Back to Journals » ClinicoEconomics and Outcomes Research » Volume 12
Analysis of the Cost-Effectiveness of Liquid Biopsy to Determine Treatment Change in Patients with Her2-Positive Advanced Breast Cancer in Colombia
Authors Sánchez-Calderón D , Pedraza A, Mancera Urrego C, Mejía-Mejía A, Montealegre-Páez AL, Perdomo S
Received 27 June 2019
Accepted for publication 26 September 2019
Published 13 February 2020 Volume 2020:12 Pages 115—122
DOI https://doi.org/10.2147/CEOR.S220726
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Dean Smith
Diana Sánchez-Calderón,1 Adriana Pedraza,2 Catalina Mancera Urrego,2 Aurelio Mejía-Mejía,2 Ana Lorena Montealegre-Páez,3 Sandra Perdomo3
1Faculty of Medicine, Universidad El Bosque, Bogotá, Colombia; 2Universidad del Rosario, Bogotá, Colombia; 3Institute of Nutrition, Genetics, and Metabolism Research, Faculty of Medicine, Universidad El Bosque, Bogotá, Colombia
Correspondence: Sandra Perdomo
International Agency for Research on Cancer (IARC), Lyon, France
Tel +33 07 7895 5680
Email [email protected]
Background: Breast cancer is highly prevalent worldwide and leads to high health-care costs. HER2-positive subtype represents 30% of all breast cancers and is associated with a poor prognosis. Patients treated with anti-HER2 therapies frequently develop resistance and require pharmacological treatment change. Liquid biopsy is a minimally invasive and an easily accessible technique, with high sensitivity and specificity, to detect molecular treatment resistance even before the onset of clinical manifestations and can thus be used to reduce unnecessary anti-HER2 treatment costs.
Objective: To evaluate the cost-effectiveness of using liquid biopsy (ctDNA detection) to determine treatment change in women with HER2-positive advanced breast cancer in Colombia.
Methodology: We performed an economic evaluation using decision tree modeling and deterministic analyses based on literature search for first and second lines of treatment (trastuzumab, pertuzumab, docetaxel, and TDM1); resistance; outcomes; and sensitivity and specificity of tests detecting molecular resistance. The effectiveness was measured using quality-adjusted life year (QALY) score, and costs were obtained from databases with national validity, suppliers, the Colombian Drug Price Information System (SISMED), and local studies.
Results: The use of liquid biopsy (ctDNA detection) with conventional treatment was more expensive and less effective than conventional treatment without liquid biopsy (US $177,985.35 and 0.533889206 QALY, respectively). The incremental cost with liquid biopsy was US $7,333.17 and the incremental effectiveness was 0.00042256 QALY relative to the conventional method.
Conclusion: Including liquid biopsy in the treatment of HER2-positive advanced breast cancer was considered currently inapplicable in Colombia because it was not cost effective. Our results open a window of opportunity to improve the development and implementation of ctDNA testing in Colombia, potentially reducing current costs. More evidence is required on the utility of this test, depending on the financial capacity of Colombia and other countries.
