12 hours. The harvested cells were directly lysed in RIPA buffer with protease inhibitors and phosphatase inhibitors. Extracts were clarified by centrifugation at 14,000 rpm for 10 minutes at 4 °C. Western blotting was performed to evaluate the protein expression of STAT1, ρ-STAT1, STAT3, and ρ-STAT3 (see online supplement).
Statistical Analyses
Categorical data are presented as number (percentage). Continuous variables are presented as means and standard deviations or medians and interquartile ranges (IQRs). To compare the categorical outcomes, chi-square tests were used, and Mann–Whitney tests were used to compare the continuous outcomes across the groups. Correlations were analyzed using Pearson’s correlation coefficient test. Pairwise comparisons were carried out with the Wilcoxon matched pairs test. P <0.05 indicated statistically significant differences.
Results
Demographics and Patient Characteristics
To assess the effect of anti–IFN-γ autoantibodies on T-lymphocyte responses in patients with T. marneffei infections, 16 patients with T. marneffei infections (including 11 anti-IFN-γ autoAb-positive patients and five anti-IFN-γ autoAb-negative patients) and 12 healthy controls were included in the analysis. Participant characteristics are detailed in Table 1. Table 2 shows the anti-IFN-γ autoAb titers of individual participants. The anti-IFN-γ autoAb titers in anti-IFN-γ autoAb-positive patients (median 1316.22 ng/mL) were significantly higher than those in anti-IFN-γ autoAb-negative patients (median 332.40 ng/mL, P<0.001) and healthy controls (median 300.5 ng/mL, P<0.001) (Table 1). Most anti-IFN-γ autoAb-positive patients had extrapulmonary involvement, including bone (n=4, 36.4%), lymph nodes (n=7, 63.6%), liver (n=2, 9.1%), spleen (n=2, 9.1%), and skin disorders (n=3, 27.3%).
 |
Table 1 Characteristics of the 16 Patients with T. marneffei Infections and 12 Healthy People During the First Visit |
 |
Table 2 Anti-IFN-γ autoAb Titer for Each Participant |
All patients were treated with intravenous amphotericin B (0.6–1.0 mg/kg/day) as standard initial therapy, followed by oral itraconazole or voriconazole at 400 mg/day for maintenance. Among anti-IFN-γ autoAb-positive patients, 3 of the 11 patients developed worsening multiorgan failure and died because of disease progression, and 6 patients had persistent talaromycosis due to poor therapeutic response (Table 1).
Anti-IFN-γ Autoantibodies Blunt the Immune Response of CD8+ and CD4+ T Cells
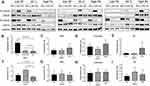
To assess the effect of anti-IFN-γ autoAbs on CD8+ T cell immune response, the PBMCs of 3 healthy donors were cultured with medium containing serum from 10 anti-IFN-γ autoAb-positive patients and 12 healthy donors for 10 days. The results show that after stimulation with the M158−66 peptide followed by 10-day culture and re-stimulation, the activation of influenza-specific T cells, represented by the frequencies of IFN-γ+TNF + CD8+ T cells, was significantly suppressed in cells cultured with medium containing IFN-γ autoantibodies. To better observe the effects of autoantibodies, we next classified four patients (TM1, TM2, TM3, and TM8) with autoantibody titers higher than 2000 ng/mL into the high-titer autoantibody group and the remaining anti-IFN-γ autoAb-positive patients into the medium-titer autoantibody group. The percentage of IFN-γ+TNF + CD8+ T cells in PBMCs of healthy donor A was significantly decreased when cultured in the serum of anti-IFN-γ autoAb-positive patients than when cultured in healthy donor serum (Figure 1A, Figure 2A), and the percentage correlated negatively with anti-IFN-γ autoAb titer (r=−0.683, P<0.05, Figure 2D). Similarly, the percentage of IFN-γ+TNF + CD8+ T cells in PBMCs of healthy donor B was significantly decreased when cultured in the serum of patients in the high-titer autoantibody group (Figures 1B and 2B) and correlated negatively with anti-IFN-γ autoAb titers (r=−0.634, P<0.05, Figure 2E). The percentage of IFN-γ+TNF + CD8+ T cells in the PBMCs of healthy donor C was also significantly decreased when cultured in the serum of patients in the high-titer autoantibody group (Figures 1C and 2C), but significant correlation was not present (P>0.05, Figure 2F). These results indicate that anti-IFN-γ autoantibodies suppressed CD8+ T cell immune response, which may lead to weakened clearance of pathogens.
