Back to Journals » Journal of Pain Research » Volume 16
Analysis of the Efficacy of Percutaneous Endoscopic Interlaminar Discectomy for Lumbar Disc Herniation with Different Types/Grades of Modic Changes
Authors Shi Z, Li P, Wu W, Jiang Y, Wang Y
Received 4 January 2023
Accepted for publication 27 May 2023
Published 6 June 2023 Volume 2023:16 Pages 1927—1940
DOI https://doi.org/10.2147/JPR.S403266
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor E Alfonso Romero-Sandoval
Zhen Shi,1 Pengfei Li,1 Wentao Wu,1 Yunduo Jiang,1 Yansong Wang1– 3
1Department of Orthopedics, The First Affiliated Hospital of Harbin Medical University, Harbin, Heilongjiang Province, People’s Republic of China; 2NHC Key Laboratory of Cell Transplantation, Harbin Medical University, Harbin, Heilongjiang Province, People’s Republic of China; 3Heilongjiang Provincial Key Laboratory of Hard Tissue Development and Regeneration, Harbin Medical University, Harbin, Heilongjiang Province, People’s Republic of China
Correspondence: Yansong Wang, Email [email protected]
Background: Percutaneous endoscopic interlaminar discectomy (PEID), one of the main techniques of spinal endoscopy, has achieved excellent results in treating lumbar disc herniation (LDH). However, its efficacy has not been systematically described in patients with LDH accompanied by Modic changes (MC).
Purpose: The purpose of this study was to observe the clinical efficacy of PEID treatment of LDH accompanied by MC.
Patients and Methods: A total of 207 patients who underwent PEID surgery for LDH were selected. According to the existence and type of MC on preoperative lumbar magnetic resonance images (MRI), they were divided into normal group (no MC, n=117), M1 group (MC I, n=23), and M2 group (MC II, n=67). According to the severity of MC, they were divided into MA group (grade A, n=45) and MBC group (grade B and C, n=45). The visual analog scale (VAS) score, Oswestry disability index (ODI) score, Disc height index (DHI), Lumbar lordosis angle (LL) and modified Macnab criteria were used to assess clinical outcomes.
Results: Postoperative back pain and leg pain VAS scores and ODI scores were significantly improved in all groups compared with preoperative scores. Patients with MC showed a deterioration in postoperative back pain VAS scores and ODI scores as time went by, and postoperative DHI decreased significantly compared with preoperative. Postoperative LL did not change significantly in each group. There was no significant difference in complications, recurrence rate and excellent rate between the groups.
Conclusion: Whether accompanied by MC or not, the efficacy of PEID for LDH was significant. However, postoperative back pain and functional status of patients with MC tend to deteriorate as time went by, especially those with type I or severe MC.
Keywords: Modic changes, MC type, MC grade, percutaneous endoscopic interlaminar discectomy, lumbar disc herniation, low back pain
Introduction
The incidence of lumbar disc herniation (LDH) has been increasing year by year, and about 10–15% of patients have to undergo surgical treatment because conservative treatment is ineffective or pain seriously affects life.1 However, there is still considerable controversy regarding the treatment of LDH with Modic changes (MC) in the corresponding segment.2,3 MC were abnormal signal changes in the endplate and subendplate bone marrow on magnetic resonance imaging (MRI). According to these signal changes and histopathology, MC was divided into three types by Modic et al:4,5 In patients with MC I, the endplate-vertebral interface is filled with vascular granulation tissue, which represents the inflammatory response of the bone marrow. MC II bone marrow space is replaced by fat. MC III is associated with subchondral osteosclerosis. In order to reduce the heterogeneity of the research, Udby et al6,7 proposed that MC grade is more clinically meaningful than MC type. According to the proportion of cumulative vertebral body height of MC, the severity is divided into three grades. Current studies generally believe that MC are closely related to low back pain and vertebral instability.8 The traditional lumbar fenestration laminectomy surgery will undoubtedly further damage the stability of the lumbar spine. Although the fixed fusion surgery can deal with the degenerated endplate and restore the stability of the vertebral body, the surgical trauma is relatively destructive, and the changes in postoperative biomechanics make adjacent segments degenerate. To make matters worse, Li et al9 found that type II sclerotic MC can affect bone fusion in patients undergoing lumbar posterior surgery, Wang et al10 reported that MC increase the risk of Cage sinking. Therefore, the treatment of LDH with MC becomes particularly complicated.