Keywords: cost-effectiveness, liquid biopsy, biomarkers, breast cancer
Introduction
Breast cancer is a pathology of great interest in public health due to its increased prevalence, incidence, and mortality rates in recent decades. According to GLOBOCAN, an initiative by the International Agency for Research on Cancer, breast cancer is considered the most frequent cause of cancer-related death in women and the second most frequent cause of deaths worldwide, with 1.67 million new cases diagnosed annually.1
In 2017, the mortality rate due to malignant breast cancer in Colombia was 5.7 per 100,000 women,2 and the overall survival at 2 years was 80%.3 However, overall survival can decrease due to delay in initiating treatment, simultaneously increasing health-care costs associated with advanced clinical stages.4
Health-care costs for breast cancer are high. It has been estimated that by 2035, the United States will spend between 3.6 and 7.6 trillion dollars on patients with breast cancer.5 For 2016 in Colombia, metastatic cancer was associated with a total cost of US $53,000 per patient during a 5-year follow-up period, while regional breast cancer reached a cost close to US $25,000, without taking relapses into account.4 In addition, costs of chemotherapy were estimated to represent 77–84% of the total cost according to the disease stage, which is consistent with other reports worldwide in which these costs were estimated to be 42–87% of the total costs.6,7
Breast cancer with amplification of the ERBB2 gene and/or overexpression of transmembrane proteins of the HER2 family of epidermal growth receptors has been identified in 10–34% of the invasive breast cancers,8 and it is considered to be associated with a poor prognosis due to its high proliferative and histological characteristics.9 Defects in cell signaling, disruption of proapoptotic and antiapoptotic protein expression, and other biochemical mechanisms and mutations (e.g., PI3K, PIK3CA genes) can lead to the development of resistance to chemotherapeutic agents and anti-HER2 therapies.10,11
Genetic alterations that lead to poor treatment response are usually detected through direct tumor resection or tissue biopsy. Liquid biopsy has been reported as an alternative test for detecting circulating tumor cells (CTC) or nucleic acids fractions [circulating tumor DNA (ctDNA), micro RNAs (miRNA), etc.], both sources of the complete tumor genome in different fluids (blood, urine, cerebrospinal fluid, and saliva).12 Liquid biopsy facilitates follow-up and supports decision-making and prognosis during treatment13 because it is minimally invasive, highly concordant with tissue biopsy, and is more sensible in capturing tumor heterogeneity.14–16
Considering the high heterogeneity of solid breast tumors and thanks to the ability to detect CTC and ctDNAs through tests with 80% sensitivity and close to 100% specificity, it has been possible to advance therapeutic monitoring, which directly impacts prognosis and survival.16–22 Other advantages include elucidating molecular abnormalities in cancer patients23,24 achieving personalized treatment according to specific mutations detected in ctDNA, and highly selective therapeutic schemes.24 In particular, identification of resistant mutations to targeted therapies using ctDNA analysis allows direct and continuous monitoring of treatment response.
Monoclonal therapy with anti-HER2 drugs is considered the first-line treatment for advanced breast cancer.17,25 Thus, the implementation of ctDNA mutation testing during the follow-up of patients with advanced breast cancer may favor earlier change of pharmacological management and avoid prolonged treatment periods while awaiting clinical evidence of resistance, which typically occurs within less than a year of treatment with anti-HER2 drugs.26,27
In Colombia, although the Clinical Practice Guideline for early detection, comprehensive treatment, follow-up, and rehabilitation of breast cancer has considered the inclusion of target therapies within the management of HER2-positive breast cancer,28 it does not consider liquid biopsy as a routine test to determine change of pharmacological therapy upon detection of resistance. Considering limited health resources, it is necessary to prioritize decisions for the inclusion of new technologies in health-care systems based on proven evidence; available resources; ethical aspects; and the local, regional, national, or international context.29–31 However, there is a gap in the literature regarding costs of cancer treatment using biomarkers, which impact therapeutic decisions from clinical and financial perspectives. In this study, we propose an economic evaluation to support decision-making regarding the use and relevance of liquid biopsy, specifically ctDNA detection, in the treatment of HER2-positive advanced breast cancer in Colombia.
Materials and Methods
Study Design
This study corresponds to a cost-effectiveness-type economic evaluation designed by means of the PICO strategy and is based on the methodological recommendations of the Methodological Manual of the Institute for Economic Evaluation in Health IETS.32
Target Population
Women with advanced HER2-positive breast cancer without hormone receptors (Stages III and IV according to the American Joint Committee on Cancer Staging System for Breast Cancer) who were candidates for management with molecular-targeted drugs in Colombia were included.
Intervention
Liquid biopsy was added to the first-line treatment, involving a test for the detection of ctDNA in plasma and early resistance mutations in order to proceed to treatment change.
Comparison
Conventional first-line treatment (pertuzumab, trastuzumab, and docetaxel) for HER2-positive advanced breast cancer in Colombia was used for comparison.