Since, we confirmed that patients with autoantibody titers higher than 2000 ng/mL showed stronger inhibition of CD8+ T cells. Therefore, we used serum of these patients (TM1, TM2, TM3) for further experiments to explore the effect of anti-IFN-γ autoAbs on CD4+ T cell immune response. PBMCs of 5 patients with talaromycosis were cultured for 10 days in media containing serum from 3 anti-IFN-γ autoAb-positive patients and from 1 healthy donor. Serum used in this set of assays were increased to 20% for optimal effects of the autoantibodies. After stimulation with heat-inactivated T. marneffei, followed by in vitro expansion, the T. marneffei -reactive CD4+ T cells, depicted by the expression of IFN-γ were significantly reduced in the culture supplemented with serum of anti-IFN-γ autoAb-positive patients (P1-P3) than those with the normal serum (HC) (Figure 3A and B), suggesting that the anti-IFN-γ autoAb present in the serum may play a major role in restricting the T cell response. Additionally, within the same treatment, with either high titer anti-IFN-γ autoAb serum or HC serum, no difference was observed in the frequencies of IFN-γ+ CD4+ T cells between anti-IFN-γ autoAb-positive group and negative patient group (Figure 3A-D), indicating that the T cells are not likely attribute to pathogenesis of talaromycosis with anti-IFN-γ autoAbs. Compared with those in the normal serum group, the levels of the Th1 cytokines including IL-2, IFN-γ, and TNF-α in the autoAb-positive serum group were significantly decreased, and the level of IL-10 was markedly elevated (P<0.05) (Figure 4). However, there was no significant difference between the anti-IFN-γ autoAb-positive serum and normal serum groups in terms of the levels of IL-4, IL-6 and IL-17 (P>0.05) (Figure 4). These results indicate that anti-IFN-γ autoantibodies significantly suppressed CD4+ Th1 cell immune response, causing the failure of the host to clear the pathogens.
 |
Figure 4 Cell supernatant cytokine levels in PBMCs of 5 patients at Day 10 following in vitro stimulation with heat-killed T. marneffei. * P < 0.05, *** P < 0.001, and **** P < 0.0001. |
Expression of ρ-STAT1 and ρ-STAT3 Protein in Patients with T. Marneffei Infection and Healthy Subjects
Of note, individuals with STAT1 deficiency or defects in IFN-JAK-STAT pathway exhibit increased susceptibility to mycobacterial and viral infections.21,22 To exclude whether anti-IFN-γ autoAb-positive patients have defects in STAT1 and STAT3, we performed the experiments as follows. PBMCs were cultured in RPMI-1640 medium with 10% fetal bovine serum and stimulated with anti-CD3/CD28 microbeads for 12 hours. After stimulation, the protein expression levels of ρ-STAT1 and ρ-STAT3 were significantly elevated in all experimental groups compared with the blank control group (all P<0.05). After 15 minutes of stimulation with IFN-γ, the protein expression level of ρ-STAT1 was significantly elevated in all experimental groups (all P<0.05); however, the protein expression level of ρ-STAT3 was not significantly different in any of the experimental groups (all P>0.05). In addition, after stimulation with IFN-γ or anti-CD3/CD28 magnetic beads, there was no significant difference in the protein expression level of ρ-STAT3 or ρ-STAT1 among the anti-IFN-γ autoAb-positive group, anti-IFN-γ autoAb-negative group, and healthy control group (Figure 5) (P>0.05). These results indicate that anti-IFN-γ autoAb-positive patients do not exist STAT1 and STAT3 deficiency, anti-IFN-γ autoantibody play a key role to impair the ability of T-lymphocyte to clear pathogens.
