Back to Journals » International Journal of General Medicine » Volume 17
Analysis of Risk Factors and Construction of a Predictive Model for Readmission in Patients with Coronary Slow Flow Phenomenon
Received 1 November 2023
Accepted for publication 28 February 2024
Published 4 March 2024 Volume 2024:17 Pages 791—808
DOI https://doi.org/10.2147/IJGM.S444169
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Yuriy Sirenko
Changshun Yan,1 Yankai Guo,2 Guiqiu Cao1
1Department of Cardiology, Fifth Affiliated Hospital of Xinjiang Medical University, Urumqi, The Xinjiang Uygur Autonomous Region, People’s Republic of China; 2Department of Pacing Electrophysiology, First Affiliated Hospital of Xinjiang Medical University, Urumqi, The Xinjiang Uygur Autonomous Region, People’s Republic of China
Correspondence: Guiqiu Cao, Tel +8613199954310, Email [email protected]
Background: Coronary slow flow phenomenon (CSFP) is a phenomenon in which distal vascular perfusion is delayed on angiography, but coronary arteries are not significantly narrowed and there is no other organic cardiac disease. Patients with CSFP may be repeatedly readmitted to the hospital because of chest pain or other symptoms of precordial discomfort, and there is a risk of adverse events. In order to investigate the risk factors affecting the readmission of CSFP patients, a prediction model was constructed with the aim of identifying patients at risk of readmission at an early stage and providing a reference for further clinical intervention.
Methods: In this study, we collected clinical data from 397 CSFP patients between June 2021 and January 2023 in Xinjiang Medical University Hospital. Telephone follow-up clarified whether the patients were readmitted to the hospital. A predictive model for readmission of CSFP patients was constructed using multifactorial logistic regression. Nomogram was used to visualize the model and bootstrap was used to internally validate the model. ROC, DCA and Calibration curve were plotted to evaluate the calibration and discriminative ability of the column line graphs, respectively. Calibration and resolution of the column line graphs, respectively.
Results: A total of 34 of 397 CSFP patients experienced readmission. Smoking history, creatine kinase isoenzyme-MB, total cholesterol, and left ventricular ejection fraction were the predictors of readmission in patients with CSFP. The area under the curve of the Nomogram model was 0.87, which indicated that the model had good predictive ability and differentiation, and the DCA and Calibration curves also indicated that the model had good consistency and was clinically useful.
Conclusion: A readmission prediction model for patients with CSFP may facilitate early identification of patients at potential risk for readmission and timely interventional therapy to improve patient prognosis.
Keywords: coronary slow flow phenomenon, readmission, predictive model
Introduction
Coronary slow flow phenomenon (CSFP) is a phenomenon in which the epicardial coronary arteries do not show significant stenosis on coronary angiography (CAG) and secondary conditions such as coronary artery spasm, aneffacement, or coronary dilatation have been excluded, but there is a delay in the development of distal perfusion.1 CSFP was first proposed by Tambe et al2 in 1972, and patients may be suspected of coronary artery disease and admitted to the hospital with symptoms such as chest tightness and chest pain at rest or during activity, with a prevalence rate of between 1% and 7%.
The cause of CSFP may be related to the inflammatory response, metabolic syndrome, endothelial dysfunction, calcium overload, and coronary microcirculatory dysfunction caused by oxidative stress injury, but the specific mechanism of its occurrence has not yet been clarified.3–5 Diffuse intimal thickening with atheromatous plaque formation in the coronary arteries of patients with CSFP, although no significant stenosis was detected.1 Some studies have indicated that the probability of cerebrovascular disease and death from cardiovascular events is significantly higher in patients with CSFP than in patients with normal coronary arteries.6 Among them, Zhu et al6 followed up 421 CSFP patients for a mean of 3.8 years, and 39 patients experienced major adverse cardiovascular events (MACE). Khor et al7 conducted a retrospective study of 215 patients with CSFP, the prognosis of CSFP over a period of 5 years was unclear, a large proportion of patients had persistent chest pain, 52% required hospitalization, 25% required a repeat CAG to clarify the condition, 5% of these patients subsequently developed acute myocardial infarction and 2% died. Fragasso et al8 reached similar conclusions in a cohort study of 12 CSFP patients, 4 of whom developed severe coronary artery disease and 1 of whom died during long-term follow-up. At this stage, with the prevalence of CSFP increasing year by year, the probability of patients being readmitted to the hospital for chest pain or other precordial discomfort is also higher than before, which seriously affects the quality of life, and there is a certain risk of MACE occurring in repeated readmissions.9
The purpose of this study is to identify potential risk predictors for readmission by comprehensively collecting basic information, clinical tests, auxiliary examination data, and follow-up data from CSFP patients, so as to construct a Nomogram model in order to predict the probability of readmission for CSFP patients. This will help the clinic to identify high-risk patients at an early stage, further develop appropriate interventions, and guide patients to control risk factors and choose reasonable treatments.
Research Content and Methodology
Research Target
This study was a prospective study that included patients with a definitive diagnosis of CSFP who visited the First Affiliated Hospital of Xinjiang Medical University and the Fifth Affiliated Hospital of Xinjiang Medical University from June 2021 to January 2023. The study was approved by the Ethics Committee of the Fifth Affiliated Hospital of Xinjiang Medical University (XYDWFYLSH-2023-052). The requirement of written informed consent was waived because the study only collected patients’ relevant clinical data with telephone follow-up. All enrolled patients or their families agreed to be included in this study.