Outcome
Effectiveness was measured by quality-adjusted life year (QALY), and utility weightings were estimated from information extracted from the literature and discussions with experts.
Time Horizon
A one-year horizon was adopted based on determinations in the literature showing a median therapeutic failure in the trastuzumab + docetaxel regimen of 9.8 months as the first-line treatment for patients with HER2-positive breast cancer.33 Additionally, the detection of resistance using liquid biopsy generates a gain of 8 weeks compared with that through conventional imaging methods when defining treatment change.34 Resistance is expected to be detected between the 1st and 3rd cycles of medication. We did not identify studies that evaluated resistance in horizons longer than 1 year, perhaps because of the low survival of patients with advanced breast cancer worldwide. Therefore, after discussions with experts, the resistance occurring during the first year of treatment was evaluated.
Perspective
The perspective corresponds to those included in the General Social Security Health System in Colombia. Specifically, the direct medical costs associated with the first and second lines of treatment (medications, consultations, and paraclinical examinations), health outcomes in terms of mortality and those referred by patients in terms of quality of life were considered.
Discount Rate
This was not applicable considering the 1-year time horizon.
Model Structure and Assumptions
To estimate the costs and benefits of each alternative (addition of liquid biopsy-ctDNA detection, to conventional treatment), a decision tree was designed that reflected the main courses of action of the disease in a patient in the short term (Figure 1). The tree design was based on a literature review of articles indexed in databases such as SciELO, PubMed, and EMBASE; Colombian Local Management Guidelines of the National Institute of Cancerology; International Guides of American Society of Clinical Oncology; and National Comprehensive Cancer Network. We searched for publications regarding first- and second-line treatments for HER2-positive advanced breast cancer, time and percentage of resistance during the first-line treatment and change to second-line treatment. Also, we looked for outcomes according to treatment and disease phase, sensitivity, and specificity of liquid biopsy to detect resistance to the first-line treatment.
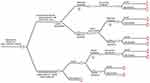 |
Figure 1 Tree decision model for cost effectiveness evaluation of liquid biopsy (ctDNA) testing to determine treatment change in women with HER2-positive advanced breast cancer in Colombia. |
Similarly, information on pharmacological treatment was validated by a clinical oncologist and a cancer genomics researcher. Bibliographic search included economic evaluations of liquid biopsy (ctDNA detection) for breast cancer in Colombia and worldwide, which did not yield any results. Finally, the proposed model was developed based on current literature, clinical practice guidelines and expert consultation. Model assumptions are listed in the supplementary material.
Transition Probabilities
The search for model probabilities in EBSCO and PubMed databases included the following keywords: “breast cancer HER2 positive,” “liquid biopsy,” “ctDNA,” “first-line treatment,” and/or “second-line treatment” (Search date: August 2018). We searched for studies that reported the probability of molecular and clinical resistance, the latter was estimated by progression-free duration with first-line management. Moreover, we searched for studies reporting probabilities of survival during the first- and second-line treatments.
Five studies that met the search criteria were selected. The transition probability extracted from the studies reported was 12 months. The CLEOPATRA study was used as a basis for the determination of first-line treatment in patients with HER2-positive advanced breast cancer35 and similar studies consistent with the time horizon of the study, were reviewed (Table 1). Other reviewed studies36,37 were discarded because they did not have accurate data at 12 months of follow-up or the study populations were rather small.
 |
Table 1 Model Probabilities of Molecular, Clinical Resistance and Survival to Anti-HER2 Therapy in Advance Breast Cancer |
Outcome Assessment
Effectiveness was measured in QALY as a measure of outcomes to assess the impact of methods included in the economic evaluation of quality of life and life expectancy of the target population.32
The utility weights were estimated from information extracted from the literature and further discussed with experts. The search was performed on PubMed, EBSCO, and Google Scholar using the following keywords: “cost-effectiveness,” “first-line treatment,” “QALY,” AND “breast cancer HER2+.” We found 34 articles, of which 28 were excluded as they did not fulfill the search criteria and 1 was excluded since full text was not accessible (Table 2).
