Back to Journals » OncoTargets and Therapy » Volume 14
Anti-γ-Aminobutyric Acid Type β Receptor Antibody-Associated Encephalitis After 2 Cycles of Durvalumab Therapy in a Patient with Small Cell Lung Cancer: A Case Report
Authors Li J , Jiang J, Lv J, Zhang R , Chen W , Li S, Jin Q, Wang G
Received 9 September 2021
Accepted for publication 21 October 2021
Published 3 November 2021 Volume 2021:14 Pages 5227—5236
DOI https://doi.org/10.2147/OTT.S337331
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Arseniy Yuzhalin
Jiamo Li,1 Jianing Jiang,1,2 Jinyan Lv,1 Ru Zhang,1 Wenting Chen,1,2 Shiyi Li,1 Qianchen Jin,1 Gang Wang1,2
1Department of Medical Oncology, The Affiliated Zhongshan Hospital of Dalian University, Dalian, Liaoning Province, People’s Republic of China; 2The Key Laboratory of Biomarker, High-Throughput Screening and Target Translation of Breast and Gastrointestinal Cancer, Dalian, Liaoning Province, People’s Republic of China
Correspondence: Gang Wang
Department of Medical Oncology, The Affiliated Zhongshan Hospital of Dalian University, Dalian, Liaoning Province, 116001, People’s Republic of China
Tel/Fax +86-411-6289737
Email [email protected]
Abstract: Anti-γ-aminobutyric acid type β receptor (anti-GABABR) antibody-associated encephalitis is a type of autoimmune encephalitis. According to current literature, its pathogenesis is reported to be closely related to tumor factors. However, diagnosis can be difficult because of the rarity of cases, limited clinical understanding, and a lack of specificity in clinical manifestation and imaging presentation. Clinical trials have demonstrated that immunotherapy can prolong the survival of patients with small cell lung cancer; however, in some cases, immunotherapy may induce anti-GABABR antibody-associated encephalitis. Patients who develop this encephalitis during immunotherapy often delay treatment because the cause is not clearly identified. In this study, we report a case of a 61-year-old man with a confirmed diagnosis of small cell lung cancer who had acute onset of cognitive impairment and seizures after two cycles of durvalumab (AstraZeneca UK Limited) combination chemotherapy. This reaction was initially considered as an immune-related adverse event (irAE) caused by durvalumab treatment, and the patient was eventually considered to have a paraneoplastic neurological disorder caused by the primary tumor. This report raises awareness of the symptoms of cognitive impairment and seizures in patients with small cell lung cancer, and the possible adverse events associated with immunotherapy. This case also highlights the importance of detecting anti-GABABR antibodies in patients with small cell lung cancer.
Keywords: anti-GABABR antibody, autoimmune encephalitis, immunotherapies, small cell lung cancer, durvalumab
Introduction
Autoimmune encephalitis (AE) is an important part of non-infectious encephalitis,1 which generally refers to a large group of diseases caused by immune responses against central nervous system (CNS) antigens. anti-GABAB receptor encephalitis is caused by anti-GABAB receptors, a cell-surface antibody, which are widely distributed in the limbic lobe system,2,3 accounting for approximately 4% to 5% of autoimmune encephalitis with anti-neural cell surface antigen antibodies.4 Dysfunction of the limbic system can cause epilepsy, cognitive impairment, and other symptoms.5 In addition, nearly 50% of the patients with anti-GABABR antibody-associated encephalitis also suffer from small cell lung cancer (SCLC) or pulmonary neuroendocrine tumors, and thus this type of encephalitis has also been thought to be a form of small cell lung cancer paraneoplastic syndrome.
