Back to Journals » Cancer Management and Research » Volume 13
Analysis of Factors Related to Lymph Node Metastasis in Early-Stage Type 1 Endometrial Cancer: Verifying the Clinical Value of Positive Threshold of the Immunohistochemical Parameter Ki67
Received 19 April 2021
Accepted for publication 5 June 2021
Published 10 August 2021 Volume 2021:13 Pages 6319—6328
DOI https://doi.org/10.2147/CMAR.S316211
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Harikrishna Nakshatri
Peng Jiang, Rui Yuan
Department of Gynecology, The First Affiliated Hospital of Chongqing Medical University, Chongqing, People’s Republic of China
Correspondence: Rui Yuan
Department of Gynecology, The First Affiliated Hospital of Chongqing Medical University, Chongqing, People’s Republic of China
Tel +86 23 89011092
Fax +86 23 89011082
Email [email protected]
Objective: Lymph node metastasis (LNM) is an important reference indicator for the prognosis of endometrial cancer (EC). Even in patients with early low-risk EC, many people still have LNM. The purpose of this study was to investigate the related factors influencing LNM in early-stage EC and determine the optimal positive threshold of immunohistochemical parameter Ki67 for predicting LNM, providing auxiliary reference indicators for clinical diagnosis and treatment.
Methods: The clinicopathological data of 651 patients with “apparent” early-stage EC who underwent standard surgical treatment were included. Univariate and multivariate logistics regression were used to analyze the correlation between each clinicopathological factor and LNM. Receiver operating characteristic curve (ROC curve) and Youden index were used to determine the optimal positive threshold of Ki67 for predicting LNM. Finally, correlation between Ki67 and various clinicopathological factors was analyzed, and the predictive value of each prognostic factor was compared.
Results: Multivariate analysis found that histologic grade (P=0.023), lymphatic vessel space invasion (LVSI) (P < 0.001), serological index Ca125 (P=0.002), immunohistochemical parameter Ki67 (P < 0.001), ER (P < 0.001) and P53 (P=0.001) were independent prognostic factors of LNM. ROC curve and Youden index showed that the optimal positive thresholds of Ki67 to predict LNM were 40%. Based on this, ROC curve showed that the area under the curve (AUC) of Ki67 (AUC=0.714) was larger than other single predictors, and Ki67 combined with other predictors can significantly increase the AUC value (AUC= 0.847 and 0.868, respectively).
Conclusion: Ki67 was an important predictor for predicting the LNM in early-stage EC and taking a positive percentage of about 40% can be used as the positive threshold of the immunohistochemical parameter Ki67. On this basis, Ki67 combined with other predictive indicators can significantly improve prediction performance and can be used for segmentally predicting LNM of early-stage EC.
Keywords: Ki67, positive threshold, predict, early-stage endometrial cancer, lymph node metastasis
Introduction
Endometrial cancer (EC) is the fourth and ninth most common cancer of the new cases in the United States and China, respectively.1,2 Although the 5-year overall survival rate of patients exceeds 80%, many patients still have a poor prognosis and even die due to recurrence.3 Related literature reports that even in early stage (stage I) patients, 2–15% of them will relapse after treatment.4 While whether the patient has lymph node metastasis (LNM) is an important reference index for treatment and prognosis,5 the prognosis of patients with LNM is poor, which is more likely to lead to recurrence of the pelvic cavity and distant metastasis after treatment. Therefore, systemic lymph node dissection is an important intervention in the standard surgery for EC.5 At present, there is considerable controversy regarding the surgical treatment of systemic lymph node dissection in patients with early EC, it is reported that for patients with early type I (endometrioid cancer) EC at low or intermediate risk, the risk of performing systemic lymph node dissection outweighs the benefits.6 Therefore, most international guidelines no longer recommend systemic lymph node dissection for such patients.5 But on the other hand, even in early low-risk EC patients, around 10% of them still have LNM according to the current risk stratification standards, which indicates that many patients have not got adequate treatment.7
To predict LNM more accurately in EC and to maximize the identification of low-risk patients for LNM, various risk stratification systems and prediction models have been developed, most of which were developed based on classical clinicopathological parameters.5,8 For example, Michael9 defined EC patients with the following specific criteria as low-risk group for LNM to guide treatment planning for reoperation in patients with incomplete surgical staging information: endometrioid histology, well or moderately differentiated, myometrial invasion <50%, and tumor size <2 cm; Capozzi10 have established a scoring system based on lymphatic vessel space invasion (LVSI) to predict the risk of LNM before surgery, thereby avoiding unnecessary lymph node resection. However, the predictive index types of these risk stratification systems are relatively single and prediction accuracy is limited.11 Especially for patients with early low-risk EC (patients with stage IA and without the above-mentioned obvious high-risk clinicopathological factors), the above predictive indicators may no longer be applicable, so there is an urgent need to find new prognostic markers that are independent of classic clinicopathological parameters.