Inclusion Criteria
Patients who presented to the clinic with clinical manifestations of chest pain, chest tightness and precordial discomfort; Meet the diagnostic criteria for CSFP proposed by GIBSON et al;10 Age was 18–80 years old; Complete follow-up information was available;
Exclusion Criteria
Age < 18 years; Presence of coronary-related diseases such as dilated cardiomyopathy (left ventricular end-diastolic internal diameter (LVEDd) >5.0 cm (women) and >5.5 cm (men), left ventricular ejection fraction (LVEF) <45%), hypertrophic cardiomyopathy (ventricular wall thickness ≥15 mm in any one or more segments of the left ventricle), aortic valve stenosis (aortic orifice area <1.0 cm2. peak flow velocity ≥4.0 m/s or mean transvalvular pressure difference ≥40 mmHg across the aortic valve), mitral stenosis (mitral valve orifice area <2.0 cm2, mean transvalvular pressure difference ≥5 mmHg across the mitral valve), pulmonary valve lesions (peak pressure difference ≥36 mmHg across the pulmonary valve, peak flow velocity ≥3.0 m/s), coronary artery thrombosis or entrapment, spasm or aneurysm dilatation of the coronary arteries, New York Society of Cardiology cardiac function classification (NYHA) ≥ grade III; History of coronary thrombolysis, post coronary stent implantation status therapy; Connective tissue disease, such as severe myositis, carbon dioxide diffusion function of less than 70%, pulmonary hypertension, lung biopsy showing proliferative vascular injury, positive antinuclear antibodies with a titer >1:320, and/or positive ENA antibodies. History of acute renal disease, such as blood creatinine greater than 400 µmmol/L and urine output less than 400 mL/d, chronic renal disease, such as renal unit damage greater than 50% of normal, blood creatinine greater than 133 µmol/L, and malignancy; Current treatment with glucocorticoids; Allergy to contrast media; Incomplete follow-up information.
Diagnostic Criteria for Patients with CSFP
CAG was accomplished in all patients via radial artery access using the standard Judkins technique. Patients were given nitroglycerin injection of 100–200 μg prior to imaging, followed by manual injection of hypotonic nonionic contrast agent (iohexol injection in doses of less than 20 mL at a time), and the image acquisition speed was set at 30 frames/s during imaging and projected in multiple positions, ie, orthostatic plus cephalic position 30°, cephalic position 30° plus 30° in the right shoulder position (anterior descending branch of the left coronary artery), 30° in the hepatic position (gyratory branch of the left coronary artery), 30° in the cephalic position and 45° in the left anterior oblique (right coronary artery). The Thrombolysis in Myocardial Infarction (TIMI) flowmetry method is based on the following criteria: the first frame is counted as the first frame when the contrast agent completely or nearly completely fills the beginning of the observed vessel (at least 70% of the lumen of the vessel is occupied by the contrast agent), and there is a tendency for the contrast agent to fill steadily, and the next frame is counted as the first frame when the contrast agent reaches the distal branches of the observed vessel and shows a steady filling trend. The last frame is counted as the last frame when the contrast reaches the distal branches of the observed vessel and shows its specific anatomical structure. The diagnostic criteria for CSFP are a normal CAG or stenosis of <40%; filling of at least one distal coronary artery for more than 3 cardiac cycles, or a corrected TIMI frame count (TFC) of >27 frames in no less than one coronary artery branch.10
Sample Size Estimation
The sample size was estimated according to the rough sample size formula proposed by Richard D Riley et al:11 sample size is usually expressed in terms of events per variable (EPV).10 EPV is widely used in the estimation of clinical prediction models, and the preliminary estimation of the independent variables was 36 items, and 10 patients were needed for each independent variable. Therefore, the sample size required for this study is 36 × 10 = 360 cases. In this study, 294 CSFP patients from the First Affiliated Hospital of Xinjiang Medical University and 103 CSFP patients from the Fifth Affiliated Hospital of Xinjiang Medical University who met the inclusion and exclusion criteria from June 2021 to January 2023 were selected for the study, and after analyzing the mean values of each clinical data of the two groups of patients with no significant differences (Supplementary Table 1), a total of 397 CSFP patients in the combined two groups met the estimated sample size requirements.
General Information Collection
The basic clinical data of the patients included age, gender, smoking history (smoking history definition: ≥1 cigarette/d, continuous smoking >6 months or previously met the smoking standard, and quit smoking less than half a year), drinking history (drinking history definition: ≥1 time per week on average, continuous drinking >6 months or previously met the drinking standard, and quit drinking less than half a year), systolic pressure (SBP), Accompanying diseases include hyperlipidemia (HLP) (diagnostic criteria for hyperlipidemia: LDL-C ≥ 3.64 mmol/L; TG ≥ 3.64 mmol/L; LDL-C ≥ 3.64 mmol/L; TG ≥3.64 mmol/L; TG ≥ 3.64 mmol/L; TG ≥ 3.64 mmol/L; TG ≥ 3.64 mmol/L; TG ≥ 3.64 mmol/L; TG ≥ 2.64 mmol/L; TG ≥ 4.64 mmol/L; TG ≥ 2.64 mmol/L). (Diagnostic criteria for hyperlipidemia: LDL-C ≥ 3.64 mmol/L; TG ≥ 1.70 mmol/L; TC ≥ 5.72 mmol/L), diabetes mellitus (DM) (Diagnostic criteria for diabetes mellitus: Random blood glucose ≥ 11.1 mmol/L; Fasting blood glucose level ≥ 7.0 mmol/L; Oral glucose tolerance test 2-hour glucose level ≥ 11.1 mmol/L), Hypertension (HTN) (Diagnostic criteria for hypertension: taking antihypertensive drugs or blood pressure ≥140/90 mmHg (1 mmHg = 0.133 kPa) in this measurement).
Collection of Biochemical Indicators
All patients were fasted for 8h, and 5–10 mL of venous blood was collected and placed in an anticoagulation tube. White blood cell (WBC), Neutrophil (Neu), Lymphocyte (Lym), Red blood cell (Red), Hemoglobin (Hem), Platelet (Plt) were analyzed by automatic blood cell analyzer. Blood potassium (potassium, K), blood sodium (sodium, Na), blood urea nitrogen (BUN), creatinine (Cre), uric acid (UA), fasting plasma glucose (FPG), albumin (ALB), creatine kinase (CK), creatine kinase isoenzymes-MB (CK-MB), Triglyceride (TG), Total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C) were determined by centrifuging the whole blood of the patients at 3000 r/min, with a centrifugation radius of 13.5 cm, for 10 min, and the serum was separated and analyzed by a fully automated chemical analyzer.