SCLC accounts for approximately 15% of all lung cancer,6 and it is an aggressive tumor characterized by rapid doubling time and high propensity for early development of disseminated disease.7 Immunotherapy is the most promising SCLC treatment in recent years.6 Three randomized clinical trials have demonstrated the efficacy of the combination of atezolizumab (IMPOWER 133) or durvalumab (CASPIAN) or pembrolizumab (Keynote-604) with chemotherapy in the first-line treatment of patients with ES-SCLC (extensive stage-small cell lung cancer). Among them, Durvalumab plus chemotherapy significantly improved median OS compared with the chemotherapy arm (13.0 versus 10.3 months; HR 0.73, 95% CI: 0.59–0.91; p=0.0047). Median PFS was 5.1 months (95% CI: 4.7–6.2) with durvalumab plus platinum–etoposide versus 5.4 months (95% CI:4.8–6.2) with platinum–etoposide alone.8 Immunotherapies targeting immune checkpoint inhibitors (ICIs) have led to a major paradigm shift in cancer treatment, improving overall survival rates for a wide range of cancers.9,10 However, despite the improved outcomes for cancer treatment, ICIs treatment has also been associated with a large number of toxicities, described as immune-related adverse events (irAEs).11,12 Among them, neurotoxicity involving the nervous system belongs to a rare category of immune-related adverse events called adverse neurological events (NAES). A large-scale analysis of the combined safety results of the CheckMate 069 Phase II and CheckMate 067 Phase III clinical trials showed a 2.2% incident rate of NAEs compared to an overall incident rate of 38.3% adverse reactions.13 Multiple neurological syndromes are associated with ICI treatment: polyneuropathy, facial palsy, optic neuritis, Guillain–Barré syndrome, myasthenia gravis, transverse myelitis, encephalitis, and aseptic meningitis.12 Autoimmune encephalitis due to treatment with ICIs is a type of NAES,14 which also includes the occurrence of anti-γ-GABABR antibody-associated encephalitis. We report the diagnosis and treatment of anti-GABABR antibody-associated encephalitis that was reported in a small cell lung cancer patient receiving immunotherapy. This case report provides a reference for the identification of different etiologies in the clinical setting and for targeted treatment.
Case Report
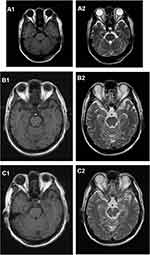
A 61-year-old Chinese male who was a long-term smoker. The chief complaint was cough with no obvious inducement for more than 1 month, mainly dry cough with blood in the sputum and occasional left-sided chest tightness. Chest CT showed possible malignant space occupying in the left lung, multiple lymph nodes enlargement in the left hilum and mediastinum, and inflammatory changes in the lower lobe of the left lung (Figure 1A1 and A2). After admission to our hospital, the imaging examination result shows that head MRI: white matter change (DWMH (deep white matter hyperintensities) grade I); Cysts of the right maxillary sinus may be involved (Figure 2A1 and A2). The result of pathological histopathology and immunohistochemistry by local bronchoscopy biopsy performed showed that: Small-cell endocrine cancer,TTF1 (+), Napsin-A (-), CK7 (-), ck5/6 (-), p63 (-), CK20 (-), CD56 (+), Syn(partially weak +), CgA (+), Ki-67 index50% (Figure 3A1 and A2). PETCT result showed that: hyperplasia masses of FDG metabolism increasing mass had been spotted in the left lower hilum, the maximum cross section about 4.6×6.2 with radiation uptake increased, and the SUVmax was about 18.1. Bronchial stenosis by compression in the left lower lung lobe was observed, and high density patch shadow could be seen at the distal end of the masses, with the radiation uptake uneven and increased, and the SUVmax was about 4.5, which was in line with the signs of central lung cancer with atelectasis of partial distal lung tissue, as well as the signs of obstructive inflammation: multiple lymph nodes with increased FDP metabolism in the left hilum and mediastinum had been observed, metastases were thus considered (Figure 4A1 and A2). Based on the patient’s pathology and imaging, the patient’s current diagnosis