 |
Table 2 Utility Weights Retrieved from the Literature |
The study chosen was that of Diaby et.al, 2017, which considers a cost-effectiveness analysis of 4 different sequences of treatments for advanced HER2-positive breast cancer in Mexican patients. We used as reference, the first sequence described in this study.21 It was also considered that the Mexican population is similar to the Colombian population because both are Latin American. A study by the same author carried out in the North American population in 2016, took the same useful assessments from the study of Llyod et al as reference.42
Identification, Measurement, and Evaluation of Costs
All costs associated with the evaluated methods and health outcomes included in the proposed decision model were considered. Institutional databases with national validity and direct consultation with experts were used as sources of information, as stipulated in the IETS methodological manual.32
Medication costs were obtained from the Drug Price Information System (SISMED) through “The drug price thermometer” of the Ministry of Health of Colombia, which displays the range of prices of each active principle according to trademark and manufacturer. The active principle of medicines was filtered obtaining their cost.43
Costs of medical consultations; diagnostic tests and follow-up (clinical laboratory, imaging, and tissue biopsy); extension studies; surgical treatment; adjuvant chemotherapy; radiotherapy; and rehabilitation were obtained from the study of Gamboa (2016). Since this study estimated costs for the treatment of cancer in Colombia at all stages, we used the annual average cost for stages III and IV (according to the American Joint Committee on Cancer Staging System for Breast Cancer).4 All costs related to diagnostic tests and procedures common to both interventions selected for this study were excluded, as well as costs associated with hormone therapy, since the target population is not eligible for this type of treatment.
Cost of liquid biopsy was obtained directly from international suppliers (Foundation Medicine, Archer DX, Oncomine Thermofisher, and Sophia DM). Costs for detection of a single mutation and panels of 28, 42, 58, and 62 genes were taken into account. The value, initially expressed in US $, was converted to Colombian pesos (COP) with the representative exchange rate of 1 USD = 3,000 COP.
Incremental Analysis
Calculation of the incremental cost-effectiveness ratio was proposed, compared to a threshold of 1 GDP per capita and 3 GDP per capita, following the recommendations of the World Health Organization, considering that Colombia does not have a threshold estimate for economic evaluations.32
Sensitivity Analysis
A deterministic sensitivity analysis was conducted to estimate effects of uncertainty on model conclusions. Variable cost of liquid biopsy was modified, considering a minimum value of US $500 corresponding to detection of a single mutation and a maximum value of US $1,500, taking into account different prices reported by suppliers and number of mutations reported in each panel to verify cost-effectiveness. Variations in the performance of liquid biopsy were not considered in the deterministic analysis because it has no therapeutic implications per se. The literature does not report a specific range of sensitivity and specificity, as no variation was considered on that variable.
Results
Results of the Baseline Case
Table 3 summarizes the result of a baseline case, comparing the two health methods proposed in this study and based on the minimum cost of liquid biopsy test (US $500). Not using liquid biopsy was the least expensive alternative, with a total cost of US $177,985.35 and an effectiveness of 0.533889206 QALY for the treatment of a patient with a time horizon of 1 year. In contrast, using liquid biopsy was the most expensive alternative, with a total cost of US $185,318.52 (incremental value = US $7,333.17 versus alternative therapy without liquid biopsy) and an effectiveness of 0.533466647 QALY.
 |
Table 3 Results of the Baseline Case Evaluation of Liquid Biopsy Testing in Anti-HER2 Therapy in Advance Breast Cancer |
Considering the scenario that addition of liquid biopsy testing is more expensive and less effective than conventional treatment without liquid biopsy, it is a dominated alternative. Therefore, the incremental cost-effectiveness relationship was not calculated.
Results of Sensitivity Analysis
The cost variable was modified and liquid biopsy was calculated at US $500, US $1,000 and US $1,500. Regardless of the variation of the value of this variable, it continued to be a dominated alternative, even with cost of US $0.