The cell proliferation index Ki67 is a well-known marker used to assess cell proliferation. A large number of studies have shown that the Ki67 index can independently predict the progression of cancer, and its expression can be easily observed by immunohistochemistry, which has led to it being widely used to reflect the prognosis of many malignant tumors.12 For example, in breast cancer, the positive percentage of Ki67 immunohistochemistry ≥20% is defined as a high Ki67 state, which was used to evaluate the prognosis of the patient and guide the decision-making of adjuvant treatment options.13 In endometrial cancer, Ki67 is also closely related to the prognosis of patients, especially in early stages of tumors, Ki67 is a powerful predictor that cannot be ignored,14 but unfortunately, Ki67 still lacks an acceptable immunohistochemical positive threshold in EC. In summary, this study aimed to explore the prognostic factors associated with LNM in early-stage EC by including “apparent” early-stage EC patients, and to determine the optimal positive threshold of the immunohistochemical parameter Ki67 for predicting LNM in early-stage EC, to provide an auxiliary reference index for treatment and prognosis management of patients.
Materials and Methods
Study Population
The clinicopathological data of patients with FIGO (2009 guidelines15) stages I–III EC who underwent primary surgery at the First Affiliated Hospital of Chongqing Medical University from 2013 to 2020 were collected, including age, body mass index (BMI), serum index Ca125, surgical procedures, postoperative pathological results (pathological type and grade of tumor, depth of myometrial invasion, LVSI status, number of removed pelvic and para-aortic lymph nodes, presence of LNM) and immunohistochemical results (Ki67, ER, PR and P53). Then these patients were first excluded from the study: patients without standard surgical treatment or lymph node dissection, patients with other malignant tumors, patients with missing medical records, patients who were lost to follow-up after surgery.
For the determination of patients with “apparent” early-stage EC for LNM, the current NCCN guidelines recommend systemic lymph node dissection should be applied for patients with the following high-risk factors: deeply invasive lesions, high-grade histology, and tumor of serous carcinoma, clear cell carcinoma, or carcinosarcoma; while for FIGO staging, deep myometrial invasion and cervical stromal invasion are important staging criteria for early-stage (stage IB and stage II) EC. Therefore, according to NCCN guidelines16 and FIGO guidelines,15 patients who do not have the following high-risk factors were defined as “apparent” early-stage patients for LNM of EC and included in the study: non-endometrioid carcinoma, high-grade (grade 3) endometrioid carcinoma, accompanied by deep myometrial invasion (infiltration depth ≥1/2), accompanied by cervical stromal invasion. All patients underwent a comprehensive staging operation including at least total uterine and bilateral salpingo-oophorectomy with pelvic lymph node dissection with or without abdominal para-aortic lymph node dissection.5 Similarly, according to the standard of AlHilli,17 the number of removed pelvic lymph nodes >10 with or without para-aortic lymph nodes >5 was defined as an effective lymph node dissection.