is extensive stage small cell lung cancer.The patient was treated with chemotherapy combined with immunotherapy according to NCCN guidelines. Subsequently, two cycles of etoposide (Qilu Pharmaceutical Co., Hainan, China) + carboplatin (Qilu Pharmaceutical Co., Hainan, China) + durvalumab regimen had been operated: etoposide 100mg d1-5 ivgtt; Carboplatin AUG5 d1 ivgtt; durvalumab1500mg d1 ivgtt; Q21d/C, and the patient had digestive tract reaction of I degree, as well as the numbness symptoms of hands and feet. Active antiemetic and neurotrophic symptomatic treatment were operated then tolerated by the patient. After two cycles of chemotherapy, the patient got the imaging evaluation result as PR (Figure 1B1 and B2). Experiencing his first epileptic seizure at night, the patient suffered convulsions and loss of consciousness occurred after sudden screaming in sleep. His head turning to the left, both eyes gazing to the right, the patient frothed at the mouth. The symptom lasted for seconds, and later he showed obstacles in conversation. Urgent examination of head CT and EEG showed no obvious abnormality (Figures 2B1, B2 and 5A), and electrolyte tests showed the result of Na+129mmol/L and Cl- 93.2mmol /L. At that time, considering the adverse reactions of immunotherapy drugs, hyponatremia and hypochloremia, Samsca (Zhejiang Otsuka Pharmaceutical Co., China) of 15mg qd and Sodium valproate (Hunan Xiangzhong Pharmaceutical, China) of 0.2 tid were actively given to correct the electrolyte disorder and to prevent epileptic seizures, respectively. The patient was then in stable condition and left the hospital. After 3 days, he suffered seizures twice, both of which improved spontaneously, and was readmitted to hospital, conscious but unresponsive. Then lumbar puncture was performed. After 2 days, the patient suffered another epileptic seizure, which improved spontaneously 2 minutes later, and then transient apnea occurred. The blood oxygen level dropped to about 50%, and showed obstacles in responding. EEG showed abnormal situation with sharp and slow waves (Figure 5B); brain MRI showed that there were strip-shaped DWI signals with slightly high intensity and reduced ADC in the left hippocampus. Spots and small patches seen in the center of bilateral semi-oval, T1WI was slightly lower while T2WI was slightly higher, and FLAIR showed high signal (Figure 2C1 and C2). Cerebrospinal fluid examination result of lumbar puncture indicated that: Pandy test: positive (+), total cell count (Tcell) 20*10^6/L, transparency: transparent, color: colorless. Glucose: 2.48mmol/L, chlorine: 111.4mmol/L, aspartate aminotransferase (AST): 14IU/L, L-lactate dehydrogenase (LDH): 27U/L, adenosine dehydrogenase: 2U/L, creatine kinase (CK): 0U/L, protein quantification (PRO): 794.96mg/L. We use a cell-based indirect immunofluorescence (IIF) assay to detect antibodies against neuronal cell surface antigens and antibodies against intracellular antigens, and the external cerebrospinal fluid test showed that the anti-γ-aminobutyric acid type β receptor (GABABR) antibody IgG was positive (Table 1).
 |
Table 1 Cerebrospinal Fluid Test Showed That the Anti-γ-Aminobutyric Acid Type B Receptor (GABAB) Antibody IgG Was Positive |
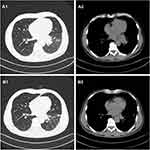 |
Figure 1 (A1 and A2) The baseline CT scan of the patient’s chest in August 2020. (B1 and B2) CT scan of the chest after two cycles of durvalumab in October 2020. |
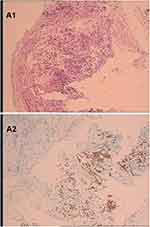 |
Figure 3 (A1 and A2) The result of the lump in the left lung pathological histopathology and immunohistochemistry by local bronchoscopy biopsy performed showed that is small-cell endocrine cancer. |
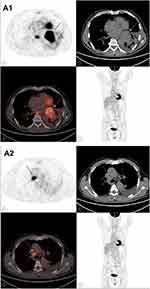 |
Figure 4 (A1 and A2) PETCT on August 27, 2020; The increased mass of FDG metabolism in the hyperplastic block indicated by the arrow. |