Echocardiography Data Collection
Echocardiograms of CSFP patients were performed using Siemens ultrasound, model X700, Philips EPIQ7C, or GEE90 color Doppler ultrasound, with a probe frequency of 2 to 4 MHz, and conventional anterior and lateral wall scans were performed to examine parasternal short-axis views and left ventricular long-axis views, and apical four-chamber ventricular views, which were analyzed by the software that was included with the diagnostic ultrasound device. Left ventricular fraction shortening, LVFS, and left ventricular ejection fraction (LVEF) were obtained, and all the results were recorded by two specialized ultrasonographers and summarized to take the median value.
Heart Rate Variability Data Collection
Heart rate variability data were collected using the DHD-6000, DHD-6020 or DHD-6024 24-hour ambulatory electrocardiogram detector. The detector complies with the standards set by the European Cardiovascular Society and the North American Society of Pacing and Electrophysiology for HRV testing, and uses a 5-minute, short-duration frequency-domain analysis at 500 Hz to select 256 consecutive cardiac cycles, and then calculates the spectral waveforms by Fourier transform method in either chronological or beat-ordered order to obtain the electrocardiographic signals and record them. In this collection Standard deviation of the normal-to-normal interval (SDNN) for all the intervals of normal sinus heart rate (normal-to-normal, NN) shows the sympathetic activation and its normal value is (141 ± 39) ms, when the value decreases, it means that sympathetic activation increases and the body adapts to the changes in the external environment decreases. Percentage of adjacent normal-to-normal intervals with standard deviation of ≥50ms (PNN50) represents the ratio between the number of adjacent NN intervals and the total number of NN intervals, with lower values implying less vagal activity. All results were recorded separately by 2 electrocardiographers and summarized to take the median record.
Follow Up
Data on whether CSFP patients were readmitted to the hospital after discharge and all follow-up information were obtained through telephone follow-up visits by the researchers to the patients or their relatives. The start of follow-up was after the patients with a definite diagnosis of CSFP were discharged from the hospital and enrolled in the study, followed by regular follow-up at intervals of 1, 3, and 6 months. During the follow-up process, it was clarified whether the patients with CSFP were re-hospitalized, and whether the relevant blood tests, auxiliary examinations and CAG were improved; if the patients were re-investigated in the First Affiliated Hospital of Xinjiang Medical University and the Fifth Affiliated Hospital of Xinjiang Medical University, then the medical record system was checked to clarify the patients’ re-admission conditions and diagnoses, and if the patients were re-investigated in other hospitals, the patients were asked for details of the conditions and diagnoses of the other hospitals over the phone, and in the process mentioned above, those who were re-hospitalized and had a clear diagnosis were considered to be the patients who had been re-invested in hospital. Follow-up was performed up to 06/2023. The occurrence of readmission during the follow-up of CSFP patients was the primary endpoint event, and the occurrence of MACE, such as acute myocardial infarction, severe arrhythmia, heart failure, and death due to coronary heart disease, during the follow-up of CSFP patients was the secondary endpoint event.
Statistical Analysis
R language 4.2.0 software was used to process the follow-up data. For measured data that conformed to a normal distribution, the independent samples t-test was used and expressed as Mean ± SD, whereas for non-normally distributed data, the rank sum test was used and expressed as M (Q1, Q3). Count data were analyzed using the χ2 test or Fischer’s test and expressed as n (%), and were considered statistically different when they yielded a result of P < 0.05. Subsequently, statistically significant clinical data were subjected to the Least absolute shrinkage and selection operator (LASSO) regression method using the “glmnet” package to screen for key characteristic variables. The final variables to be included were determined after considering the LASSO regression results and the clinical significance of each variable. Characteristic variable ratio (OR) and two-sided 95% confidence intervals (CI) were calculated and predictive models were constructed using multifactor logistic regression analysis. Finally, the “rms” package was used to visualize the model by constructing line plots, and the “pROC” package was used to assess the predictive power of the nomogram and the ability to discriminate between the various predictors by plotting the Receiver operating characteristic curve (ROC), and calculating the Area Under the Curve (AUC) to assess the accuracy of the model. The “rms” package was utilized to plot clinical calibration curves, bootstrap bootstrap validation was used for internal validation of the model, and a consistency C-index was obtained to assess the consistency of the model. The “rmda” package was utilized to plot a decision curve analysis (DCA) to clarify the net clinical benefit of using the model. A Restricted cubic spline (RCS) model was constructed using the “ggplot” package to explore the dose-response relationship between predictors and readmission of CSFP patients. If P-overall < 0.05, it indicates that there is a dose-response relationship between predictors and readmission of CSFP patients; if P-overall < 0.05 and P-non-linear > 0.05, it indicates that there is a linear dose-response relationship between predictors and readmission of CSFP patients; and if P-overall < 0.05 and P-non-linear < 0.05, it indicates that there is a nonlinear dose-response relationship between predictors and readmission of CSFP patients. The test level α=0.05.