Postoperative Pathology and Immunohistochemical Analysis
The postoperative specimens of all patients were immediately fixed with formalin tissue fixative and sent to the Pathology Laboratory Center of Chongqing Medical University within 20 minutes for embedding, sectioning, H&E staining and immunohistochemical analysis in accordance with uniform standards. Pathological results (tumor size, extent of tumor invasion, pathological type and grade of tumor, depth of myometrial invasion, LVSI status, number of removed pelvic and para-aortic lymph nodes, presence of LNM) were initially judged by the primary physician and reviewed by the superior physician.
Immunohistochemical studies of ER, PR, Ki67 and P53 were carried out with an optimized and validated IHC protocol of immunohistochemistry autostainer (Leica Bond-Max, Milton Keynes, UK).18 Briefly, paraffin sections were deparaffinized and hydrated, which were then subjected to antigen retrieval with microwave. Next, sections were cooled and treated with peroxidase blocker for 5 minutes to block endogenous peroxidase activity. The following primary mouse monoclonal antibodies (ready-to-use) were used: Ki67 (clone MX006), ER (clone SP1), PR (clone MX009) and P53 (clone MX008) (all purchased from Maixin Biotech, Fuzhou, China) were applied separately and incubated overnight at 4°C. Then, specimens were incubated at room temperature for 30 minutes with using a biotin-labeled anti-mouse secondary antibody. Diaminobenzidine (DAB) was used for color development and hematoxylin was used for section counterstaining. Known positive tissue sections were used as positive control, phosphate-buffered saline (PBS) was used to replace the primary antibody as a negative control.
Interpretation of immunohistochemistry: five high-power fields were randomly observed in the most active tumor area (“hottest spot” of tumor), tumor cells with strong nuclear immunostaining were defined as positive cells, 100 tumor cells were evaluated in each field, and the average positive percentage (0–100%) of each immunohistochemical parameter (Ki67, ER, PR and P53) was calculated in five fields.18,19 This process was independently evaluated by two experienced pathologists and the results were recorded separately. If the difference between the count results of two observers ≤10%, the observation results were considered to be consistent; if the count results between two observers differed >10%, the counts would be re-evaluated, and a consensus was obtained. Finally, the average of the results of the two observers represented the final result of interpretation of immunohistochemistry.20
Referring to other similar studies, ER and PR were defined as negative if the proportion of positive tumor cells ≤5%.21,22 According to the 3-tier system for immunohistochemistry interpretation of P53,23 overexpression (the proportion of positive tumor cells ≥75%) and complete absence of expression (no obvious positive tumor cell staining) were defined as abnormal (aberrant/mutation-type) expression of P53, on the contrary, positive expression between the two extremes (positive tumor cell ratio 0–75%) was defined as normal (wild-type) expression of P53. Ki67 was expressed as the percentage of positive tumor cells (0–100%).
Statistical Analysis
Measurement data were represented by the mean, median and range, and comparisons between groups were performed by Student’s t-test. Categorical data were expressed as frequency and percentage, and chi-square test was used for comparison between groups. Univariate and multivariate analysis were realized by logistic regression model. The optimal threshold of positive percentage of Ki67 was determined by the receiver operating characteristic curve (ROC) and Youden index (Youden Index=Sensitivity + Specificity −1, range from 0 to 1).24 Patients with Ki67 index ≥ the positive threshold were defined as high-Ki67 group, and patients with Ki67 index < the positive threshold were defined as low-Ki67 group. Differences of the clinicopathological parameters between the two groups were compared. Finally, area under the curve (AUC) of ROC was used to compare the prediction accuracy of each predictor.5 The predictor has a valid predictive value only when the AUC is greater than 0.5, the predictor has a fair or good predictive performance when AUC lies between 0.6 and 0.7 or is greater than 0.8, respectively.5 SPSS 25.0 (IBM Statistics, Chicago, IL, USA) software was used for statistical analysis of the data. P < 0.05 was considered statistically significant.