Combined with the clinical manifestations, the patient was diagnosed as anti-GABAB receptor encephalitis, and was diagnosed to continue taking sodium valproate orally, with gamma globulin (Guangdong Weilun Biopharmaceutical Co., China) (0.4g/kg) 30g/day and levetiracetam tablets (UCB Pharma S.A., Belgium) of 0.5g qd treatment supplemented. On November 1, 2020, intermittent convulsions occurred several times during the day and spontaneously relieved. At 15:00, the patient had another convulsion and was immediately given diazepam 10mg of intravenous push. A few minutes later, the patient suddenly suffered from respiratory and cardiac arrest and was immediately given cardiopulmonary resuscitation. At 16:05, the patient was transferred to MICU treatment, and high-dose hormone with methylprednisolone (Pharmacia Upjohn Inc, China) 500mg was injected intravenously once a day, supplemented by continuing human immunoglobulin intravenous injection and antiepileptic treatment. The patient’s condition was in remission than before. At 6:00 on November 2, 2020, the patient had sudden convulsions and spontaneously relieved within a few seconds, followed by ventricular tachycardia, ventricular fibrillation and sudden death. CPR was actively performed, and the rescue was successful. The patient was then treated by intravenous injection impact therapy with increasing dose of methylprednisolone 1.0/day for 2 days, reducing dose of methylprednisolone by 500mg for 2 days, and reducing dose of methylprednisolone by 80mg for 2 days. At 12:10 on November 7, 2020, the patient reappeared with convulsions. After relief for seconds, the blood pressure dropped, and blood oxygen saturation dropped to 60–70%. The heart rate was 110 times/min, and the pulse was weak. Then the oxygen flow adjusted to increase, the intermittent sputum suction operated, the metaraminol increased to the maximum of 10mL/h, the ventilator was adjusted to the highest ventilation pressure, the symptoms were no remission; dopamine (Shanghai Hefei Pharmaceutical, China), nikethamide (Tianjin Gold Pharmaceutical Co., China), and lobeline (Shanghai Hefei Pharmaceutical, China) were used to injected intravenously, the symptoms were still no remission. The rescuing failed.
Discussion
Anti-GABABR antibody-associated encephalitis is usually characterized clinically by acute seizures, memory loss, and impaired consciousness.15 Epilepsy is the prominent and critical clinical manifestation of anti-GABABR antibody-associated encephalitis, with epilepsy or intractable epilepsy often the first symptom.3,16 GABAB is widely distributed in the central nervous system of rodents and humans, and thus there is a high density of GABABR binding sites in the brain and spinal cord, especially in the hippocampus, thalamus, and cerebellum.17,18 GABAB1R and GABAB2R heterodimers constitute G protein-coupled receptors, which exert mainly inhibitory effects through potassium and calcium channels. Anti-GABABR antibody-associated encephalitis acts on GABABR through autoantibodies that form GABABR complexes. These complexes inhibit the highly active neural network cells and prevent normal synchronous activity of neuron cells, while allowing new stimuli to disrupt the synchronous activity of neuron cells. As an inhibitory synaptic receptor, GABABR is widely distributed in the limbic lobe system, which is associated with the regulation of cognition, emotion, and autonomic nervous function. This explains how GABABR can cause epileptic seizures and changes in memory and behavior.5
Nearly 50% of the patients diagnosed with Anti-GABABR antibody encephalitis were later clinically diagnosed with SCLC. In this case, the patient was first pathologically diagnosed as SCLC, without suffering from psychiatric symptoms such as epilepsy and abnormalities on head magnetic resonance imaging (MRI), when he was hospitalized. The first symptom was acute onset of epilepsy after two cycles of durvalumab treatment. Laboratory tests indicated hyponatremia and hypochloremia, therefore the initial diagnosis was seizures caused by electrolyte imbalance. Moreover, further cognitive symptoms such as unresponsiveness and loss of calculation power gradually appeared. Seizures were frequent, including severe and intractable seizures, with poor response to antiepileptic drugs, and the patient eventually displayed neurobehavioral abnormalities and clinical manifestations of memory decline.