Results
Clinical Characteristics of the Study Population
Three hundred and forty-eight and 167 patients with CSFP treated in the First Affiliated Hospital of Xinjiang Medical University and the Fifth Affiliated Hospital of Xinjiang Medical University, respectively, and 37 and 26 patients were excluded based on the inclusion and exclusion criteria, respectively. Eleven and three patients were excluded due to duplication of patient readmission data, respectively. Ten and seven patients were lost due to failure of patients and their families to complete the telephone follow-up, respectively. Sixteen and eight patients were excluded due to incomplete clinical examination data of patients and missing data, respectively. Finally, 294 patients with CSFP in the First Affiliated Hospital of Xinjiang Medical University were included with a prevalence rate of 1.8%, and 103 patients with CSFP in the Fifth Affiliated Hospital of Xinjiang Medical University were included with a prevalence rate of 2.9% (Figure 1), with a total of 397 patients with CSFP in the two groups with an age of 55.50 ± 11.30 years, spanning from 29 years old to 72 years old, and with 242 (60.96%) male patients and 155 (39.04%) female patients, and 141 (35.52%) patients with combined hypertension. A total of 155 (39.04%) patients, 141 (35.52%) patients with comorbid hypertensive disease, 28 (7.05%) patients with comorbid hyperlipidemia, 41 (10.33%) patients with comorbid diabetes mellitus, 103 (25.94%) patients with history of smoking, 125 (31.49%) patients with history of alcohol intake, and systolic blood pressure of 128.00 (118.00, 137.00) mmHg, diastolic blood pressure was 78.00 (70.00,86.00) mmHg and body mass index was 26.97 ± 4.25 kg/m2. Thirty-four patients (8.56%) with CSFP were readmitted to the hospital, with a mean readmission time of 14.56 ± 4.27 months, of whom 20 patients (58.82%) remained CSFP with perfected CAG due to chest pain, chest tightness, and precordial discomfort, 5 patients (14.71%) with coronary atherosclerotic heart disease, 4 patients (11.76%) with episodic ventricular preterm contraction, 3 patients (8.82%) with episodic atrial precontraction, 1 patient (2.94%) with atrial fibrillation, and 1 patient (2.94%) with non-ST-segment elevation myocardial infarction (8.82%), 1 (2.94%) with atrial fibrillation, and 1 (2.94%) with non-ST-segment elevation myocardial infarction.
 |
Figure 1 Patient Screening and Grouping Flowchart. |
Predictors Associated with Readmission in Patients with CSFP
The results of the analysis of variance showed that compared with the non-readmission group, the differences in smoking history, SBP, UA, FPG, CK-MB, TG, TC, HDL-C, Neu, PNN50, and LVEF, eleven clinical indices, were statistically significant (P < 0.01), and the differences in SDNN, FS, LCX, three clinical indices, were statistically significant (P < 0.05), and the differences in the remaining indicators were not statistically significant (Table 1). Categorization of variables for further screening of clinical indicators of variability(Table 2). Fourteen clinical factors potentially affecting readmission were downscaled by LASSO regression. A 3-fold cross-validation was used to select the optimal Lambda parameter, with the optimal value being the optimal Lambda value within the one-variance range of the minimum cross-validation error, to screen out the most representative risk factors for the occurrence of readmission. The results showed that the corresponding nonzero regression coefficient variables at this time were smoking history, CK-MB, TC, and LVEF (Figure 2A and B).
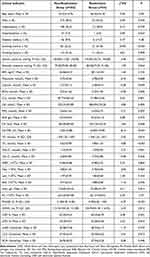 |
Table 1 Comparison of Clinical Data Between Two Groups of Patients with Coronary Slow Flow Phenomenon |
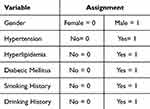 |
Table 2 Assignment of General Information on Patients with Coronary Slow Flow Phenomenon |
Constructing a Predictive Model for Readmission of Patients with CSFP
A multifactorial logistic regression analysis was performed with whether the patients were readmitted to the hospital for treatment as the dependent variable, and four characteristic variables screened by LASSO regression as the independent variables, and the results showed that the smoking history [β=1.609, OR=4.998, 95% CI:(2.132~12.218)], CK-MB [β=0.176, OR=1.193, 95% CI:(1.084~1.333)], TC [β=0.829, OR=2.169,95% CI:(1.568~3.474)], and LVEF [β=−0.136, OR=0.873,95% CI:(0.812~0.940)] were the risk predictors of re-admission of patients with CSFP (Table 3). The above four characteristic indicators are brought into the R language using the “rms” package structure to draw forest plots, multi-model ROC and column line graphs to visualize the model. The forest plot shows that smoking history, CK-MB, and TC OR greater than 1 are located on the right side of the dotted line and are risk factors for readmission in patients with CSFP, and LVEF OR less than 1 is located on the left side of the dotted line and is a protective factor for readmission in patients with CSFP (Figure 3). The area under the curve (AUC) of the four characteristic indicators seen in the multi-model ROC were smoking history [AUC=0.696,95% CI:(0.610~0.782)], CK-MB [AUC=0.761,95% CI:(0.671~0.851)], TC [AUC= 0.734,95% CI:(0.640~0.828)], LVEF [AUC=0.637,95% CI:(0.547~0.728)] (Figure 4). Plotting columns to visualize the predictive model for ease of clinical use (Figure 5).
 |
Table 3 Multifactorial Logistic Regression Analysis of Readmission in Patients with Coronary Slow Flow Phenomenon |
Evaluating Predictive Model Performance
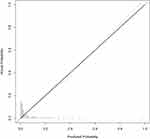
The ROC curve and Calibration curve were used to evaluate the predictive ability of the prediction model and for internal validation. The area under the ROC curve of the model was 0.875, 95% CI was 0.802–0.948, sensitivity and specificity were 0.882 and 0.749, respectively, the cutoff value was 0.077, and Yoden’s index was 1.632, which made the predictive ability good (Figure 6). Internal validation of the model through 5-fold cross-validation bootstrap validation 1000 times to obtain a corrected consistency C-index of 0.863, sensitivity, specificity of 0.989 and 0.278, respectively, indicating that the model overall prediction of the accuracy of the model is higher, the results of the Hosmer-Lemeshow goodness-of-fit test was Chi-squared = 10.42, P-value = 0.24, suggesting a better model fit.
Decision curve analysis (DCA) and Calibration curves were plotted to evaluate the clinical benefit of the model. The results of the Calibration curve suggest that the overall prediction accuracy of the model is relatively high, and the predicted probability of the model is consistent with the actual probability of occurrence when the probability of the event is 28% and 49%, respectively. However, when the probability of occurrence is less than 28% or between 49% and 100%, the model may overestimate the risk. Conversely, when the probability of occurrence is between 28% and 49%, the model may underestimate the risk (Figure 7).
 |
Figure 7 DCA curve of the readmission prediction model for patients with coronary slow flow phenomenon. |
DCA was used to measure the clinical value of the predictive model. In the model, the area under the curve within the black dashed, blue solid, and black solid lines represents the net benefit of the model, whereas the black dashed and black solid lines represent whether or not a readmission occurred or did not occur for all patients. The DCA suggests that the use of a column chart for predicting the occurrence of CSF can yield greater benefit if the threshold probability is >6%. Therefore, this prediction model can be used to predict the likelihood of CSF in patients with high accuracy and a wide range of threshold probabilities, which is clinically valuable in clinical applications (Figure 8).