Discussion
We examined the effects of anti-IFN-γ autoAb level on T lymphocyte responses in Chinese patients with T. marneffei infections for the first time. In addition, we studied phosphorylation of STAT1 and STAT3 in anti-IFN-γ autoAb-positive patients with T. marneffei infection and compared it with healthy patients. These findings provide further evidence of the pathophysiology among patients with anti-IFN-γ autoAb-associated immunodeficiency.
The clinical characteristics reported herein were consistent with our previous findings12 and those published previously.10,11 Patients with defective T lymphocyte function due to anti-IFN-γ autoAbs tended to have severe disseminated infection and poor clinical outcomes. We found that anti-IFN-γ autoAb-positive patients suffer from more serious disease due to systemic multiorgan disseminated infection and poor response to antibiotic treatment. A previous study revealed an association of the low levels of CD4+ T cells with the severity and disease progression in T. marneffei infection in Southeast Asia.23 However, in our study, the CD4+/CD8+ T cell count in patients was at an average level or elevated, suggesting that although the number of T cells in patients has not decreased, they may be impaired in function.
Hence, we assumed that an increase in autoantibodies leads to impaired T cell function, which predisposes patients to infection. We initially focused on the healthy subjects. Influenza is one of the most common acute respiratory illness, caused by influenza A, B, and C viruses, that occurs in local outbreaks or seasonal epidemics.24 Antigen-specific memory CD8+ T cell immune response can be formed in healthy people that had been infected with influenza.25 Influenza-specific CD8+ T cells play a key role in broadly cross-reactive immunity to influenza viruses because they recognize peptide fragments of 8–11 amino acids in length that are derived from highly conserved internal viral proteins in the context of MHC class I molecules.26 Following activation, CD8+ T cells also secrete antiviral cytokines such as IL-2, IFN-γ, and TNF-α, which further recruit innate and adaptive immune cells to the sites of viral dissemination and induce antiviral responses in infected cells.19,20 Therefore, influenza T cell memory is an ideal model to study the impact of anti-IFN-γ autoantibodies on memory T cell response. Does the serum of patients with anti-IFN-γ autoantibodies inhibit influenza-specific CD8+ T cell response? Here, we cultured PBMCs from healthy controls with high-titer anti-IFN-γ autoAbs for 10 days. After M158−66 peptide stimulation followed by 10-day culture in the presence of serum with different levels of IFNg autoantibodies, the reactivation of the T cells, represented by the dual expression of IFNg and TNF, in the high-titer autoantibodies was clearly suppressed. The levels of IFN-γ+TNF+ CD8+ T cells in two healthy controls (A and B) negatively correlated with the antibody titers, and there was a trending negative correlation between the T cells response and healthy control C. These findings indicated that the immune function of CD8+ T cells was significantly inhibited by the anti-IFN-γ autoAbs, which may impose an increased risk of pathogen infection. Browne and colleagues found that when PBMCs from healthy controls were cultured in 10% plasma from anti-IFN-γ autoAb-positive patients, lipopolysaccharide-induced PBMC secretion of TNF-a was inhibited,9 which was consistent with the results of our study. In addition, previous study have shown that IFN-γ plays a major role in CD8+ T cell immune response after infection, which mediate pathogen clearance.27 In a study by Hufford and colleagues, short-term acute CD80 and CD86 blockade led to a significant decline in IFN-γ secretion, which coincided with a reduction in IFN-γ+CD8+ T cells in vivo.28 Therefore, anti-IFN-γ autoAbs could affect CD8+ T cell counts through different molecules or signaling pathways, although the specific mechanisms remain to be further explored.