Results
General Clinicopathological Data Characteristics of Patients
From 2013 to 2020, a total of 1384 patients received standard surgical treatment at the First Affiliated Hospital of Chongqing Medical University, 651 patients with “apparent” early-stage EC were finally included in this study according to the inclusion and exclusion criteria (Figure 1). The basic characteristics of patients are summarized in Table 1. The median age of patients was 52 (range 24–81) years. Four hundred and seventy-eight (73.4%) patients only underwent pelvic lymph node dissection, while 173 (26.6%) patients underwent pelvic and para-aortic lymph node dissection. The median number of removed lymph nodes was 32 (10–93). A total of 83 (12.7%) patients had LNM, of which 13 (2.0%) patients also had abdominal para-aortic LNM, and no solitary abdominal para-aortic LNM was observed. The immunohistochemical staining results of ER, PR, P53 and Ki67 were shown in Supplementary Figures 1–4. The expression distribution of ER, PR, P53 and Ki67 could be seen in Table 1. The distribution range of the value of immunohistochemical parameter Ki67 was 0–90% (median 30%) (Table 1).
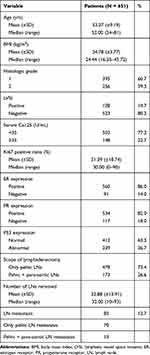 |
Table 1 Baseline Characteristics of the Patients |
 |
Figure 1 Flow chart for patient inclusion. |
Univariate and Multivariate Analysis of LNM
Univariate analysis showed age (P=0036), histologic grade (P=0.046), lymphatic vessel space invasion (LVSI) (P < 0.001), serological index Ca125 (P < 0.001), immunohistochemical parameter Ki67 (P < 0.001), ER (P < 0.001), PR (P < 0.001) and P53 (P=0.005) were associated with LNM. These factors were further included in multivariate analysis, while the BMI (P=0.743) was not included in multivariate analysis due to P > 0.05 in univariate analysis. Further multivariate analysis found that histologic grade (P=0.023), lymphatic vessel space invasion (LVSI) (P < 0.001), serological index Ca125 (P=0.002), Ki67 (P < 0.001), ER (P < 0.001) and P53 (P=0.001) were independent prognostic factors for LNM (Table 2).
 |
Table 2 Univariate and Multivariate Analysis of Predictive Factors for Lymph Node Metastases |
The Optimal Positive Threshold of Ki67 Associated with LNM
Univariate and multivariate analysis confirmed that Ki67 was an independent prognostic factor for LNM in early-stage EC. Furthermore, ROC curve and Youden index revealed that the optimal positive threshold of Ki67 for predicting LNM was 40% (AUC = 0.767; sensitivity = 72.3%; specificity = 70.4%) (Figure 2).
Comparison of Clinicopathological Parameters and Survival Analysis Between High- and Low-Ki67 Groups
According to the optimal positive threshold (40%) of Ki67, patients with Ki67 index ≥40% and <40% were defined as high-Ki67 group and low-Ki67 group, respectively. Comparison between the two groups showed that high-Ki67 expression was significantly associated with age ≥60 (P=0031), lymphatic vessel space invasion (LVSI) (P < 0.001), serological index Ca125 ≥35 (U/mL) (P=0.029), ER “negative” expression (P < 0.001) and PR “negative” expression (P < 0.001) (Table 3).
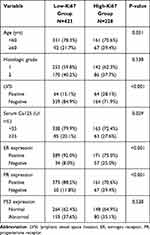 |
Table 3 Comparison of Clinicopathological Parameters Between Low-Ki67 Group and High-Ki67 Group |
To explore the relationship between Ki67 and prognosis of patients, we collected prognostic data of patients (454 patients in total) with follow-up more than two years, the median follow-up time was 48 (range 8–79) months. There were 156 patients in high-Ki67 group, of which 32 (20.5%) patients relapsed and 16 (10.3%) patients died; while there were 298 patients in low-Ki67 group, of which 16 (5.4%) patients relapsed and 12 (4.0%) patients died. Kaplan-Meier survival analysis showed that the 3-year recurrence-free survival rates of patients in high- and low-Ki67 groups were 80.5% (95% CI, 74.0–87.0%) and 94.4% (95% CI, 91.7–97.1%), respectively (P < 0.001, Figure 3A); the 3-year overall survival rates were 91.0% (95% CI, 86.3–95.7%) and 96.1% (95% CI, 93.7–98.5%), respectively (P=0.006, Figure 3B).