Evaluating the Relationship Between Readmission and Characteristic Indicators in Patients with CSFP
The dose-response relationship between readmission and characteristic indicators in CSFP patients was further explored based on the results of multifactorial logistic regression analysis. Three of the continuous characteristic indicators were categorized into CK-MB <9.90, 9.90–12.40, 12.40–16.17, >16.17, TC<3.32, 3.32–4.02, 4.02–4.79, >4.79, LVEF <61.90, 61.90–63.00, 63.00–65.36, >65.36 at four nodes, respectively. A Restricted cubic spline (RCS) model was constructed for analysis using “ggplot”, with 4 nodes, with CSFP patients’ readmission as the horizontal coordinate and the predicted OR as the vertical coordinate, and the upper and lower dashed lines representing 95% CI. The model was based on smoking history, CK-MB, TC, and LVEF crossover as adjustment variables, and there was a linear dose-response relationship between readmission and CK-MB in patients with CSFP (P-overall < 0.01, P-non-linear = 0.78) (Figure 9A). There was a linear dose-response relationship between readmission and TC in patients with CSFP (P-overall < 0.01, P-non-linear = 0.50) (Figure 9B), There was a linear dose-response relationship between readmission and LVEF in patients with CSFP (P-overall < 0.01, P-non-linear = 0.19) (Figure 9C).
Discussion
CSFP is a unique angiographic presentation characterized by delayed distal vascular perfusion without significant stenosis of the epicardial coronary arteries and in the absence of other organic cardiac disease.12 Although, the mechanism of CSFP pathogenesis has not yet been clarified, a number of possible etiologies have been proposed in conjunction with previous clinical studies. For example, Pekdemir et al13 found decreased values of FFR in patients with CSFP by intravascular ultrasound and measurement of fractional flow reserve (FFR), a negative correlation between the number of TIMI frames and FFR, and extensive intima-media thickening and atheromatous plaque formation in epicardial coronary arteries. This study reveals that CSFP patients present with symptoms of coronary atherosclerosis. Kundi et al3 indicated that the expression of endolipin is closely related to the expression of pro-inflammatory cytokines, and the blood levels of endolipin in patients with CSFP were significantly higher than those in patients with normal CAG, thus confirming that inflammatory response plays an important role in the pathogenesis of CSFP. CSFP is generally considered a benign condition, but several studies have shown that the incidence of myocardial ischemia, malignant arrhythmias, sudden cardiac death, and recurrent acute coronary syndromes in this group has increased significantly in recent years.14 A follow-up study indicated that the probability of MACE in CSFP patients was about 9.3%.6 It has also been shown that patients with CSFP have a statistically significant increase in the frequency of hospitalization due to chest pain at rest and during exercise.9 As the prevalence of CSFP and the number of readmissions for chest pain discomfort continue to rise, this not only affects patients’ quality of life but also carries the risk of developing MACE. Therefore, an accurate assessment of the risk factors for readmission of CSFP patients and precise interventions are now critical steps to reduce the readmission rate and optimize the health status of the patients.
A total of 397 patients with CSFP were included in this study, of whom 34 were readmitted, with a readmission rate of 8.56%. A total of 36 variables were collected from clinical data related to patients with CSFP, and the results of the statistical analysis indicated that smoking history, CK-MB, and TC were risk factors for the occurrence of readmission in patients with CSFP, and LVEF was a protective Factors.
A history of smoking is more common in the male population. Harmful substances contained in smoke, such as nicotine, may increase the risk of coronary artery disease by damaging the endothelial cells of blood vessels, inducing vasospasm, and decreasing the oxygen-carrying capacity of the blood.15 NO is an important vasodilator that promotes vasodilation and increased blood flow, and the harmful components of cigarettes reduce nitric oxide (NO) levels in the blood, leading to vasoconstriction of coronary arteries, which restricts blood flow.16 Smoking also induces platelets to release reactive substances, such as platelet aggregating factor and platelet-derived growth factor, which can cause platelet aggregation and thrombus formation. Although no significant stenosis was found in patients with CSFP in the CAG observations, the reactive substances released under the stimulation of smoking can cause damage to the vascular endothelial cells of the coronary arteries, thereby affecting the dilatation and contraction of the endothelial layer, and altering the coronary artery’s blood flow rate.17 In addition to this, burning tobacco and inhaling it into the body increases oxidized substances in the blood through a variety of pathways, and may lead to a decrease in the concentration of antioxidants such as reduced glutathione, triggering oxidative stress in the body and reducing the antioxidant capacity of the vascular endothelium. Oxidative stress can also lead to a chronic inflammatory state in the body, which can lead to problems such as insulin resistance and abnormal glucose tolerance, which has been shown to be one of the most important risk factors for the development of CSFP.18 Smoking can also lead to the upregulation of the expression of some inflammation-related genes, Dang et al19 found that the activation of the transcription factor NF-κB was enhanced in patients who smoked, which would further damage the vascular endothelial cells, leading to pathologic changes in coronary vasculature and the appearance of CSFP. Ghaffari et al20 found that male patients with a history of smoking were more likely to develop CSFP. In the present study the proportion of male patients with CSFP was higher than that of females and there were relatively more males in the readmission group. Though there was no statistically significant difference between the two groups, history of smoking was an independent predictor of readmission of the patients. Therefore it is necessary to emphasize the importance of smoking cessation in male patients with a history of smoking.