The Th1 cell response mediated by effector CD4+ T cells that secrete IFN- correlates with dominant protective immunity against fungal microorganisms.29 When fungi invade the initial infection site (epithelial cells) within the lungs, CD4+ T cells are activated and further differentiate into Th1 or T helper type-2 (Th2) cells.17 CD4 Th1 cells utilize a highly specific T cell receptor to identify infected cells and initiate pathogen killing via IFN-γ secretion, and secreted IFN-γ further synergistically activates NK cells and macrophages with a phagocytic killing function to eliminate fungi.30 Here, we found that the percentage of CD4+ T cells secreting IFN-γ in PBMCs from the serum containing anti-IFN-γ autoAbs culture was significantly lower than that of normal serum group, and the expression levels of IFN-γ, TNF-α and IL-2 in the cell supernatant were significantly decreased. Therefore, the decrease in IFN-γ due to blockade by anti-IFN-γ autoAbs might be associated with the significant inhibition of the Th1 cell response. In fact, previous studies on IFN-γ/IL12 pathway defects have been reported. Holland et al found that due to defects in IFN-γ receptors, the PBMCs of these patients produced only 10% of the normal amount of IFN-γ and IL-12 when stimulated with phytohemagglutinin in vitro.31 Losana et al also found that after anti-CD3 and PMA stimulation, T lymphocytes from patients with IL-12R1 or IFN-γR1 receptor gene defects produced only 33–50% of the normal levels of IFN-γ found in the corresponding cells from healthy individuals.32 Doffinger R et al reported that in the presence of 5% serum containing anti-IFN-γ autoAbs, IFN-γ secretion was reduced in the PBMCs of patients with nontuberculous mycobacteria after tuberculin and PHA stimulation and that IL-12 secretion induced by lipopolysaccharide was also impaired.33 These findings also provide support for our results, suggesting that deficiency in the IFN-γ signaling pathway might lead to an impaired CD4+ Th1 cell response in patients with an increased risk of T. marneffei infection. Additionally, we observed elevated expression levels of IL-10. IL-10 is secreted by Th2 cells and M2 macrophages34 and can antagonize Th1 cell responses by inhibiting Th1 cell differentiation and IFN-γ production.35 Although we have validated the impact of autoantibodies on the Th1 immune response, the balancing mechanism by which autoantibodies regulate the Th1/Th2 immune response still needs further study.
IFN-γ is a vital cytokine in the STAT1 signaling pathway, which results in Th1 differentiation.36 STAT3 mediates IL-23 signaling and plays a key role in the production of IFN-γ, IL-12, and IL-17. STAT1 and STAT3 immune signals mediate the balance of Th1/Th17 cytokines, which is important in protection against pathogen infections.37 Defects in the JAK-STAT signaling pathway, which regulates IFN-γ-mediated signal transduction and transcription, have been extensively reported to be associated with the development of infectious diseases.38,39 Here, we detected the phosphorylation of STAT1 and STAT3 and found no significant differences in the expression of ρ-STAT1 and ρ-STAT3 proteins between the high-autoantibody group, the low-autoantibody group, and the healthy control group in vitro. Previous studies have confirmed that the expression of ρ-STAT1 was significantly inhibited when PBMCs of infected patients were cultured in vitro with serum containing anti-IFN-γ autoAbs.9,15,40,41 These results suggest that the production of anti-IFN-γ autoAbs is key to the pathogenesis of T. marneffei infection, which contrasts with the pathogenesis of defects in the IFN-γ signaling pathway caused by congenital STAT mutations.42,43
Although we have successfully demonstrated the effect of anti-IFN-γ autoAbs on CD4+ and CD8+ T cell immune function, several limitations need to be acknowledged. First, further studies with a larger sample size are required to validate the findings. Moreover, we have considered only the context of T lymphocytes, and the possible immunoregulatory effects on other immune cell-like NK cells and on macrophages remain unclear. Finally, the present study was not able to establish the detailed molecular mechanism of anti-IFN-γ autoAb production and action. Further study is needed to deepen our understanding of the association between anti-IFN-γ autoAbs and intracellular pathogens.
Conclusion
Neither STAT1 nor STAT3 expression were altered in anti-IFN-γ autoAb-positive patients. The high titer of anti-IFN-γ autoAbs within the serum might help explain the inhibitory immune responses of CD8+ T cells and CD4+ cells that contributed to the impairment of the immune defense function and subsequent T. marneffei infection.
Data Sharing Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
The study was carried out in accordance with the Declaration of Helsinki. The study was approved by the Ethics Committee of the First Affiliated Hospital of Guangzhou Medical University (reference number 2019026). All study participants signed written informed consent forms. The authors alone are responsible for the content and writing of the paper.