The Clinical Prognostic Value of the Optimal Positive Threshold of Ki67
To further illustrate the clinical value of Ki67 for predicting LNM in early-stage EC, ROC curve and AUC were used to compare the predictive performance of various predictive markers and their combinations. From Figure 4 and Table 4, the following key results can be drawn: 1. The AUC of Ki67 (AUC=0.714) was greater than other single predictors, including pathological parameters histologic grade (AUC=0.558), LVSI (AUC=0.691), serological index Ca125 (AUC=0.625), immunohistochemical markers ER (AUC=0.682), P53 (AUC=0.580); 2. Ki67 combined with classical pathological parameters (histologic grade and LVSI) and other molecular indicators (Ca125, ER and P53) can improve predictive performance, of which the AUC was the largest (AUC=0.868); 3. Even if Ki67 only combined with molecular indicators (Ca125, ER and P53), the AUC at this status can still reach 0.847, which was close to the AUC of the above optimal combination (0.868).
 |
Table 4 The Discriminatory Power (AUC) of Each Predictors and Combination to Predict LNM |
 |
Figure 4 The ROC curve of various predictive markers and their combinations for predicting LNM. |
Discussion
LNM is an important prognostic indicator of EC. Patients with LNM in EC are defined as at least stage IIIC (or stage IV if accompanied by distant metastasis) according to FIGO staging (2009 guidelines).15 Related literature5 reported that the 5-year disease-free survival rate of FIGO stage I–II patients without lymph node metastasis was 90%, that of patients with pelvic LNM was 75%, and patients with para-aortic LNM was 38%. Similarly, the overall recurrence rate for patients with LNM was 48% while for patients without LNM was 8%. Therefore, it is particularly important to accurately predict LNM and take timely intervention measures (including systemic lymph node dissection and postoperative adjuvant radiotherapy or/and chemotherapy) during the perioperative and postoperative period. At present, the prediction of LNM in EC mainly depends on the classic clinicopathological parameters. For example, the latest European guidelines3 and NCCN guidelines16 recommend systemic lymph node dissection should be performed for patients with the following high-risk factors: non-endometrioid histological subtypes (especially for clear cell carcinoma, serous carcinoma and carcinosarcoma), myometrial invasion depth of 50% or deeper, high-grade (grade 3) endometrioid carcinoma. According to the above classification criteria, most patients who may need lymph node dissection can be correctly distinguished, but as mentioned in introduction, about 10% of patients who are identified as early low-risk patients for LNM still have not received adequate treatment.
In this study, patients with “apparent” early-stage EC were served as study subjects. Univariate and multivariate analysis found that histologic grade, LVSI, serological index Ca125, immunohistochemical parameters Ki67, ER and P53 were significantly associated with LNM. Unlike the other two immunohistochemical parameters (ER and P53), which have a clear definition of two-category expression, there is no accepted positive threshold for Ki67 in endometrial cancer. Therefore, we determined the optimal positive threshold (40%) of Ki67 for predicting LNM. Based on this threshold, we also found that the postoperative recurrence-free survival rate and overall survival rate of patients in high-Ki67 group were much lower than those in low-Ki67 group (P < 0.001 and P=0.006, respectively, Figure 3). It indicated that we could try to take 40% as the threshold to perform binary classification of Ki67, which could be used as an important auxiliary reference for predicting LNM in early-stage EC. Further analysis found that the predictive performance of Ki67 (AUC=0.714) was better than other single predictive indicators (Figure 4 and Table 4), including classic pathological parameters (histologic grade and LVSI), which indicated that for the prediction of LNM in early-stage EC, the classic clinicopathological parameters were important but not the only thing that mattered, other molecular predictors (such as Ki67) should be considered to improve the accuracy of the prediction as much as possible.