Creatine kinase is widely distributed in the cytoplasm and mitochondria of a variety of tissues, and it is closely associated with ATP regeneration, intracellular energy flow, and muscle contraction. In particular, CK-MB, the most sensitive indicator of myocardial injury, is concentrated in cardiomyocytes. When myocardial tissue is damaged by ischemia, CK-MB diffuses into the bloodstream through the cell membrane of the damaged cells.21 Microvascular dysfunction with a diameter less than or equal to 400 micrometers is considered a risk factor for the development of CSFP.22 Endothelial dysfunction in microvascular structures increases angiotensin levels, which causes myocardial ischemia and hypoxia and a range of clinical symptoms.23 Under hypoxic conditions, cardiomyocytes also produce lactic acid via the glycolytic pathway, and the increase in intracellular lactic acid content causes a change in the acidic environment of the cytosol, resulting in an increase in the release of CK-MB.24 CSFP microvascular dysfunction also leads to increased oxidative stress in cardiomyocytes.25 Oxidative stress is a process in which the production of intracellular oxygen free radicals exceeds the scavenging capacity of antioxidant defense systems, which in turn leads to intracellular oxidative damage. Oxidative stress can activate a variety of signaling pathways, such as apoptosis signaling pathways, inflammatory signaling pathways, etc. The activation of these signaling pathways also contributes to the release of CK-MB.26 CK-MB, as a sensitive marker of myocardial injury, can be used to predict the extent of coronary artery disease, while delayed coronary perfusion and CK-MB release in patients with CSFP are associated with patient readmission. Therefore, an in-depth study of the relationship between creatine kinase and CSFP is important for the prevention and treatment of cardiovascular disease.
TC is a lipid which enters the cell mainly through the transportation of lipoproteins. Elevated levels of TC lead to increased levels of apolipoproteins in the blood, increasing the risk of lipid deposits in the cardiovascular system, which can gradually form coronary plaques and impede blood flow, thus increasing the risk of cardiovascular disease.27 The effects of TC on the cardiovascular system are not only caused through plaque formation, but it has been found that high TC levels can also directly damage endothelial cells, decreasing some of the important bioactive substances they produce and reducing vasoconstriction, leading to CSFP.28 Pekdemir et al29 stated that extensive calcification was present in the coronary arteries of most patients with CSFP, suggesting that CSFP may be the result of non-obstructive coronary atherosclerosis and microvascular disease. In contrast, dyslipidemia such as increased serum TC and TG decreases the concentration of endothelium-dependent vasodilatory factor, which in turn leads to coronary microvascular sclerosis.30 Dyslipidemia levels are strongly associated with the incidence of ischemic heart disease, myocardial infarction, and all-cause mortality.31 In this study, we found that both TG and TC were higher in the readmission group of CSFP patients than in the non-readmission group and were statistically significant. However, TG is metabolized by most cells in the body, but TC is not. Previous studies have pointed out that the main cause of increased risk of chronic heart disease and death is TC, not TG.32 Therefore, early administration of statin lipid-lowering drugs in clinical practice improves vascular endothelial function by controlling dyslipidemia and reduces clinical symptoms in patients with CSFP.33
Echocardiography has been widely used to assess cardiac function in hospitalized patients.34 It was found that the majority of diseased vessels in patients with CSFP were anterior descending coronary arteries, followed by simultaneous involvement of all three coronary arteries. However, diseased vessels can affect myocardial strain in the relevant cardiac segments.35 The greater the number of diseased coronary arteries, the more severe the effect on cardiac function.36 Therefore, close follow-up monitoring of patients with CSFP is necessary, in which LV systolic function is critical to the assessment and prognosis of the disease. In a study by Balci et al,37 LVEF was found to be reduced but within the normal range in the CSFP group. This is in agreement with the present study, where LVEF was lower in patients in the readmission group than in the non-readmission group, but the mean value was 60.43 ± 6.39%. The fact that LVEF was within the normal range in patients with CSFP does not exclude that their systolic function has not yet been compromised. Two-dimensional speckle-tracking ultrasound technology has been used to detect mildly impaired left ventricular systolic function due to its ability to measure whole-heart long-axis strain capacity (GLS).38 Teerapat et al39 study indicated that GLS in the CSFP group, with a mean value of −18.2%, was lower than normal values. In addition, Liu et al36 using 3D speckle tracking technique also found decreased LV systolic function in CSFP patients. Although no significant stenosis was found in the coronary arteries of the CSFP patient in the CAG, the patient did have impaired left ventricular systolic function, which may have been one of the reasons for the patient’s readmission to the hospital with chest pain at rest or after activities.40 Cem et al41 stated that LAD is the most common artery with delayed perfusion in CSFP, and the present study also found that the number of cLAD, LCX, and RCA flow frames in the readmission group was higher than that in the non-readmission group, and CK-MB was also an independent risk factor for readmission of patients with CSFP; therefore, it is considered that long-term delayed coronary artery filling in patients with CSFP has some effect on myocardial blood supply and cardiac function, which leads to the release of CK-MB from cardiomyocytes into the bloodstream, and abnormal LVEF. Dipyridamole is clinically indicated as a treatment to improve left ventricular impairment in patients with CSFP, but further follow-up studies are needed to confirm this result in terms of long-term efficacy.42
This study has some limitations. First, the CSFP patients in this study were mainly included by Xinjiang Medical University Hospital, and the model was only internally validated using the bootstrap method without further external validation combined with multicenter data, so large samples and high-quality randomized controlled studies are still needed in the future to make the model more broadly applicable, in order to reduce the bias generated by the results and to improve the stability. Second, the clinical indicators focused on in this study are not yet comprehensive, did not assess the psychological factors of CSFP patients’ first admission and readmission, and have not yet further collected data on patients’ socioeconomic factors, nursing factors, literacy level, and medication adherence, etc. Future studies can more comprehensively explore the factors related to CSFP patients’ readmission, and improve the indicators of the column charts as a way to facilitate clinical decision-making.