Discussion and Conclusions
Breast cancer is the most frequent cause of cancer-related death in women and the second most frequent cause of death worldwide, making it a pathology of great social and economic interest for countries worldwide.1 In Colombia, based on a mortality rate of 12.79 per 100,000 women, the development of therapeutic alternatives has been crucial, enabling the use of anti-HER2 monoclonal therapy (such as trastuzumab), as the first-line treatment of advanced cancer,25,26 to achieve an overall survival of more than 48 months in patients with HER2-positive advanced breast cancer.44 Some studies have reported a 5-year overall survival of around 74.9% and 3.5 years global survival from the beginning of treatment in the metastatic stage.45,46
Liquid biopsy has been shown benefits in obtaining the genomic information of tumors, facilitating processing time of samples and overcoming invasiveness of the procedures compared with conventional pathological testing.16 Moreover, it provides additional advantages, such as detection of treatment resistance for targeted therapies and real-time evaluation of the dynamic molecular abnormalities of the tumor using serial sampling.23,24
Based on our results, liquid biopsy (ctDNA detection) may not be suitable to be included within the General System of Health Insurance in Colombia, specifically for the treatment of HER2-positive advanced breast cancer, considering that it was not cost effective. However, it is important to highlight that the difference between the two comparators' effectiveness was minimally assessed by QALY´s.
The definition of resistance to treatment with molecular-targeted drugs would allow for adjusting treatments and conventional chemotherapies in a timely manner, which is mostly relevant in early stages; however, this was not contemplated in this study. Such adjustments would, in turn, favor the health system because costs would be lowered. Moreover, this benefit applies as well to all patients in that exposure to targeted therapies is decreased, which frequently lead to cardiotoxicity, allergic reactions, lung disease, hematotoxicity, and gastrointestinal disorders.26 In addition, the costs of Liquid Biopsy may be reduced if it is performed by local-certified laboratories in Colombia and if the panels designed consider the most frequent mutations that confer treatment resistance in HER2-positive advanced breast cancer. Colombia is one of the leading countries in the Latin-American region in terms of installed genomic facilities, cancer genetic research groups and educational programs in genomics.47 It has the potential to implement centralized liquid biopsy testing in the country eventually reducing costs and time processing.
As a limitation of the present study, an uncertainty probabilistic analysis was not performed, which may lead to modifications of our results. However, the inexistence of economic studies of cost-effectiveness involving the use of liquid biopsy in breast cancer limited comparisons. Nonetheless, this scenario provides future research opportunities to consolidate better evidence of economic evaluations for liquid biopsy since the probability of the expected benefits may exceed the costs and it would not be appropriate to rule it out categorically, despite being, in the present study, a dominated alternative.
Considering that liquid biopsy sensitivity and specificity is 80% and 100%, respectively,16 as well as that genomic sequencing can reduce the proportion of false negatives by increasing its sensitivity, liquid biopsy may become the first-choice method for diagnosis, follow-up, and prognosis for breast cancer and other cancers. This current evidence urges the need to conduct further studies, taking different disease stages and limited health resources into account, to prioritize decisions in the face of evidence and context of each country and their potential to finance new health-care technologies.29,30
In conclusion, liquid biopsy is not currently applicable to be included in the treatment of HER2-positive advanced breast cancer in Colombia because it is not cost effective. These results urge more strategies to improve local sequencing capacities that could eventually reduce current costs for ctDNA test outsourcing. More evidence should be compiled regarding the utility of this test, and further economic evaluation of this method, including probabilistic analyses according to the financing capacity of Colombia and other countries.
Ethical Considerations
This is a risk-free study according to Colombian regulations and was conducted based on the Declaration of Helsinki and its latest amendments. There will be no disclosure of the results to trade companies.
Funding
This work was supported by the Faculty of Medicine and the Research Unit of Universidad El Bosque.
Disclosure
The authors declared that they do not have any conflicts of interest. There was no sponsorship or financial support by the pharmaceutical industry; and there was no mention of commercial brands.