Prof. Ruetten proposed percutaneous endoscopic interlaminar discectomy (PEID) in 2006 as a complementary procedure to percutaneous endoscopic transforaminal discectomy (PETD), which is more advantageous in treating LDH with high ilium and hypertrophic transverse process in the L5-S1 segment.11 PEID, as one of the major minimally invasive techniques, resembles the view of traditional open surgery as well as more direct intra-spinal decompression, minimizing damage to paraspinal muscle stripping and small joints to maintain spinal stability, and has now been proved to have excellent postoperative results and surgical safety.12 With the development of spinal endoscopic instruments and the improvement of surgical techniques, PEID is not limited to the treatment of L5-SI segments, and the indications have also expanded from a single LDH to lumbar spinal stenosis, lumbar vertebral metastases, lumbar disc cysts and reparation of recurrent LDH.13,14 However, its efficacy in LDH with MC has not been systematically described. The purpose of this study was to evaluate the clinical effectiveness of PEID treatment for LDH with MC of different types/grades.
Materials and Methods
Patient Selection
The research was in compliance with the Helsinki Declaration. And it was approved by the Ethics Committee of the First Affiliated Hospital of Harbin Medical University. 207 patients who underwent PEID surgery at our hospital from July 2019 to December 2021 were selected (Figure 1). Inclusion criteria were: (1) significant symptoms of low back pain and leg pain; (2) symptoms consistent with imaging; (3) ineffective strictly conservative treatment for >3 months; (4) if MC were indicated in the endplate, the segment of MC was required to be a single segment and consistent with the segment of lumbar disc herniation. Exclusion criteria: (1) multi-segment treated lumbar disc herniation, multi-segment MC, and mixed MC; (2) those with previous history of lumbar spine surgery; (3) patients with lumbar degeneration: lumbar spine tumor, lumbar spine tuberculosis, etc; (4) lumbar instability and spondylolisthesis; (5) lumbar spinal stenosis and scoliosis; (6) extreme lateral disc herniation.
 |
Figure 1 Flow chart for inclusion in the study population. |
Radiological Evaluation
The lumbar spine MRI evaluation included: MC type, MC grade (Figure 2), MSU Classification, types of lumbar disc herniation and Pfirrmann grade of disc degeneration. Sagittal images were imaged with T1-weighted and T2-weighted, and axial images were imaged at the level of the herniated lumbar disc segment with T2-weighted. The lumbar spine X-ray evaluation included: Disc height index (DHI) and Lumbar lordosis angle (LL) from standing lateral radiographs. All Imaging parameters were evaluated twice by two spine surgeons experienced in lumbar imaging reading and three times by a third surgeon if there was inconsistency after the second evaluation. The surgeons were blinded to the clinical status.
 |
Figure 2 Grade A, B, and C of MC II on T2-weighted sagittal MRI. The blue dashed line represents 25% of the vertebral height; the yellow dashed line represents 50% of the vertebral height. |
According to the traditional MC classification method:4 we observed whether there are abnormal MRI signal changes in the vertebral endplate and subendplate bone, and divided the patients into: normal group (no abnormal signal); M1 group (MC I signal: low signal in T1 image and high signal in T2 image); M2 group (MC II signal: high signal in T1 image and mild high signal in T2 image); M3 group (MC III signal: low signal in both T1 and T2 images).
In the same functional spine unit (FSU), the MC grades were classified according to the highest sagittal section of MC: Grade A: MC invade <25% of vertebral body height; Grade B: MC invade 25–50% of vertebral body height; Grade C: MC invade >50% of vertebral body height. Consider MA as mild MC (Grade A) and MBC as severe MC (Grade B and C). Only vertebral marrow signal changes extending from the endplate and involving two or more adjacent sagittal slices were classified as MC; very small areas of signal change (<5 mm in diameter) usually occur in the corners of the vertebral body and were not classified as MC in this study.15
The degree of intervertebral disc degeneration was graded by Pfirrmann:16 Grades 1 and 2 represent normal disc height with clear distinction between the nucleus pulposus and the annulus fibrosus; grade 3 has normal or slightly decreased disc height with indistinct distinction between the nucleus pulposus and the annulus fibrosus; grade 4 has moderately decreased disc height with loss of distinction between the nucleus pulposus and the annulus fibrosus; and grade 5 has collapsed disc with loss of distinction between the nucleus pulposus and the annulus fibrosus.
According to the MSU classification standard,17 the degree and location of intervertebral disc herniation are divided into: 2-A, 2-B, 2-AB, 3-A, 3-B, 3-AB. According to types of lumbar disc herniation,18 they are classified into three categories: protrusion, extrusion and sequestration.
The DHI was measured as follows:19 the average of the anterior, middle and posterior height of the vertebral space was measured as the disc height, and the ratio of the disc height to the sagittal diameter of the superior vertebral body as the DHI. The LL is the angle between the vertical line of the upper edge of the L1 vertebral body and the vertical line of the upper edge of the S1 vertebral body on the lumbar lateral radiographs20 (Figure 3).