Consent for Publication
All authors have read and approved the manuscript.
Acknowledgments
We thank the patients, the nurses and clinical staff who are providing patient care, the staff at the respiratory medicine department of the hospital, the staff at the clinical laboratory of the hospital, and the technical staff of the Department of State Key Laboratory of Respiratory Disease for excellent assistance. Furthermore, we would also like to thank AJE team for polishing the English language of this manuscript.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was funded by the open fund of State Key Laboratory of Respiratory Diseases (SKLRD-OP-201913); The independent fund of State Key Laboratory of Respiratory Diseases (SKLRD-Z-202019); The Guangzhou Institute of Respiratory Health Open Project (2019GIRHZ06).
Disclosure
The authors have no relevant financial or non-financial interests to disclose.
References
1. Barcenas-Morales G, Cortes-Acevedo P, Doffinger R. Anticytokine autoantibodies leading to infection: early recognition, diagnosis and treatment options. Curr Opin Infect Dis. 2019;32(4):330–336. doi:10.1097/QCO.0000000000000561
2. Ku CL, Chi CY, von Bernuth H, Doffinger R. Autoantibodies against cytokines: phenocopies of primary immunodeficiencies? Hum Genet. 2020;139(6–7):783–794. doi:10.1007/s00439-020-02180-0
3. Browne SK. Anticytokine autoantibody-associated immunodeficiency. Annu Rev Immunol. 2014;32:635–657. doi:10.1146/annurev-immunol-032713-120222
4. Shih HP, Ding JY, Yeh CF, Chi CY, Ku CL. Anti-interferon-gamma autoantibody-associated immunodeficiency. Curr Opin Immunol. 2021;72:206–214. doi:10.1016/j.coi.2021.05.007
5. Cao C, Xi L, Chaturvedi V. Talaromycosis (Penicilliosis) Due to Talaromyces (Penicillium) marneffei: insights into the Clinical Trends of a Major Fungal Disease 60 Years After the Discovery of the Pathogen. Mycopathologia. 2019;184(6):709–720. doi:10.1007/s11046-019-00410-2
6. Vanittanakom N, Cooper CR, Fisher MC, Sirisanthana T. Penicillium marneffei infection and recent advances in the epidemiology and molecular biology aspects. Clin Microbiol Rev. 2006;19(1):95–110. doi:10.1128/CMR.19.1.95-110.2006
7. Chan JF, Lau SK, Yuen KY, Woo PC. Talaromyces (Penicillium) marneffei infection in non-HIV-infected patients. Emerg Microbes Infect. 2016;5:e19. doi:10.1038/emi.2016.18
8. Tang BS, Chan JF, Chen M, et al. Disseminated penicilliosis, recurrent bacteremic nontyphoidal salmonellosis, and burkholderiosis associated with acquired immunodeficiency due to autoantibody against gamma interferon. Clin Vaccine Immunol. 2010;17(7):1132–1138. doi:10.1128/CVI.00053-10
9. Browne SK, Burbelo PD, Chetchotisakd P, et al. Adult-onset immunodeficiency in Thailand and Taiwan. N Engl J Med. 2012;367(8):725–734. doi:10.1056/NEJMoa1111160
10. Zeng W, Qiu Y, Tang S, Zhang J, Pan M, Zhong X. Characterization of Anti–Interferon-γ Antibodies in HIV-Negative Patients Infected With Disseminated Talaromyces marneffei and Cryptococcosis. Open Forum Infect Dis. 2019;6(10):ofz208. doi:10.1093/ofid/ofz208
11. Guo J, Ning X-Q, Ding J-Y, et al. Anti–IFN-γ autoantibodies underlie disseminated Talaromyces marneffei infections. J Exp Med. 2020;217(12):12. doi:10.1084/jem.20190502