References
1. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–86. doi:10.1002/ijc.29210
2. Instituto Nacional de Cancerología INC, Departamento Administrativo Nacional de Estadística DANE. Datos mortalidad nacional. Sistema de información de cáncer en Colombia SICC (Versión 1.0) [Internet]. Bogotá, Colombia: INC, DANE 2017. Available http://www.infocancer.co.
3. Pardo C, de Vries E. Overall survival of cancer patients in the Colombian National Cancer Institute. Rev Colomb Cancerol. 2017;21(1):12–18.
4. Gamboa Ó, Buitrago LA, Lozano T, et al. Costos directos de la atención del cáncer de mama en Colombia. Rev Colomb Cancerol. 2016;20(2):52–60.
5. Tartari F, Santoni M, Pistelli M, Berardi R. Healthcare cost of HER2-positive and negative breast tumors in the United States (2012–2035). Cancer Treat Rev. 2017;60:12–17. doi:10.1016/j.ctrv.2017.08.005
6. González-Robledo MC, Wong R, Ornelas HA, Knaul FM. Costs of breast cancer care in Mexico: analysis of two insurance coverage scenarios. Ecancermedicalscience. 2015;9:587. doi:10.3332/ecancer.2015.587
7. Daroudi R, Sari AA, Nahvijou A, Kalaghchi B, Najafi M, Zendehdel K. The economic burden of breast cancer in Iran. Iran J Public Health. 2015;44(9):1225–1233.
8. Ross JS, Slodkowska EA, Symmans WF, Pusztai L, Ravdin PM, Hortobagyi GN. The HER-2 receptor and breast cancer: ten years of targeted anti-HER2 therapy and personalized medicine. Oncologist. 2009;14(4):320–368. doi:10.1634/theoncologist.2008-0230
9. Yao M, Fu P. Advances in anti-HER2 therapy in metastatic breast cancer. Chin Clin Oncol. 2018;7(3):27. doi:10.21037/cco
10. Flores de Mera ML. Bases moleculares de la resistencia a taxanos y nuevas alternativas terapéuticas en el cáncer avanzado de mama y próstata. [Doctoral Thesis]. Depósito de Investigación Universidad de Sevilla: Universidad de Sevilla; 2016. 117.
11. de Melo Gagliato D, Jardim D, Marchesi M, Hortobagyi GN. Mechanisms of resistance and sensitivity to anti-HER2 therapies in HER2+ breast cancer. Oncotarget. 2016;7(39):64431–64446. doi:10.18632/oncotarget.7043
12. Murtaza M, Dawson SJ, Tsui DW, et al. Non-invasive analysis of acquired resistance to cancer therapy by sequencing of plasma DNA. Nature. 2013;497(7447):108–112. doi:10.1038/nature12065
13. Polasik A, Tzschaschel M, Schochter F, et al. Circulating tumour cells, circulating tumour DNA and circulating microRNA in metastatic breast carcinoma–what is the role of liquid biopsy in breast cancer? Geburtshilfe Frauenheilkd. 2017;77(12):1291–1298. doi:10.1055/s-0043-122884
14. Chu D, Park BH. Liquid biopsy: unlocking the potentials of cell-free DNA. Virchows Arch. 2017;471(2):147–154. doi:10.1007/s00428-017-2137-8
15. Diehl F, Schmidt K, Choti MA, et al. Circulating mutant DNA to assess tumor dynamics. Nat Med. 2008;14(9):985–990. doi:10.1038/nm.1789
16. Krishnamurthy N, Spencer E, Torkamani A, Nicholson L. Liquid biopsies for cancer: coming to a patient near you. J Clin Med. 2017;6(1):3. doi:10.3390/jcm6010003
17. Palacios Calvo J, Albanell J, Rojo F, et al. Consenso de la Sociedad Española de Anatomía Patológica y la Sociedad Española de Oncología Médica sobre biomarcadores en cáncer de mama. Rev Esp Patol. 2018;51(2):97–109. doi:10.1016/j.patol.2017.12.002