Surgical Procedures
The patient is placed in the prone position. The puncture entry point is marked approximately 1.0 cm next to the spinous process of the lesioned segment. 0.25% lidocaine anesthetizes the skin, subcutaneous tissue, fascia, muscle, small joints of the lumbar spine, and ligamentum flavum layer by layer. The sharp blade makes a skin incision approximately 1.0 cm long at the puncture site and incises the subcutaneous and fascia in sequence. Next place the working channel and endoscope. After endoscopic removal of part of the lamina and ligamentum flavum to access the spinal canal and removal of part of the epidural fat, the dural sac and nerve roots are exposed, and a nerve probe is used to confirm the location of the nerve roots and their relationship to the adjacent tissues, and to release the adhesions around the nerve roots and dural sac. 3–5 mL of 1.33% lidocaine is injected into the epidural space, and shortly after induction of anesthesia, the working channel is rotated to gently push the nerve root medially to reveal the herniated disc and remove the herniated nucleus pulposus, and some of the degenerated nucleus pulposus within the disc. The operation was completed after adequately decompressing the nerve root, stopping bleeding, probing for decompression, and suturing the incision (Figure 4).
Follow-Up of Postoperative Outcomes
Patients were followed up by telephone and outpatient visits at 3 months, 6 months, and 1 year after surgery. Visual analog scale (VAS) was used to assess changes in patients’ low back and lower extremity pain. The Oswestry Dysfunction Index (ODI) was used to assess the improvement of patients’ functional impairment. The imaging parameters were measured by picture archiving and communication system (PACS, version 4.1.5.1). The modified Macnab criteria were used to assess the excellent rate at the last follow-up. A period of pain-free remission of at least 6 months with re-protrusion of the same segment ipsilaterally or contralaterally is considered a recurrence.
Statistical Analysis
In this study, SPSS 26.0 software was used for statistical analysis of the data. Measurement data were expressed as 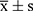 . For measurement data that followed a normal distribution and had homogeneity of variance, t-tests or one-way ANOVA were used; otherwise, non-parametric tests were used. Count data were analyzed using chi-square tests or Fisher’s exact probability tests. A P-value of less than 0.05 was considered statistically significant.
. For measurement data that followed a normal distribution and had homogeneity of variance, t-tests or one-way ANOVA were used; otherwise, non-parametric tests were used. Count data were analyzed using chi-square tests or Fisher’s exact probability tests. A P-value of less than 0.05 was considered statistically significant.
Results
Demographic Characteristics
A total of 207 patients were included in this study. Patients were divided into groups according to preoperative MRI evaluation results: with 117 cases in the normal group, 23 cases in the M1 group, and 67 cases in the M2 group. 5 cases exhibited MC III signal and were excluded from the study for the time being because the sample size was too small. According to the grade of MC, 45 cases were divided into mild MA group and 45 cases in the moderate-severe MBC group. There was no statistical difference between the groups in terms of general clinical information in the perioperative period (Table 1 and Table 2).
 |
Table 1 Preoperative Demographic Characteristics of Patients in the Normal, M1, and M2 Groups |
 |
Table 2 Demographic Characteristics of the MA and MBC Groups |
Clinical Outcomes
Improvement in VAS scores for low back pain: All patients showed significant improvement in VAS scores for low back pain at all postoperative time periods compared to preoperative. The VAS scores for low back pain in the normal group gradually decreased from 5.06±1.07 preoperatively to 1.20±0.77 at one year postoperatively. The VAS scores for low back pain in the M1 group decreased from 5.35±1.20 preoperatively to 2.13±0.69 at 6 months postoperatively and increased again to 2.3±0.88 at one year postoperatively. The VAS scores for low back pain in the M2 group decreased from 5.10±1.22 preoperatively to 2.1±0.84 at 3 months postoperatively and no significant change was observed at the subsequent follow-up. There was a significant difference in VAS scores for low back pain between the normal, M1, and M2 groups at 1 year postoperatively (P<0.001), and a two by two comparison: There was a significant difference between the normal group and the M1 group (P<0.001) and between the normal group and the M2 group (P<0.001), while there was no significant difference between the M1 group compared with the M2 group (P=0.456). In the MA group, the VAS scores for low back pain decreased from 5.13±1.10 preoperatively to 1.84±0.67 at 6 months postoperatively and did not change significantly at the subsequent one-year follow-up. The VAS scores for low back pain in the MBC group decreased from 5.20±1.32 preoperatively to 2.20±0.87 at 3 months postoperatively and increased again to 2.33±0.77 at the 1-year postoperative follow-up. There was a significant difference in VAS scores for low back pain between the MA and MBC groups at 6 months postoperatively (P=0.013) and at 1 year postoperatively (P=0.006).