Considering that the patient developed neurological symptoms during the course of immunotherapy, the occurrence of immune encephalitis due to immunotherapy cannot be excluded. As several recent studies have shown, immunotherapy has been a major advance in cancer treatment. Cancer cells evade recognition and destruction by the immune system by over-expressing various immunosuppressive molecules in the tumor microenvironment.19,20 Some of these molecules, such as cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4), programmed cell death protein 1 (PD-1) and its ligand PD-L1 have been used as targets for novel immunotherapeutic agents. These so-called ICIs allow the immune system to reactivate cytotoxic T lymphocytes and destroy tumor cells, which can produce a remarkable clinical benefit for a wide range of cancer types.21
However, altering the immune system in this way may subsequently lead to the development of various clinically relevant autoimmune manifestations, which means the occurrence of irAEs. Since these effects are thought to be caused by overall immune enhancement, they may affect any system in the body, but primarily the skin, colon, lungs, endocrine glands, and liver. Of these, neurotoxicity involving the nervous system is relatively rare. Neurological adverse events (NAEs) are uncommon compared to other adverse reactions.12,19–23
Currently, no cases of epilepsy-related symptoms caused by durvalumab have been previously reported. Information provided by the manufacturers states
the clinical manifestation of encephalitis in immune-mediated adverse reactions has the potential to trigger seizures, but the incidence of aseptic meningitis in 1889 patients treated with durvalumab was less than 1%. In a retrospective study on immune checkpoint inhibitors in central nervous system complications, including 19 patients treated with immunotherapy, the associated neurotoxicity typing and clinical signs were reported, and one patient treated with the combination of durvalumab+ ipilimumab developed cerebellar syndrome but did not develop symptoms of epilepsy.24
Reports show that the time of ICI treatment and onset of neurological syndrome episodes varies; out of six cases described in the Global Pharmacovigilance and Epidemiology Database, the median onset time was 51 days.13 Relevant literature reported that typical signs and symptoms of NAEs occur within 3 months of starting immunotherapy, with a median symptom onset at 6 weeks.10
In general, the neurological complications caused by immunotherapy involve the peripheral nervous system, and the immune-related complications of the central nervous system are rare,24,25 and there has been no report of the epilepsy-related cases caused by durvalumab. However, in this study, it took 23 days for the patient to receive durvalumab to present with acute epilepsy, which is in line with the time from ICI treatment to onset of neurological symptoms reported previously. In summary, while we believed it unlikely that the immune encephalitis was caused by immunotherapy in this case, the possibility could not be completely ruled out. The use of immunotherapy was therefore discontinued at the initial onset of acute disease.
A case of anti-GARBR antibody-associated encephalitis diagnosed in an elderly woman after three cycles of durvalumab treatment was previously reported, and the patient was considered to have irAEs due to immunotherapy because the patient stopped immunotherapy immediately after the acute onset of the disease and improved completely after hormone therapy was given. In contrast, the patient herein did not improve significantly after discontinuation of immunotherapy and active administration of hormonal therapy, so the patient herein was also not considered to have irAEs due to immunotherapy at the level of therapeutic effectiveness.26
Based on previous clinical experience and exclusion diagnosis, we considered that the patient was more likely to have autoimmune encephalitis caused by the primary tumor, namely paraneoplastic neurological disease. Paraneoplastic neurological diseases are quite common in patients with SCLC, the most common being Lambert–Eaton myasthenic syndrome (LEMS), myasthenia syndrome, sensory neuropathy, and limbic encephalitis. The clinical manifestations of anti-GABABR antibody-associated encephalitis are consistent with those of limbic encephalitis, which are usually characterized by acute epilepsy, memory loss and impaired consciousness.14
As described, there were initially EEG, CT, or MRI abnormalities, however abnormalities were observed in the later phase. Fluid attenuated inversion recovery-T2 weighted (FLAIR/T2) MRI abnormalities (no substantial enhancement) involving the medial temporal lobe often occur in patients with typical marginal encephalitis, and MRI results may be the result of seizures. Therefore, a lack of corresponding abnormal imaging results at the onset of illness is not contradictory to the appearance of imaging abnormalities at a later stage; this may be a sign of disease progression in terms of the increased frequency of seizures in the patient. In addition, MRI changes in patients cannot provide specific information for the diagnosis of anti-GABABR antibody-associated encephalitis.27 Therefore, negative brain MRI scan results cannot exclude the diagnosis of this disease. Most importantly, anti-γ-aminobutyric acid antibodies were found in the patient’s cerebrospinal fluid, which is the strong evidence for the diagnosis of anti-GABABR antibody-associated encephalitis.