18. Jacobs VR, Kates RE, Kantelhardt E, et al. Health economic impact of risk group selection according to ASCO-recommended biomarkers uPA/PAI-1 in node-negative primary breast cancer. Breast Cancer Res Treat. 2013;138(3):839–850. doi:10.1007/s10549-013-2496-z
19. Marguet S, Mazouni C, Ramaekers BL, et al. European cost-effectiveness study of uPA/PAI-1 biomarkers to guide adjuvant chemotherapy decisions in breast cancer. Eur J Cancer. 2016;63:168–179. doi:10.1016/j.ejca.2016.05.013
20. Diaby V, Adunlin G, Ali AA, et al. Cost-effectiveness analysis of 1st through 3rd line sequential targeted therapy in HER2-positive metastatic breast cancer in the United States. Breast Cancer Res Treat. 2016;160(1):187–196. doi:10.1007/s10549-016-3978-6
21. Diaby V, Ali AA, Williams KJ, et al. Economic evaluation of sequencing strategies in HER2-positive metastatic breast cancer in Mexico: a contrast between public and private payer perspectives. Breast Cancer Res Treat. 2017;166(3):951–963. doi:10.1007/s10549-017-4473-4
22. Appierto V, Di Cosimo S, Reduzzi C, Pala V, Cappelletti V, Daidone MG. How to study and overcome tumor heterogeneity with circulating biomarkers: the breast cancer case. Semin Cancer Biol. 2017;44:106–116. doi:10.1016/j.semcancer.2017.04.007
23. Colomer R, Aranda-López I, Albanell J, et al. Biomarkers in breast cancer: a consensus statement by the Spanish Society of Medical Oncology and the Spanish Society of Pathology. Clin Transl Oncol. 2018;20(7):815–826. doi:10.1007/s12094-017-1800-5
24. Alix-Panabières C, Pantel K. Clinical applications of circulating tumor cells and circulating tumor DNA as liquid biopsy. Cancer Discov. 2016;6(5):479–491. doi:10.1158/2159-8290.CD-15-1483
25. Nicolini A, Barak V, Biava P, Ferrari P, Rossi G, Carpi A. Use of immunotherapy to treat metastatic breast cancer. Curr Med Chem. 2019;16(6):941–962. doi:10.2174/0929867325666180209124052
26. Labidi S, Mejri N, Lagha A, et al. Targeted therapies in HER2-overexpressing metastatic breast cancer. Breast Care (Basel). 2016;11(6):418–422. doi:10.1159/000452194
27. Hainsworth JD, Murphy PB, Alemar JR, Daniel BR, Young RR, Yardley DA. Use of a multiplexed immunoassay (PRO Onc assay) to detect HER2 abnormalities in circulating tumor cells of women with HER2-negative metastatic breast cancer: lack of response to HER2-targeted therapy. Breast Cancer Res Treat. 2016;160(1):41–49. doi:10.1007/s10549-016-3969-7
28. Colombia, Ministerio de Salud y Protección Social. Guía de práctica Clínica para la detección temprana, tratamiento integral, seguimiento y rehabilitación del cáncer de mama Segunda edición. Guía Nº 19 [Internet]. Bogotá D.C. Available http://gpc.minsalud.gov.co/gpc_sites/Repositorio/Conv_500/GPC_cancer_mama/gpc_cancer_mama_profesionales.aspx.
29. Daniels N. Accountability for reasonableness: establishing a fair process for priority setting is easier than agreeing on principles. BMJ. 2000;321(7272):1300–1301. doi:10.1136/bmj.321.7272.1300
30. Mejía Mejía A. Evaluación económica de programas y servicios de salud. Rev Gerenc Polit Salud. 2008;7(15):91–113.
31. Sabik LM, Lie RK. Priority setting in health care: lessons from the experiences of eight countries. Int J Equity Health. 2008;7:4. doi:10.1186/1475-9276-7-4
32. Instituto de Evaluación Tecnológica en Salud. Manualpara la elaboración de evaluaciones económicas en salud. Bogotá D.C.: IETS; 2014.