12. Chen ZM, Li ZT, Li SQ, et al. Clinical findings of Talaromyces marneffei infection among patients with anti-interferon-gamma immunodeficiency: a prospective cohort study. BMC Infect Dis. 2021;21(1):587. doi:10.1186/s12879-021-06255-9
13. Pithukpakorn M, Roothumnong E, Angkasekwinai N, et al. HLA-DRB1 and HLA-DQB1 Are Associated with Adult-Onset Immunodeficiency with Acquired Anti-Interferon-Gamma Autoantibodies. PLoS One. 2015;10(5):e0128481. doi:10.1371/journal.pone.0128481
14. Ku CL, Lin CH, Chang SW, et al. Anti-IFN-gamma autoantibodies are strongly associated with HLA-DR*15:02/16:02 and HLA-DQ*05:01/05:02 across Southeast Asia. J Allergy Clin Immunol. 2016;137(3):945–948e948. doi:10.1016/j.jaci.2015.09.018
15. Lin CH, Chi CY, Shih HP, et al. Identification of a major epitope by anti-interferon-gamma autoantibodies in patients with mycobacterial disease. Nat Med. 2016;22(9):994–1001. doi:10.1038/nm.4158
16. Kak G, Raza M, Tiwari BK. Interferon-gamma (IFN-gamma): exploring its implications in infectious diseases. Biomol Concepts. 2018;9(1):64–79. doi:10.1515/bmc-2018-0007
17. Bartemes KR, Kita H. Innate and adaptive immune responses to fungi in the airway. J Allergy Clin Immunol. 2018;142(2):353–363. doi:10.1016/j.jaci.2018.06.015
18. Verma A, Wuthrich M, Deepe G, Klein B. Adaptive immunity to fungi. Cold Spring Harb Perspect Med. 2014;5(3):a019612. doi:10.1101/cshperspect.a019612
19. Jansen JM, Gerlach T, Elbahesh H, Rimmelzwaan GF, Saletti G. Influenza virus-specific CD4+ and CD8+ T cell-mediated immunity induced by infection and vaccination. J Clin Virol. 2019;119:44–52. doi:10.1016/j.jcv.2019.08.009
20. Auladell M, Jia X, Hensen L, et al. Recalling the Future: immunological Memory Toward Unpredictable Influenza Viruses. Front Immunol. 2019;10:1400. doi:10.3389/fimmu.2019.01400
21. Patel SY, Doffinger R, Barcenas-Morales G, Kumararatne DS. Genetically determined susceptibility to mycobacterial infection. J Clin Pathol. 2008;61(9):1006–1012. doi:10.1136/jcp.2007.051201
22. Sancho-Shimizu V, Perez de Diego R, Jouanguy E, Zhang SY, Casanova JL. Inborn errors of anti-viral interferon immunity in humans. Curr Opin Virol. 2011;1(6):487–496. doi:10.1016/j.coviro.2011.10.016
23. Supparatpinyo K, Khamwan C, Baosoung V, Sirisanthana T, Nelson KE. Disseminated Penicillium marneffei infection in Southeast Asia. Lancet. 1994;344(8915):110–113. doi:10.1016/s0140-6736(94)
24. Paules C, Subbarao K. Influenza. Lancet. 2017;390(10095):697–708. doi:10.1016/s0140-6736(17)
25. Del Campo J, Bouley J, Chevandier M, et al. OVX836 Heptameric Nucleoprotein Vaccine Generates Lung Tissue-Resident Memory CD8+ T-Cells for Cross-Protection Against Influenza. Front Immunol. 2021;12:678483. doi:10.3389/fimmu.2021.678483
26. Nussing S, Sant S, Koutsakos M, Subbarao K, Nguyen THO, Kedzierska K. Innate and adaptive T cells in influenza disease. Front Med. 2018;12(1):34–47. doi:10.1007/s11684-017-0606-8
27. Ostler T, Davidson W, Ehl S. Virus clearance and immunopathology by CD8(+) T cells during infection with respiratory syncytial virus are mediated by IFN-gamma. Eur J Immunol. 2002;32(8):2117–2123. doi:10.1002/1521-4141(200208)32:8<2117::
28. Hufford MM, Kim TS, Sun J, Braciale TJ. Antiviral CD8+ T cell effector activities in situ are regulated by target cell type. J Exp Med. 2011;208(1):167–180. doi:10.1084/jem.20101850