Conclusion
This rare case demonstrates that patients with SCLC can have sudden acute onset neurological symptoms, manifested as cognitive impairment and seizures, which may be combined with anti-GABABR antibody-associated encephalitis. Our case report will assist with the etiology of patients with anti-GABABR antibody-associated encephalitis. The case also highlights the importance of testing for anti-γ-aminobutyric acid antibodies in the detection of small cell lung cancer. Other neurotoxic reactions of the nervous system caused by immunotherapy also deserve to be taken into account. It is important to recognize these conditions because timely diagnosis and treatment directly affect the prognosis of patients.
Ethics Statements
The Affiliated Zhongshan Hospital of Dalian University approved publishing the case details.
Acknowledgments
We thank the patient in this report and her family. Written informed consent was obtained from the patient for publication of this case report and any accompanying images. This work was supported by grants from the National Natural Science Foundation of China (#81803109) and the Doctor Study-up Foundation of Liaoning Province (2019-BS-010).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Dutra LA, Abrantes F, Toso FF, Pedroso JL, Barsottini OGP, Hoftberger R. Autoimmune encephalitis: a review of diagnosis and treatment. Arq Neuropsiquiatr. 2018;76(1):41–49. doi:10.1590/0004-282x20170176
2. Armangue T, Leypoldt F, Dalmau J. Autoimmune encephalitis as differential diagnosis of infectious encephalitis. Curr Opin Neurol. 2014;27(3):361–368. doi:10.1097/WCO.0000000000000087
3. Zeng W, Cao L, Zheng J, Yu L. Clinical characteristics and long-term follow-up of seven cases of anti-GABABR encephalitis in patients of Han Chinese descent. Neurol Sci. 2020;41(2):373–378. doi:10.1007/s10072-019-04095-9
4. Lancaster E, Martinez-Hernandez E, Dalmau J. Encephalitis and antibodies to synaptic and neuronal cell surface proteins. Neurology. 2011;77(2):179–189. doi:10.1212/WNL.0b013e318224afde
5. Esposito S, Principi N, Calabresi P, Rigante D. An evolving redefinition of autoimmune encephalitis. Autoimmun Rev. 2019;18(2):155–163. doi:10.1016/j.autrev.2018.08.009
6. Yang S, Zhang Z, Wang Q. Emerging therapies for small cell lung cancer. J Hematol Oncol. 2019;12(1):47. PMID: 31046803; PMCID: PMC6498593. doi:10.1186/s13045-019-0736-3
7. Waqar SN, Morgensztern D. Treatment advances in small cell lung cancer (SCLC). Pharmacol Ther. 2017;180:16–23. PMID: 28579387. doi:10.1016/j.pharmthera.2017.06.002
8. Esposito G, Palumbo G, Carillio G, et al. Immunotherapy in small cell lung cancer. Cancers. 2020;12(9):2522. PMID: 32899891; PMCID: PMC7565004. doi:10.3390/cancers12092522
9. Onoi K, Chihara Y, Uchino J, et al. Immune checkpoint inhibitors for lung cancer treatment: a review. J Clin Med. 2020;9(5):1362. doi:10.3390/jcm9051362
10. Reynolds KL, Guidon AC. Diagnosis and management of immune checkpoint inhibitor-associated neurologic toxicity: illustrative case and review of the literature. Oncologist. 2019;24(4):435–443. doi:10.1634/theoncologist.2018-0359
11. Nakamura Y. Biomarkers for immune checkpoint inhibitor-mediated tumor response and adverse events. Front Med. 2019;6:119. doi:10.3389/fmed.2019.00119