Improvement in VAS scores for leg pain: All patients showed significant improvement in VAS scores for leg pain at all postoperative time periods compared to preoperative time. The VAS scores for leg pain of the normal, M1, and M2 groups gradually decreased from 6.70±1.23, 6.96±1.40, and 6.88±1.25 before surgery to 0.81±0.78, 1.13±0.69, and 1.00±0.67 one year after surgery, and there was no statistical difference between the various time periods. The VAS scores for leg pain of MA and MBC groups decreased from 6.78±1.17 and 7.02±1.39 preoperatively to 0.93±0.75 and 1.13±0.59 one year postoperatively, and there was no statistical difference between the various time periods.
Improvement in ODI scores: The ODI scores of each group of patients at different follow-up time points were normally distributed. Analysis of variance was used to compare the data between groups, and t-tests were used to compare the data within groups. All patients showed significant improvement in ODI scores at all postoperative time periods compared to preoperative time. The ODI scores in the normal group gradually decreased from 57.37±8.44 preoperatively to 11.41±5.14 at one year postoperatively. The ODI in the M1 and M2 groups decreased from 59.57±9.16 and 57.84±10.04 preoperatively to 15.13±4.55 and 14.30±4.41 at 6 months postoperatively and increased again to 24.09±8.37 and 22.19±6.32 at one year postoperatively. There was a significant difference in ODI scores between the normal, M1, and M2 groups at 1 year postoperatively (P<0.001), and a two by two comparison: There was a significant difference between the normal group and the M1 group (P<0.001) and between the normal group and the M2 group (P<0.001), while there was no significant difference between the M1 group compared with the M2 group (P=0.57). The ODI scores in the MA and MBC groups decreased from 57.71±10.73 and 58.84±8.87 preoperatively to 13.51±4.48 and 15.51±4.20 at 6 months postoperatively, and then increased to 20.38±5.48 and 24.98±7.44 at 1 year postoperatively. The ODI scores in the MA and MBC groups were significantly different at 6 months (P=0.032) and 1 year postoperatively (P=0.001) (Tables 3, 4 and Figure 5).
 |
Table 3 Preoperative and Postoperative VAS and ODI Scores in the Normal, M1, and M2 Groups |
 |
Table 4 Preoperative and Postoperative VAS and ODI in the MA and MBC Groups |
Changes in imaging parameters: There were no significant difference in DHI between the groups before surgery. At the final follow-up, DHI decreased in each group compared with that before surgery: there was no significant difference in DHI in the normal group compared with that before surgery, and there was a statistically significant decrease in DHI in patients with MC compared with that before surgery (P < 0.05). At the final follow-up, DHI was significantly lower in the MBC group than in the MA group (P < 0.05), while there was no statistical difference between the normal, M1, and M2 groups. There were no significant differences in LL among groups before surgery. At the last follow-up, there were no statistically significant differences in LL compared to preoperative values within each group, and no statistically significant differences were found among the groups.
Complications
There were 11 patients presented with transient postoperative sensory disturbances, 6 cases in the normal group, 1 case in the M1 group, and 4 cases in the M2 group. There were 2 cases presented dural sac rupture, 1 case in normal group and 1 case in M2 group. There was 1 case of epidural hematoma in the normal group. The complication rates in the normal, M1 and M2 groups were 11.11%, 13.04% and 14.93%, respectively. The complication rates in the MA and MBC groups were 11.11% and 17.78%, respectively. There was no statistical difference in the incidence of complications between the different types and grades of groups. No cases of intervertebral space infection or nerve root injury were found.
Recurrence
There were 3 patients who suffered from recurrence in the normal group, 2 patients suffered from recurrence in the M1 group, and 4 patients suffered from recurrence in the M2 group. The recurrence rates were 2.56%, 8.70%, and 5.97%, respectively. There were 2 cases of recurrence in the MA group and 4 cases in the MBC group, with recurrence rates of 4.44% and 8.89%, respectively. At the final follow-up, there was no statistical difference in the recurrence rate between the different types and grades of groups (Table 5).
 |
Table 5 Complications and Recurrences |
E/G Rate
In the final follow-up, according to the modified MacNab criteria, the percentages of excellent and good results in the normal, M1, and M2 groups were: 93.16%, 82.61%, and 83.58%, respectively. The percentages of excellent and good in the MA and MBC groups were: 88.89% and 77.78%, respectively. There was no statistical difference in the percentages of excellent and good between different types and grades of groups.
Discussion
MC is not uncommon in patients with LDH, about 19–59%,21 and up to 43.48% in this study, and the data of the frequency and distribution of MC are consistent with previous findings.22 PEID causes less damage to the paravertebral muscles, the small joints are preserved to some extent, the herniated discs are removed without destabilizing the spine, the nerve roots are sufficiently decompressed with or without MC, and the VAS scores for leg pain is similarly improved with no trend of deterioration.