Although Ki67 as a single predictor had a relatively good predictive performance, compared with taking Ki67 alone to predict LNM, we preferred to recommend that Ki67 combined with other prognostic markers should be used for predicting LNM to guide clinical work. For example, Ki67 combined with other molecular indicators (Ca125, ER and P53) can greatly optimize predictive performance (AUC=0.847), and even adding pathological parameters (histologic grade and LVSI) on this basis can greatly optimize predictive performance (AUC=0.868) (Figure 4 and Table 4). However, the accurate assessment of classical pathological parameters often requires postoperative pathological examination, especially for LVSI, which can only be determined precisely after a full pathological review of a hysterectomy specimen.25 Therefore, we propose to implement “segment prediction” (preoperative prediction and postoperative prediction) for LNM. That is, before surgery, Ki67 combined with other molecular indicators (Ca125, ER and P53) could be used (preoperative immunohistochemical results can be obtained from preoperative curettage specimens) to roughly predict LNM of patients. If the above-mentioned molecular indicators of a patient indicate a great risk of LNM, then the patient should undergo sentinel lymph node biopsy (appropriately increase the number of lymph nodes for biopsy and expand the scope of biopsy) to confirm whether the patient has LNM, or even systemic lymph node dissection should be applied if necessary. After surgery, when there is a complete and reliable postoperative pathological examination, pathological parameters (histologic grade and LVSI) should be added to the above molecular indicators (Ca125, Ki67, ER and P53) to predict LNM. If the postoperative predictors (histologic grade, LVSI, Ca125, Ki67, ER and P53) of a patient suggest that the risk of LNM is high, appropriate adjuvant therapy (radiotherapy and chemotherapy) might should be considered for patients, or even a second operation may be required to supplement the lymph node dissection if necessary (short-term postoperative imaging or other auxiliary examinations with high suspicion of LNM). Of course, there is still a lack of models or risk stratification systems that comprehensively use various predictive indicators, and our study may be the preliminary basis for such research.
The study also has certain limitations. First of all, the results of this study were derived from postoperative pathological specimens. Some studies25,26 have shown that postoperative pathological results are often more adequate and reliable than preoperative biopsy results. However, predicting LNM before surgery by using preoperative biopsy specimens was still encouraged and recommended.18 Of course, the consistency of the diagnosis of preoperative biopsy specimens and postoperative pathological specimens still needs to be evaluated by prospective studies.18 Secondly, there is no unified standard procedure for the interpretation of immunohistochemical parameter Ki67. The “hot spot” method of assessment was used in this study, which was also applied in most similar studies,27,28 but it is still necessary to establish an international evaluation standard to unify the interpretation of immunohistochemistry results. Finally, the study is a single-center retrospective study and needs to be verified by a multi-center prospective experiment.
In summary, it is important and necessary to perform immunohistochemical analysis of certain classic molecules (such as Ki67, ER, PR and P53) on postoperative specimens of patients with EC. The results of immunohistochemistry can be used as a supplement to clinicopathological parameters, helping clinicians and pathologists to better interpret postoperative tumor specimens, and even serve as an important reference index for postoperative adjuvant treatment.13 Especially in the early stages of tumors, the known clinicopathological parameters have limited predictive performance, but combined with immunohistochemical analysis can carry out more accurate risk stratification of early patients.29 In this study, we analyzed the relevant factors of LNM in patients with “apparent” early-stage EC and determined the optimal positive threshold (40%) of Ki67 for predicting LNM. On this basis, we can make a preliminary evaluation of the risk of LNM of patients before and after surgery to formulate more complete surgical plans and prognostic management measures.
Abbreviations
EC, endometrial cancer; LNM, lymph node metastasis; ER, estrogen receptor; PR, progesterone receptor; LVSI, lymphatic vessel space invasion; FIGO, International Federation of Gynecology and Obstetrics; ROC, Receiver Operating Characteristic; AUC, area under the curve; BMI, body mass index.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
Ethics Committee of Chongqing Medical University approved this study (Ethics approval number: 2020-166). All patients provided their informed consent before starting the treatment and gave consent to have their data published. As it was a retrospective clinical study, all the patients were contacted by telephone to obtain verbal informed consent and it was approved by the ethics committee. All data about the patients was anonymized or maintained with confidentiality.