29. Romani L. Immunity to fungal infections. Nat Rev Immunol. 2011;11(4):275–288. doi:10.1038/nri2939
30. Li Z, Lu G, Meng G. Pathogenic Fungal Infection in the Lung. Front Immunol. 2019;10:1524. doi:10.3389/fimmu.2019.01524
31. Holland SM, Dorman SE, Kwon A, et al. Abnormal regulation of interferon-gamma, interleukin-12, and tumor necrosis factor-alpha in human interferon-gamma receptor 1 deficiency. J Infect Dis. 1998;178(4):1095–1104. doi:10.1086/515670
32. Losana G, Rigamonti L, Borghi I, et al. Requirement for both IL-12 and IFN-γ signaling pathways in optimal IFN-γ production by human T cells. Eur J Immunol. 2002;32:3. doi:10.1002/1521-4141(200203)32:3<693::
33. Doffinger R, Helbert MR, Barcenas-Morales G, et al. Autoantibodies to interferon-gamma in a patient with selective susceptibility to mycobacterial infection and organ-specific autoimmunity. Clin Infect Dis. 2004;38(1):e10–14. doi:10.1086/380453
34. Saraiva M, O’Garra A. The regulation of IL-10 production by immune cells. Nat Rev Immunol. 2010;10(3):170–181. doi:10.1038/nri2711
35. de Waal Malefyt R, Haanen J, Spits H, Roncarolo MG. Interleukin 10 (IL-10) and viral IL-10 strongly reduce antigen-specific human T cell proliferation by diminishing the antigen-presenting capacity of monocytes via downregulation of class II major histocompatibility complex expression. J Exp Med. 1991;174(4):915–924. doi:10.1084/jem.174.4.915
36. Rosenzweig SD, Holland SM. Defects in the interferon-gamma and interleukin-12 pathways. Immunol Rev. 2005;203:38–47. doi:10.1111/j.0105-2896.2005.00227.x
37. Olbrich P, Freeman AF. STAT1 and STAT3 mutations: important lessons for clinical immunologists. Expert Rev Clin Immunol. 2018;14(12):1029–1041. doi:10.1080/1744666X.2018.1531704
38. Filipe-Santos O, Bustamante J, Chapgier A, et al. Inborn errors of IL-12/23- and IFN-gamma-mediated immunity: molecular, cellular, and clinical features. Semin Immunol. 2006;18(6):347–361. doi:10.1016/j.smim.2006.07.010
39. Woellner C, Gertz EM, Schaffer AA, et al. Mutations in STAT3 and diagnostic guidelines for hyper-IgE syndrome. J Allergy Clin Immunol. 2010;125(2):424–432 e428. doi:10.1016/j.jaci.2009.10.059
40. Xie YL, Rosen LB, Sereti I, et al. Severe Paradoxical Reaction During Treatment of Disseminated Tuberculosis in a Patient With Neutralizing Anti-IFNgamma Autoantibodies. Clin Infect Dis. 2016;62(6):770–773. doi:10.1093/cid/civ995
41. Yerramilli A, Huang GKL, Griffin DWJ, et al. Disseminated Nontuberculous Mycobacterial Infection Associated With Acquired Immunodeficiency Due to Anti-Interferon gamma Autoantibodies. Open Forum Infect Dis. 2019;6(4):ofz131. doi:10.1093/ofid/ofz131
42. Pan M, Qiu Y, Zeng W, Tang S, Wei X, Zhang J. Disseminated Talaromyces marneffei infection presenting as multiple intestinal perforations and diffuse hepatic granulomatous inflammation in an infant with STAT3 mutation: a case report. BMC Infect Dis. 2020;20(1):394. doi:10.1186/s12879-020-05113-4
43. Zhang W, Ye J, Qiu C, et al. Rapid and precise diagnosis of T. marneffei pulmonary infection in a HIV-negative patient with autosomal-dominant STAT3 mutation: a case report. Ther Adv Respir Dis. 2020;14:1753466620929225. doi:10.1177/1753466620929225
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.