Conclusion
In conclusion, this study developed a readmission risk prediction model for CSFP patients using four common and easily available clinical data (smoking history, CK-MB, TC, and LVEF) and visualized the model by drawing a nomogram. The results showed that the model has good predictive ability and clinical value, which can help physicians identify CSFP patients at risk of readmission and provide early intervention to address the risk factors, improve the prognosis of patients, and reduce the occurrence of adverse cardiovascular events.
Ethical Approval and Consent to Participate
The studies involving human participants were reviewed and approved by the Medical Ethics Committee, the Fifth Affiliated Hospital of Xinjiang Medical University (No XYDWFYLSH-2023-052). As this study is retrospective and presents no risk of harm to subjects, and no privacy of individuals is exposed, informed consent was waived. All procedures adhered to the ethical standards and the Helsinki Declaration (as revised in 2013).
Funding
This work was funded by the Xinjiang Uygur Autonomous region Science and technology support project plan (No. 2020E0274), the Natural Science Foundation of Xinjiang Uygur Autonomous Region (2022D01C578) and the Natural Science Foundation of Xinjiang Uygur Autonomous Region (2021D01C450).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Alvarez C, Siu H. Coronary slow-flow phenomenon as an underrecognized and treatable source of chest pain: case series and literature review. J Investig Med High Impact Case Rep. 2018;6:2324709618789194.
2. Hawkins BM, Stavrakis S, Rousan TA, et al. Coronary slow flow--prevalence and clinical correlations. Circ J. 2012;76(4):936–942. doi:10.1253/circj.CJ-11-0959
3. Kundi H, Gok M, Kiziltunc E, et al. The relationship between serum endocan levels with the presence of slow coronary flow: a Cross-Sectional Study. Clin Appl Thromb Hemost. 2017;23(5):472–477. doi:10.1177/1076029615618024
4. Zhu YT, Zhu LP, Wang ZY, et al. Plasma choline as a diagnostic biomarker in slow coronary flow. Cardiol Res Pract. 2020;2020:7361434. doi:10.1155/2020/7361434
5. Baysal SS, Koc S. Oxidant-antioxidant balance in patients with coronary slow flow. Pak J Med Sci. 2019;35(3):786–792. doi:10.12669/pjms.35.3.162
6. Heianza Y, Ma W, Manson JE, et al. Gut microbiota metabolites and risk of major adverse cardiovascular disease events and death: a systematic review and meta-analysis of prospective studies. J Am Heart Assoc. 2017;6(7):e004947. doi:10.1161/JAHA.116.004947
7. Aksoy S, Öz D, Öz M, et al. Predictors of long-term mortality in patients with stable angina pectoris and coronary slow flow. Medicina. 2023;59(4):763. doi:10.3390/medicina59040763
8. Fragasso G, Chierchia SL, Arioli F, et al. Coronary slow-flow causing transient myocardial hypoperfusion in patients with cardiac syndrome X: long-term clinical and functional prognosis. Int J Cardiol. 2009;137(2):137–144. doi:10.1016/j.ijcard.2008.06.070
9. Kaplan M, Abacıoğlu ÖÖ, Yavuz F, et al. Slow flow phenomenon impairs the prognosis of coronary artery ectasia as well as coronary atherosclerosis. Braz J Cardio Surg. 2021;36(3):346–353. doi:10.21470/1678-9741-2020-0618
10. Gibson CM, Cannon CP, Daley WL, et al. TIMI frame count: a quantitative method of assessing coronary artery flow. Circulation. 1996;93(5):879–888. doi:10.1161/01.CIR.93.5.879
11. Dhiman P, Ma J, Qi C, et al. Sample size requirements are not being considered in studies developing prediction models for binary outcomes: a systematic review. BMC Med Res Methodol. 2023;23(1):188. doi:10.1186/s12874-023-02008-1
12. Beltrame JF. Defining the coronary slow flow phenomenon. Circ J. 2012;76(4):818–820. doi:10.1253/circj.CJ-12-0205
13. Pekdemir H, Cin VG, Ciçek D, et al. Slow coronary flow may be a sign of diffuse atherosclerosis. Contribution of FFR and IVUS. Acta Cardiol. 2004;59(2):127–133. doi:10.2143/AC.59.2.2005166
14. Cutri N, Zeitz C, Kucia AM, et al. ST/T wave changes during acute coronary syndrome presentation in patients with the coronary slow flow phenomenon. Int J Cardiol. 2011;146(3):457–458. doi:10.1016/j.ijcard.2010.10.120
15. Hackshaw A, Morris JK, Boniface S, et al. Low cigarette consumption and risk of coronary heart disease and stroke: meta-analysis of 141 cohort studies in 55 study reports. BMJ. 2018;360:jr5855. doi:10.1136/bmj.j5855
16. Mesaik MA, Jabeen A, Saeed M, et al. Effects of smokeless tobacco samples from Tabuk Saudi Arabia on nitric oxide production: a potential risk for cancer and cardiovascular diseases. Curr Comput Aided Drug Des. 2022;18(2):110–119. doi:10.2174/1573409917666211118094840
17. Dai XT, Kong TZ, Zhang XJ, et al. Relationship between increased systemic immune-inflammation index and coronary slow flow phenomenon. BMC Cardiovasc Disord. 2022;22(1):362. doi:10.1186/s12872-022-02798-0
18. Binak E, Gunduz H, Sahin M, et al. The relation between impaired glucose tolerance and slow coronary flow. Int J Cardiol. 2006;111(1):142–146. doi:10.1016/j.ijcard.2005.09.007
19. Dang X, He B, Ning Q, et al. Alantolactone suppresses inflammation, apoptosis and oxidative stress in cigarette smoke-induced human bronchial epithelial cells through activation of Nrf2/HO-1 and inhibition of the NF-κB pathways. Respir Res. 2020;21(1):95. doi:10.1186/s12931-020-01358-4