The mechanism of MC occurrence is mainly closely related to biomechanical and biochemical alterations, the alteration of local stress in the endplate leads to microfracture of the endplate and subendplate bone, forming local inflammation, and the nucleus pulposus contacts the circulatory system through the ruptured endplate, producing inflammatory mediators and exacerbating local inflammation of the endplate.23–26 MC I is considered to be an acute-subacute repair response to vertebral bone marrow injury with a more severe inflammatory response.27–29 It has been reported that MC I and II have an increased density of sensory nerve fibers in the endplates, bone marrow and annulus fibrosus compared to normal subjects.28,30 It has also been reported that patients with MC are closely related to lumbar instability.31 All of the above may be the source of low back pain in patients with MC. However, this does not appear to be the primary cause of back pain in LDH patients with MC. Our findings showed no significant difference in preoperative low back pain whether accompanied by MC or not, and surprisingly, similar relief of short-term postoperative low back pain is achieved without treatment of the endplate. This is similar to the results of a prospective study by Ohtori et al,2 who performed discectomy without fusion in patients with LDH, patients with and without Modic type 1 changes showed a similar improvement in the low back pain score, that lower back pain appears to mainly originate from disc or nerve root compression. Therefore, the author believes that PEID can achieve good postoperative results even in the presence of MC. A retrospective study by Xu et al32 showed that postoperative back pain and functional status tended to worsen over time in patients with LDH with MC treated by PETD, especially with MC I. The results of this study showed that patients with MC had a deterioration trend in low back pain scores and lumbar spine function from 6 months to 1 year postoperative follow-up, which is possibly related to untreated lesion endplates.
There is increasing evidence suggesting that the size and structure of MC correlate with low back pain and the degree of degeneration.33–35 A retrospective study by Udby et al,7 in which patients with LDH with various severity of MC were evaluated preoperatively, concluded that MC grade was significantly associated with more severe disability and reduced health-related quality of life. Our results showed no significant differences in efficacy indicators between MC of different types at all postoperative time periods. While MC with different grades showed significant differences in back pain relief and lumbar spine functional recovery at 6 months-1 year postoperatively, severe MC with significantly higher postoperative back pain scores and ODI scores than mild MC. The authors suggest that (1) vertically larger MC is more prevalent in MC I. In this study, MC I accounted for 8.89% in the MA group and up to 40% in the MBC group. (2) The vertebrobasilar nerve enters the vertebral body from the foramen of the vertebrobasilar vein and branches uniformly to the upper and lower vertebral bodies to innervate the bony endplates. The larger the vertical MC is, the more inflammatory factors stimulate the nerves in the sagittal plane. (3) With vertically larger MC, the greater the endplate involvement tends to be, the more nerves are stimulated by inflammatory factors in the cross-sectional plane. In this study, MC that invaded the anterior-posterior diameter of the endplate accounted for 13.33% in the MA group and up to 35.56% in the MBC group. The above is also consistent with the views of Mättä36 and Saukkonen et al,37 which may explain the relatively poor outcome after surgery with severe MC.
It is generally believed that the loss of disc height is closely related to disc degeneration. The results of this study showed that the patients with MC had a statistically significant decrease in DHI at the last follow-up, indicating that the patients with MC might have continuous disc degeneration after PEID. At the last follow-up, there was no statistical difference in DHI between the normal group, M1 group, and M2 group, while the DHI of the MBC group was significantly lower than that of the MA group, which may indicate that the MBC group had more severe disc degeneration. Some scholars believe that the decrease or disappearance of lumbar lordosis is a sign of spinal instability, which can lead to chronic low back pain.38,39 In this study, there was no statistical difference in LL between each group at the last follow-up compared with before surgery, and there was no statistical difference in LL between each group of patients. This suggests that PEID has no significant effect on spinal stability, even in patients with MC. Therefore, we believe that for patients with LDH accompanied by MC, postoperative residual low back pain may be caused by residual MC, and the aggravation of postoperative low back pain may also be related to persistent disc degeneration. However, for LDH mainly manifested as radicular pain, solving the pain stimulation caused by disc protrusion may be the primary consideration. Studies have shown that postoperative transient sensory disturbance is a common complication of percutaneous endoscopic lumbar discectomy (PELD),40,41 accounting for approximately 5%-15%, with the incidence of 5.31% shown in the results of this study. It may be due to the compression of the dorsal root nerve by the placement of the working channel, the pulling of the nerve root as it is pushed toward the midline, and the electrical stimulation of the nerve root by the radio frequency tip. A total of 2 cases of dural sac tears were observed, 1 case of accidental injury by scissors of the ligamentum flavum in the normal group and the other case was a tear caused by stripping the epidural adhesions in the M2 group, and fluid gelatin was finally injected, and no cerebrospinal fluid leakage or other adverse effects were observed after surgery. One 32-year-old male patient presented epidural hematoma on the first postoperative day, and the hematoma was again removed endoscopically via the posterior approach and a drainage tube was placed, and the patient’s symptoms were relieved immediately after surgery. No significant differences in postoperative complications were observed between the various groups.