33. Marty M, Cognetti F, Maraninchi D, et al. Randomized Phase II trial of the efficacy and safety of trastuzumab combined with docetaxel in patients with human epidermal growth factor receptor 2–positive metastatic breast cancer administered as first-line treatment: the M77001 study group. J Clin Oncol. 2005;23(19):4265–4274. doi:10.1200/JCO.2005.04.173
34. Ma F, Zhu W, Guan Y, et al. ctDNA dynamics: a novel indicator to track resistance in metastatic breast cancer treated with anti-HER2 therapy. Oncotarget. 2016;7(40):66020–66031. doi:10.18632/oncotarget.v7i40
35. Swain SM, Kim SB, Cortés J, et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA study): overall survival results from a randomised, double-blind, placebo-controlled, Phase 3 study. Lancet Oncol. 2013;14(6):461–471. doi:10.1016/S1470-2045(13)70130-X
36. Perez EA, Barrios C, Eiermann W, et al. Trastuzumab emtansine with or without pertuzumab versus trastuzumab plus taxane for human epidermal growth factor receptor 2-positive, advanced breast cancer: primary results from the phase III MARIANNE study. J Clin Oncol. 2017;35(2):141–148. doi:10.1200/JCO.2016.67.4887
37. Yeo W, Luk MY, Soong IS, et al. Efficacy and tolerability of trastuzumab emtansine in advanced human epidermal growth factor receptor 2-positive breast cancer. Hong Kong Med J. 2018;24(1):56–62. doi:10.12809/hkmj176808
38. Baselga J, Cortes J, Im SA, et al. Biomarker analyses in CLEOPATRA: a Phase III, placebo-controlled study of pertuzumab in human epidermal growth factor receptor 2-positive, first-line metastatic breast cancer. J Clin Oncol. 2014;32(33):3753–3761. doi:10.1200/JCO.2013.54.5384
39. Sakai H, Tsurutani J, Iwasa T, et al. HER2 genomic amplification in circulating tumor DNA and estrogen receptor positivity predict primary resistance to trastuzumab emtansine (T-DM1) in patients with HER2-positive metastatic breast cancer. Breast Cancer. 2018;25(5):605–613. doi:10.1007/s12282-018-0861-9
40. Verma S, Miles D, Gianni L, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012;367:1783–1791. doi:10.1056/NEJMoa1209124
41. Le QA, Bae YH, Kang JH. Cost-effectiveness analysis of trastuzumab emtansine (T-DM1) in human epidermal growth factor receptor 2 (HER2): positive advanced breast cancer. Breast Cancer Res Treat. 2016;159(3):565–573. doi:10.1007/s10549-016-3958-x
42. Lloyd A, Nafees B, Narewska J, Dewilde S, Watkins J. Health state utilities for metastatic breast cancer. Br J Cancer. 2006;95(6):683–690. doi:10.1038/sj.bjc.6603326
43. Ministerio de Salud y Protección Social. Termómetro de precios de medicamentos [Internet]. Minsalud, Colombia. Available https://www.minsalud.gov.co/salud/MT/Paginas/termometro-de-precios.aspx.
44. Blanchette PS, Desautels DN, Pond GR, et al. Factors influencing survival among patients with HER2‑positive metastatic breast cancer treated with trastuzumab. Breast Cancer Res Treat. 2018;170(1):169–177. doi:10.1007/s10549-018-4734-x
45. Yardley DA, Tripathy D, Brufsky AM, et al. Long-term survivor characteristics in HER2-positive metastatic breast cancer from registHER. Br J Cancer. 2014;110(11):2756–2764. doi:10.1038/bjc.2014.174
46. Maffuz-Aziz A, Labastida-Almendaro S, Sherwell-Cabello S, et al. Supervivencia de pacientes con cáncer de mama. Análisis por factores pronóstico, clínicos y patológicos. Ginecol Obstet Mex. 2016;84(8):498–506.
47. Torres Á, Oliver J, Frecha C, et al. Cancer genomic resources and present needs in the Latin American Region. Public Health Genomics. 2017;20(3):194–201. doi:10.1159/000479291
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