20. Ghaffari S, Tajlil A, Aslanabadi N, et al. Clinical and laboratory predictors of coronary slow flow in coronary angiography. Perfusion. 2017;32(1):13–19. doi:10.1177/0267659116659918
21. Aksakal E, Yapici O, Yazici M, et al. Apical hypertrophic cardiomyopathy: a case of slow flow in lad and malign ventricular arrhythmia. Int J Cardio Imag. 2005;21(2–3):185–188. doi:10.1007/s10554-004-2457-z
22. Oksen D, Aslan M, Ozmen E, et al. Ranolazine improved left ventricular diastolic functions and ventricular repolarization indexes in patients with coronary slow flow. Front Cardiovasc Med. 2023;10:1207580. doi:10.3389/fcvm.2023.1207580
23. Beltrame JF, Limaye SB, Wuttke RD, et al. Coronary hemodynamic and metabolic studies of the coronary slow flow phenomenon. Am Heart J. 2003;146(1):84–90. doi:10.1016/S0002-8703(03)00124-8
24. Yazdani AN, Pletsch M, Chorbajian A, et al. Biomarkers to monitor the prognosis, disease severity, and treatment efficacy in coronary artery disease. Expert Rev Cardiovasc Ther. 2023;21(10):675–692. doi:10.1080/14779072.2023.2264779
25. Aparicio A, Cuevas J, Morís C, et al. Slow coronary blood flow: pathogenesis and clinical implications. Eur Cardiol. 2022;17:e08. doi:10.15420/ecr.2021.46
26. Tang L, Yu J, Zhuge S, et al. Oxidative stress and Cx43-mediated apoptosis are involved in PFOS-induced nephrotoxicity. Toxicology. 2022;478:153283. doi:10.1016/j.tox.2022.153283
27. Caselli C, De Caterina R, Smit JM, et al. Triglycerides and low HDL cholesterol predict coronary heart disease risk in patients with stable angina. Sci Rep. 2021;11(1):20714. doi:10.1038/s41598-021-00020-3
28. Kanikowska D, Malińska A, Mickiewicz A, et al. Effect of flaxseed (Linum usitatissimum L.) supplementation on vascular endothelial cell morphology and function in patients with dyslipidaemia-a preliminary observation. Nutrients. 2022;14(14):2879. doi:10.3390/nu14142879
29. Pekdemir H, Polat G, Cin VG, et al. Elevated plasma endothelin-1 levels in coronary sinus during rapid right atrial pacing in patients with slow coronary flow. Int J Cardiol. 2004;97(1):35–41. doi:10.1016/j.ijcard.2003.06.025
30. Mäkinen PI, Ylä-Herttuala S. Therapeutic gene targeting approaches for the treatment of dyslipidemias and atherosclerosis. Curr Opin Lipidol. 2013;24(2):116–122. doi:10.1097/MOL.0b013e32835da13c
31. Langsted A, Freiberg JJ, Tybjaerg-hansen A, et al. Nonfasting cholesterol and triglycerides and association with risk of myocardial infarction and total mortality: the Copenhagen City heart Study with 31 years of follow-up. J Intern Med. 2011;270(1):65–75. doi:10.1111/j.1365-2796.2010.02333.x
32. Ginsberg HN, Packard CJ, Chapman MJ, et al. Triglyceride-rich lipoproteins and their remnants: metabolic insights, role in atherosclerotic cardiovascular disease, and emerging therapeutic strategies-A consensus statement from the European Atherosclerosis Society. Eur Heart J. 2021;42(47):4791–4806. doi:10.1093/eurheartj/ehab551
33. Demir H, Kahraman G, Isgoren S, et al. Evaluation of post-stress left ventricular dysfunction and its relationship with perfusion abnormalities using gated SPECT in patients with cardiac syndrome X. Nucl Med Commun. 2008;29(3):208–214. doi:10.1097/MNM.0b013e3282f52c49
34. Javadi DH, Sotudeh DS, Javadi DA, et al. Echocardiographic evaluation of left and right ventricular function in patients with coronary slow flow syndrome: a Comparative Study. Curr Probl Cardiol. 2022;47(9):100925. doi:10.1016/j.cpcardiol.2021.100925
35. Kemaloğlu ÖT, Eren M, Atasoy I, et al. Are biventricular systolic functions impaired in patient with coronoray slow flow? A prospective study with three dimensional speckle tracking. Int J Cardio Imag. 2017;33(5):675–681. doi:10.1007/s10554-016-1054-2
36. Liu S, Wang Y, Li J, et al. Incremental value of three-dimensional speckle-tracking echocardiography for evaluating left ventricular systolic function in patients with coronary slow flow. Curr Probl Cardiol. 2022;47(9):100928. doi:10.1016/j.cpcardiol.2021.100928
37. Balci MM, Arslan U, Kocaoğlu I, et al. Right ventricular functions in patients with slow coronary flow. Acta Cardiol. 2013;68(2):161–166. doi:10.1080/AC.68.2.2967273
38. Smiseth OA, Torp H, Opdahl A, et al. Myocardial strain imaging: how useful is it in clinical decision making?. Eur Heart J. 2016;37(15):1196–1207. doi:10.1093/eurheartj/ehv529
39. Yingchoncharoen T, Agarwal S, Popović ZB, et al. Normal ranges of left ventricular strain: a meta-analysis. J Am Soc Echocardiogr. 2013;26(2):185–191. doi:10.1016/j.echo.2012.10.008
40. Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American society of echocardiography and the European association of cardiovascular imaging. J Am Soc Echocardiogr. 2015;28(1):1–39.e14. doi:10.1016/j.echo.2014.10.003
41. Özde C, Aktüre G, Aytekin S, et al. Assessment of the relationship between coronary flow rates and myocardial perfusion abnormality in patients with nonobstructive coronary artery disease: an observational study in cardiac syndrome X and coronary slow flow. Nucl Med Commun. 2019;40(11):1122–1129. doi:10.1097/MNM.0000000000001080
42. Suner A, Cetin M. Is dipyridamole useful in improving left ventricular systolic and diastolic function in patients with coronary slow flow?. Echocardiography. 2016;33(10):1472–1478. doi:10.1111/echo.13290
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.