In a meta-analysis by Brooks et al,42 MC was one of the predictors of recurrent lumbar disc herniation. In a prospective study by Kim et al,43 MC was shown to be a risk factor for early recurrence of percutaneous endoscopic lumbar discectomy. In this study, the recurrence rate of patients with concomitant MC was significantly higher than the normal group, although there was no statistical difference in the results. In surgery for combined MC, the removed nucleus pulposus often contained detached cartilage endplates and was less sticky and elastic. In patients with recurrence of combined MC who underwent surgery again, the reherniated disc often contained endplate components, and this may be one of the reasons for the relatively high recurrence rate of combined MC patients. It has been suggested that the recurrence rate can be reduced by removing the protruding disc from the fibrous ring rupture to avoid further damage to the normal fibrous ring and then using bipolar radiofrequency electrocoagulation to shrink the fibrous ring rupture.44,45
This study is the first to systematically describe the postoperative efficacy and characteristics of PEID for LDH with different MC grades/types, and it was found that MC severity had a more significant effect on clinical outcomes. As with any clinical study, our study has limitations: due to the small number of MC patients, further grading of each type of MC was not performed and was not compared with other surgical. In future studies, it is recommended to refine the phenotypes of MC and to validate the findings of existing studies with multicenter, large sample and multilevel prospective studies, with the aim of obtaining higher quality evidence-based medicine and providing better guidance to clinical practice.
Conclusion
The efficacy of PEID in treating patients with lumbar disc herniation with or without MC is remarkable, and the surgery is safe and reliable. MC affects the improvement of low back pain and lumbar spine function after PEID, especially MC I or severe MC. In patients with MC, the choice of surgery should be carefully considered, not only the MC type but also the MC grade.
Abbreviations
PEID, Percutaneous endoscopic interlaminar discectomy; PTED, Percutaneous transforaminal endoscopic discectomy; LDH, Lumbar disc herniation; MC, Modic changes; MRI, Magnetic resonance imaging; VAS, Visual analog scale; ODI, Oswestry disability index; DHI, Disc height index; LL, Lumbar lordosis angle; Preop, Preoperation.
Data Sharing Statement
The data used to support the findings of this study are available from the corresponding author upon request.
Ethics Approval and Consent to Participate
This research was approved by the ethics committee of The First Affiliated Hospital of Harbin Medical University. The research was in compliance with the Helsinki Declaration. Written informed consent was obtained from all the participants. All procedures were performed in accordance with relevant guidelines.
Acknowledgments
We would like to thank all the participants in the study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The authors have received no external funding in order to support this project.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Yoon WW, Koch J. Herniated discs: when is surgery necessary? EFORT Open Rev. 2021;6(6):526–530. doi:10.1302/2058-5241.6.210020
2. Ohtori S, Yamashita M, Yamauchi K, et al. Low back pain after lumbar discectomy in patients showing endplate Modic type 1 change. Spine. 2010;35(13):E596–E600. doi:10.1097/BRS.0b013e3181cd2cb8
3. Kumarasamy D, Rajasekaran S, Anand KSV, et al. Lumbar disc herniation and preoperative Modic changes: a prospective analysis of the clinical outcomes after microdiscectomy. Global Spine J. 2022;12(5):940–951. doi:10.1177/2192568220976089
4. Modic MT, Steinberg PM, Ross JS, Masaryk TJ, Carter JR. Degenerative disk disease: assessment of changes in vertebral body marrow with MR imaging. Radiology. 1988;166(1 Pt 1):193–199. doi:10.1148/radiology.166.1.3336678
5. Modic MT, Masaryk TJ, Ross JS, Carter JR. Imaging of degenerative disk disease. Radiology. 1988;168(1):177–186. doi:10.1148/radiology.168.1.3289089
6. Udby PM, Samartzis D, Carreon LY, Andersen MØ, Karppinen J, Modic M. A definition and clinical grading of Modic changes. J Orthop Res. 2022;40(2):301–307. doi:10.1002/jor.25240
7. Udby PM, Modic M, Elmose S, et al. The clinical significance of the Modic changes grading score. Global Spine J. 2022:21925682221123012. doi:10.1177/21925682221123012
8. Rahme R, Moussa R. The Modic vertebral endplate and marrow changes: pathologic significance and relation to low back pain and segmental instability of the lumbar spine. AJNR Am J Neuroradiol. 2008;29(5):838–842. doi:10.3174/ajnr.A0925
9. Li H, Chen S, Wei H-Y, et al. Type 2 sclerotic Modic change affect fusion result in patients undergoing PLIF with pedicle screw instrumentation: a retrospective study. BMC Musculoskelet Disord. 2021;22(1):598. doi:10.1186/s12891-021-04461-9
10. Wang MY, Xu L, Qiu Y, et al. Modic改变对腰椎经椎间孔椎间融合效果和融合器沉降的影响 [Effect of Modic changes on fusion rate and cage subsidence after transforaminal lumbar interbody fusion]. Zhonghua Yi Xue Za Zhi. 2019;99(47):3703–3709. Chinese. doi:10.3760/cma.j.issn.0376-2491.2019.47.006
11. Ruetten S, Komp M, Godolias G. A New full-endoscopic technique for the interlaminar operation of lumbar disc herniations using 6-mm endoscopes: prospective 2-year results of 331 patients. Minim Invasive Neurosurg. 2006;49(2):80–87. doi:10.1055/s-2006-932172
12. Zhou C, Zhang G, Panchal RR, et al. Unique complications of percutaneous endoscopic lumbar discectomy and percutaneous endoscopic interlaminar discectomy. Pain Physician. 2018;21(2):E105–E112.