Funding
There is no funding to report.
Disclosure
The authors declared no conflicts of interest in this study.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30. doi:10.3322/caac.21590
2. Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi:10.3322/caac.21338
3. Colombo N, Creutzberg C, Amant F, et al.; Group E-E-EECCW. ESMO-ESGO-ESTRO Consensus. Conference on endometrial cancer: diagnosis, treatment and follow-up. Ann Oncol. 27;2016:16–41. doi:10.1093/annonc/mdv484
4. Salani R, Nagel CI, Drennen E, Bristow RE. Recurrence patterns and surveillance for patients with early stage endometrial cancer. Gynecol Oncol. 2011;123:205–207. doi:10.1016/j.ygyno.2011.07.014
5. Bendifallah S, Canlorbe G, Laas E, et al. A predictive model using histopathologic characteristics of early-stage type 1 endometrial cancer to identify patients at high risk for lymph node metastasis. Ann Surg Oncol. 2015;22:4224–4232. doi:10.1245/s10434-015-4548-6
6. Konno Y, Todo Y, Minobe S, et al. A retrospective analysis of postoperative complications with or without para-aortic lymphadenectomy in endometrial cancer. Int J Gynecol Cancer. 2011;21:385–390. doi:10.1097/IGC.0b013e3182094e09
7. Ballester M, Canlorbe G, Cortez A, et al. Histological and immunohistochemical profiles predict lymph node status in women with low-intermediate risk endometrial cancer. Gynecol Oncol. 2013;130(3):457–462. doi:10.1016/j.ygyno.2013.06.001
8. Kang S. Comparing prediction models for lymph node metastasis risk in endometrial cancer: the winner may not take it all. J Gynecol Oncol. 2017;28(6):e92. doi:10.3802/jgo.2017.28.e92
9. Milam MR, Java J, Walker JL, Metzinger DS, Parker LP, Coleman RL. Nodal metastasis risk in endometrioid endometrial cancer. Obstet Gynecol. 2012;119(2, Part 1):286. doi:10.1097/AOG.0b013e318240de51
10. Capozzi VA, Sozzi G, Uccella S, et al. Novel preoperative predictive score to evaluate lymphovascular space involvement in endometrial cancer: an aid to the sentinel lymph node algorithm. Int J Gynecol Cancer. 2020;30(6):806–812. doi:10.1136/ijgc-2019-001016
11. Boyraz G, Atalay FO, Salman MC, et al. Comparison of Mayo and Milwaukee risk stratification models for predicting lymph node metastasis in endometrial cancer. Int J Gynecol Cancer. 2018;28:869–874. doi:10.1097/IGC.0000000000001261
12. Penault-Llorca F, Radosevic-Robin N. Ki67 assessment in breast cancer: an update. Pathology. 2017;49:166–171. doi:10.1016/j.pathol.2016.11.006
13. Ohara M, Matsuura K, Akimoto E, et al. Prognostic value of Ki67 and p53 in patients with estrogen receptor-positive and human epidermal growth factor receptor 2-negative breast cancer: validation of the cut-off value of the Ki67 labeling index as a predictive factor. Mol Clin Oncol. 2016;4:648–654. doi:10.3892/mco.2016.776
14. Jiang P, Jia M, Hu J, et al. Prognostic value of Ki67 in patients with stage 1–2 endometrial cancer: validation of the cut-off value of Ki67 as a predictive factor. Onco Targets Ther. 2020;13:10841–10850. doi:10.2147/OTT.S274420
15. Pecorelli S. Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int J Gynaecol Obstet. 2009;105(2):103–104. doi:10.1016/j.ijgo.2009.02.012
16. NCCN. NCCN Guidelines Version 2.2019 Endometrial Carcinoma. NCCN Clinical Practice Guidelines in Oncology; Version 2.2019: NCCN Guidelines for Patients®; 2018. Available from: www.nccn.org.