12. Kumar V, Chaudhary N, Garg M, Floudas CS, Soni P, Chandra AB. Current diagnosis and management of immune related adverse events (irAEs) induced by immune checkpoint inhibitor therapy. Front Pharmacol. 2017;8:49. doi:10.3389/fphar.2017.00049
13. Pan PC, Haggiagi A. Neurologic immune-related adverse events associated with immune checkpoint inhibition. Curr Oncol Rep. 2019;21(12):108. doi:10.1007/s11912-019-0859-2
14. Bien CG. Diagnosing autoimmune encephalitis based on clinical features and autoantibody findings. Expert Rev Clin Immunol. 2019;15(5):511–527. doi:10.1080/1744666X.2019.1573676
15. Newman MP, Blum S, Wong RC, et al. Autoimmune encephalitis. Intern Med J. 2016;46(2):148–157. doi:10.1111/imj.12974
16. Vogrig A, Joubert B, André-Obadia N, Gigli GL, Rheims S, Honnorat J. Seizure specificities in patients with antibody-mediated autoimmune encephalitis. Epilepsia. 2019;60(8):1508–1525. doi:10.1111/epi.16282
17. Nosadini M, Mohammad SS, Ramanathan S, Brilot F, Dale RC. Immune therapy in autoimmune encephalitis: a systematic review. Expert Rev Neurother. 2015;15(12):1391–1419. doi:10.1586/14737175.2015.1115720
18. Heine J, Prüss H, Bartsch T, Ploner CJ, Paul F, Finke C. Imaging of autoimmune encephalitis–relevance for clinical practice and hippocampal function. Neuroscience. 2015;309:68–83. doi:10.1016/j.neuroscience.2015.05.037
19. Tian Y, Gao A, Wen Q, et al. Immune-related neurological toxicities of PD-1/PD-L1 inhibitors in cancer patients: a systematic review and meta-analysis. Front Immunol. 2020;11:595655. doi:10.3389/fimmu.2020.595655
20. Barquín-García A, Molina-Cerrillo J, Garrido P, Garcia-Palos D, Carrato A, Alonso-Gordoa T. New oncologic emergencies: what is there to know about immunotherapy and its potential side effects? Eur J Intern Med. 2019;66:1–8. doi:10.1016/j.ejim.2019.05.020
21. Tison A, Quéré G, Misery L, et al. Safety and efficacy of immune checkpoint inhibitors in patients with cancer and preexisting autoimmune disease: a nationwide, multicenter cohort study. Arthritis Rheumatol. 2019;71(12):2100–2111. doi:10.1002/art.41068
22. Choi J, Lee SY. Clinical characteristics and treatment of immune-related adverse events of immune checkpoint inhibitors. Immune Netw. 2020;20(1):e9. doi:10.4110/in.2020.20.e9
23. Hahn AW, Gill DM, Agarwal N, Maughan BL. PD-1 checkpoint inhibition: toxicities and management. Urol Oncol. 2017;35(12):701–707. doi:10.1016/j.urolonc.2017.08.005
24. Vogrig A, Muñiz-Castrillo S, Joubert B, et al. Central nervous system complications associated with immune checkpoint inhibitors. J Neurol Neurosurg Psychiatry. 2020;91(7):772–778. doi:10.1136/jnnp-2020-323055
25. Johnson DB, Manouchehri A, Haugh AM, et al. Neurologic toxicity associated with immune checkpoint inhibitors: a pharmacovigilance study. J Immunother Cancer. 2019;7(1):134. doi:10.1186/s40425-019-0617-x
26. Shechtman Y, Shalata W, Khoury R, Mahajna A, Weller B, Agbarya A. Encephalitis induced by durvalumab during treatment of metastatic small-cell lung cancer: illustrative case and review of the literature. J Immunother. 2021;44(7):243–247. PMID: 33734140. doi:10.1097/CJI.0000000000000362
27. Zhao XH, Yang X, Liu XW, Wang SJ. Clinical features and outcomes of Chinese patients with anti-γ-aminobutyric acid B receptor encephalitis. Exp Ther Med. 2020;20(1):617–622. doi:10.3892/etm.2020.8684
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.