13. Chen K-T, Tseng C, Sun L-W, Chang K-S, Chen C-M. Technical considerations of interlaminar approach for lumbar disc herniation. World Neurosurg. 2021;145:612–620. doi:10.1016/j.wneu.2020.06.211
14. Kim CH, Chung CK, Jahng T-A, Yang H-J, Son Y-J. Surgical outcome of percutaneous endoscopic interlaminar lumbar diskectomy for recurrent disk herniation after open diskectomy. J Spinal Disord Tech. 2012;25(5):E125–E133. doi:10.1097/BSD.0b013e31825bd111
15. Wang Y, Videman T, Battié MC. Modic changes: prevalence, distribution patterns, and association with age in white men. Spine J. 2012;12(5):411–416. doi:10.1016/j.spinee.2012.03.026
16. Pfirrmann CW, Metzdorf A, Zanetti M, Hodler J, Boos N. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine. 2001;26(17):1873–1878. doi:10.1097/00007632-200109010-00011
17. Mysliwiec LW, Cholewicki J, Winkelpleck MD, Eis GP. MSU classification for herniated lumbar discs on MRI: toward developing objective criteria for surgical selection. Eur Spine J. 2010;19(7):1087–1093. doi:10.1007/s00586-009-1274-4
18. Costello RF, Beall DP. Nomenclature and standard reporting terminology of intervertebral disk herniation. Magn Reson Imaging Clin N Am. 2007;15(2). doi:10.1016/j.mric.2006.12.001
19. Inoue H, Ohmori K, Miyasaka K, Hosoe H. Radiographic evaluation of the lumbosacral disc height. Skeletal Radiol. 1999;28(11):638–643. doi:10.1007/s002560050566
20. Barrey C, Jund J, Noseda O, Roussouly P. Sagittal balance of the pelvis-spine complex and lumbar degenerative diseases. A comparative study about 85 cases. Eur Spine J. 2007;16(9):1459–1467. doi:10.1007/s00586-006-0294-6
21. Karchevsky M, Schweitzer ME, Carrino JA, Zoga A, Montgomery D, Parker L. Reactive endplate marrow changes: a systematic morphologic and epidemiologic evaluation. Skeletal Radiol. 2005;34(3):125–129. doi:10.1007/s00256-004-0886-3
22. Albert HB, Kjaer P, Jensen TS, Sorensen JS, Bendix T, Manniche C. Modic changes, possible causes and relation to low back pain. Med Hypotheses. 2008;70(2):361–368. doi:10.1016/j.mehy.2007.05.014
23. Dudli S, Fields AJ, Samartzis D, Karppinen J, Lotz JC. Pathobiology of Modic changes. Eur Spine J. 2016;25(11):3723–3734. doi:10.1007/s00586-016-4459-7
24. Liu J, Hao L, Suyou L, et al. Biomechanical properties of lumbar endplates and their correlation with MRI findings of lumbar degeneration. J Biomech. 2016;49(4):586–593. doi:10.1016/j.jbiomech.2016.01.019
25. Schroeder GD, Markova DZ, Koerner JD, et al. Are Modic changes associated with intervertebral disc cytokine profiles? Spine J. 2017;17(1):129–134. doi:10.1016/j.spinee.2016.08.006
26. Crockett MT, Kelly BS, van Baarsel S, Kavanagh EC. Modic type 1 vertebral endplate changes: injury, inflammation, or infection? AJR Am J Roentgenol. 2017;209(1):167–170. doi:10.2214/AJR.16.17403
27. Lurie JD, Moses RA, Tosteson ANA, et al. Magnetic resonance imaging predictors of surgical outcome in patients with lumbar intervertebral disc herniation. Spine. 2013;38(14):1216–1225. doi:10.1097/BRS.0b013e31828ce66d
28. Ohtori S, Inoue G, Ito T, et al. Tumor necrosis factor-immunoreactive cells and PGP 9.5-immunoreactive nerve fibers in vertebral endplates of patients with discogenic low back Pain and Modic Type 1 or Type 2 changes on MRI. Spine. 2006;31(9):1026–1031. doi:10.1097/01.brs.0000215027.87102.7c
29. Fields AJ, Liebenberg EC, Lotz JC. Innervation of pathologies in the lumbar vertebral end plate and intervertebral disc. Spine J. 2014;14(3):513–521. doi:10.1016/j.spinee.2013.06.075