17. AlHilli MM, Podratz KC, Dowdy SC, et al. Preoperative biopsy and intraoperative tumor diameter predict lymph node dissemination in endometrial cancer. Gynecol Oncol. 2013;128(2):294–299. doi:10.1016/j.ygyno.2012.10.009
18. Yang B, Shan B, Xue X, et al. Predicting lymph node metastasis in endometrial cancer using serum CA125 combined with immunohistochemical markers PR and Ki67, and a comparison with other prediction models. PLoS One. 2016;11(5):e0155145. doi:10.1371/journal.pone.0155145
19. Yu X, Guo S, Song W, et al. Estrogen receptor alpha (ERalpha) status evaluation using RNAscope in situ hybridization: a reliable and complementary method for IHC in breast cancer tissues. Hum Pathol. 2017;61:121–129. doi:10.1016/j.humpath.2016.12.005
20. Smith D, Stewart CJR, Clarke EM, et al. ER and PR expression and survival after endometrial cancer. Gynecol Oncol. 2018;148:258–266. doi:10.1016/j.ygyno.2017.11.027
21. Ferrandina G, Ranelletti FO, Gallotta V, et al. Expression of cyclooxygenase-2 (COX-2), receptors for estrogen (ER), and progesterone (PR), p53, ki67, and neu protein in endometrial cancer. Gynecol Oncol. 2005;98(3):383–389. doi:10.1016/j.ygyno.2005.04.024
22. van der Putten LJM, Visser NCM, van de Vijver K, et al. Added value of estrogen receptor, progesterone receptor, and L1 cell adhesion molecule expression to histology-based endometrial carcinoma recurrence prediction models: an ENITEC collaboration study. Int J Gynecol Cancer. 2018;28:514–523. doi:10.1097/IGC.0000000000001187
23. Kobel M, Ronnett BM, Singh N, Soslow RA, Gilks CB, McCluggage WG. Interpretation of P53 Immunohistochemistry in endometrial carcinomas: toward increased reproducibility. Int J Gynecol Pathol. 2019;38(Suppl 1):S123–S131. doi:10.1097/PGP.0000000000000488
24. Schisterman EF, Perkins NJ, Liu A, Bondell H. Optimal cut-point and its corresponding youden index to discriminate individuals using pooled blood samples. Epidemiology. 2005;16:73–81. doi:10.1097/01.ede.0000147512.81966.ba
25. Anton C, Baracat EC, Dogan NU, Köhler C, Carvalho JP, Di Favero GM. A novel model to estimate lymph node metastasis in endometrial cancer patients. Clinics. 2017;72:30–35. doi:10.6061/clinics/2017(01)06
26. Koskas M, Fournier M, Vanderstraeten A, et al. Evaluation of models to predict lymph node metastasis in endometrial cancer: a multicentre study. Eur J Cancer. 2016;61:52–60. doi:10.1016/j.ejca.2016.03.079
27. Dowsett M, Nielsen TO, A’Hern R, et al.; International Ki-67 in Breast Cancer Working G. Assessment of Ki67 in breast cancer: recommendations from the International Ki67 in Breast Cancer working group. J Natl Cancer Inst. 2011;103(22):1656–1664. doi:10.1093/jnci/djr393
28. Honma N, Horii R, Iwase T, et al. Ki-67 evaluation at the hottest spot predicts clinical outcome of patients with hormone receptor-positive/HER2-negative breast cancer treated with adjuvant tamoxifen monotherapy. Breast Cancer. 2015;22:71–78. doi:10.1007/s12282-013-0455-5
29. Jiang P, Jia M, Hu J, Huang Z, Deng Y, Hu Z. A nomogram model involving immunohistochemical markers for predicting the recurrence of stage I-II endometrial cancer. Front Oncol. 2020;10:586081. doi:10.3389/fonc.2020.586081
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