30. Brown MF, Hukkanen MV, McCarthy ID, et al. Sensory and sympathetic innervation of the vertebral endplate in patients with degenerative disc disease. J Bone Joint Surg Br. 1997;79(1):147–153. doi:10.1302/0301-620X.79B1.0790147
31. Hayashi T, Daubs MD, Suzuki A, et al. Motion characteristics and related factors of Modic changes in the lumbar spine. J Neurosurg Spine. 2015;22(5):511–517. doi:10.3171/2014.10.SPINE14496
32. Xu J, Li Y, Wang B, et al. Percutaneous endoscopic lumbar discectomy for lumbar disc herniation with Modic changes via a transforaminal approach: a retrospective study. Pain Physician. 2019;22(6):E601–E608.
33. Kjaer P, Leboeuf-Yde C, Korsholm L, Sorensen JS, Bendix T. Magnetic resonance imaging and low back pain in adults: a diagnostic imaging study of 40-year-old men and women. Spine. 2005;30(10):1173–1180. doi:10.1097/01.brs.0000162396.97739.76
34. Määttä JH, Karppinen J, Paananen M, et al. Refined phenotyping of Modic changes: imaging biomarkers of prolonged severe low back pain and disability. Medicine. 2016;95(22):e3495. doi:10.1097/MD.0000000000003495
35. Mera Y, Teraguchi M, Hashizume H, et al. Association between types of Modic changes in the lumbar region and low back pain in a large cohort: the Wakayama spine study. Eur Spine J. 2021;30(4):1011–1017. doi:10.1007/s00586-020-06618-x
36. Määttä JH, Karppinen JI, Luk KDK, Cheung KMC, Samartzis D. Phenotype profiling of Modic changes of the lumbar spine and its association with other MRI phenotypes: a large-scale population-based study. Spine J. 2015;15(9):1933–1942. doi:10.1016/j.spinee.2015.06.056
37. Saukkonen J, Määttä J, Oura P, et al. Association between Modic changes and low back pain in middle age: a Northern Finland Birth Cohort study. Spine. 2020;45(19):1360–1367. doi:10.1097/BRS.0000000000003529
38. Luoma K, Riihimäki H, Luukkonen R, Raininko R, Viikari-Juntura E, Lamminen A. Low back pain in relation to lumbar disc degeneration. Spine. 2000;25(4):487–492. doi:10.1097/00007632-200002150-00016
39. Inanami H, Iwai H, Kaneko T, et al. Relationship between lumbar lordosis and the ratio of the spinous process height to the anterior spinal column height. Sci Rep. 2020;10(1):6718. doi:10.1038/s41598-020-63648-7
40. Wang H, Zhou Y, Li C, Liu J, Xiang L. Risk factors for failure of single-level percutaneous endoscopic lumbar discectomy. J Neurosurg Spine. 2015;23(3):320–325. doi:10.3171/2014.10.SPINE1442
41. Ahn Y. Transforaminal percutaneous endoscopic lumbar discectomy: technical tips to prevent complications. Expert Rev Med Devices. 2012;9(4):361–366. doi:10.1586/erd.12.23
42. Brooks M, Dower A, Abdul Jalil MF, Kohan S. Radiological predictors of recurrent lumbar disc herniation: a systematic review and meta-analysis. J Neurosurg Spine. 2020;1–11. doi:10.3171/2020.6.SPINE20598
43. Kim HS, You JD, Ju CI. Predictive scoring and risk factors of early recurrence after percutaneous endoscopic lumbar discectomy. Biomed Res Int. 2019;2019:6492675. doi:10.1155/2019/6492675
44. Liu K-C, Yang S-K, B-R O, et al. Using percutaneous endoscopic outside-in technique to treat selected patients with refractory discogenic low back pain. Pain Physician. 2019;22(2):187–198.
45. Kim HS, Park JY. Comparative assessment of different percutaneous endoscopic interlaminar lumbar discectomy (PEID) techniques. Pain Physician. 2013;16(4):359–367.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.