Back to Journals » Cancer Management and Research » Volume 15
Analysis of Baseline Serum Lipid Profile for Predicting Clinical Outcomes of Patients with Extensive-Stage Small Cell Lung Cancer
Received 24 April 2023
Accepted for publication 18 July 2023
Published 27 July 2023 Volume 2023:15 Pages 773—783
DOI https://doi.org/10.2147/CMAR.S418487
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Bilikere Dwarakanath
Mingshuang Wu,1 Yi He,1 Chenxi Pan2
1Department of Urology, The Second Affiliated Hospital of Dalian Medical University, Dalian, Liaoning, People’s Republic of China; 2Department of Breast Surgery, The Second Affiliated Hospital of Dalian Medical University, Dalian, Liaoning, People’s Republic of China
Correspondence: Yi He; Chenxi Pan, The Second Affiliated Hospital of Dalian Medical University, 467, Zhongshan Road, Shahekou District, Dalian, 116044, People’s Republic of China, Tel/Fax +86 411 84671291, Email [email protected]; [email protected]
Purpose: Serum lipids were reported to be the prognostic factors of various cancers, but their prognostic value in small cell lung cancer (SCLC) patients remains unclear. This study investigated the relationship between lipid profiles and clinical outcomes in extensive-stage (ES) SCLC by establishing a predictive risk classification model.
Patients and Methods: We retrospectively analyzed the prognostic values of pretreatment serum lipids and their derivatives in patients with a confirmed diagnosis ES-SCLC. Independent factors of progression-free survival (PFS) were determined by univariate and multivariate cox analysis. Then, prognostic nomograms were established, of which predictive performance was evaluated by concordance index (C-index), calibration curves, receiver operating characteristic (ROC) curves, and decision curve analyses (DCA).
Results: A total of 158 patients was included in this study. Four optimal PFS-related factors, total cholesterol (TC) ≥ 5.30, high-density lipoprotein cholesterol (HDL-C) > 1.30, triglycerides (TG)/HDL-C > 2.18, and ki67 expression > 70%, were included to construct the predictive nomogram. The C-indexes in training and validation sets were 0.758 and 0.792, respectively. ROC curves, calibration plots, and DCA all suggested favorable discrimination and predictive ability. Besides, the nomogram also performed better predictive ability than ki67 expression. Nomogram-related risk score divided the patients into two groups with significant progression disparities.
Conclusion: The promising prognostic nomogram based on lipid parameters could help clinicians to conveniently and accurately evaluate the prognosis of ES-SCLC patients and identify high-risk groups, so as to formulate individualized therapeutic regimens and follow-up strategies in time.
Keywords: small cell lung cancer, serum lipid, ki67, chemotherapy, nomogram
Introduction
The most lethal illness in the world continues to be lung cancer. Small cell lung cancer (SCLC), which makes up 15% of all cases of lung cancer and is categorized as a high-grade neuroendocrine tumor, is a particularly fatal and aggressive kind of the disease that spreads quickly, metastasizes early, and rapidly develops therapeutic resistance.1 Approximately two-thirds of all diagnoses of SCLC fall within the extensive-stage (ES) SCLC classification.2
Standard-of-care platinum chemotherapy (carboplatin or cisplatin) combined with the topoisomerase II inhibitor etoposide is the first-line treatment for extensive-stage (ES) SCLC.3 The overall 5-year survival rate has remained between 1% and 5% for decades4 despite the fact that chemotherapy might produce a response in 60% to 80% of ES-SCLC patients, complete remission was only seen in 15% to 20% of patients, and the majority of patients relapsed shortly after starting treatment. On the other hand, SCLC is divided into four subgroups, each of which responds differently to platinum-based chemotherapy.5 The proportion of each subtype in the tumor determines its overall sensitivity to platinum-based treatment. However, the bulk of SCLC tissue test samples is acquired via needle biopsy, which unavoidably leads in test variance and predictability. The multidisciplinary treatment of cancer patients is essential for better results. Finding novel tumor indicators to better select individuals who can use already available treatments (such combining immunotherapy) would considerably aid clinical decision-making due to the disease’s dismal prognosis and propensity for recurrence.
Lipids are crucial elements of cell membranes and have been linked to the fundamental mechanisms underlying the development of cancer, such as excessive proliferation and abnormal signaling. In addition, cellular energy supply, signaling, and other critical elements of tumor cell proliferation are all influenced by lipid metabolism. Dyslipidemia is frequently noted in individuals with a range of tumor forms because of the accelerated metabolism and proliferation of tumor cells.6 Recent researches have shown that the serum lipid profile is useful in predicting the prognosis of tumors,7,8 and as a result, it has been viewed as a prospective therapeutic target.9 There has not been a definite study on the relationship between cholesterol markers and prognosis in SCLC patients, nevertheless.
In this work, we looked and examined whether lipid profiles can be used as prognostic indicators for ES-SCLC. In addition to developing a lipid profile-based model that was proven to have increased prognostic precision, we retrospectively analyzed the lipid characteristics of patients and investigated their relevance in predicting disease prognosis when combined with current clinical indicators.
Materials and Methods
Patients
The patients were retrospectively identified from our patient database after the review and approval of the ethics committee of the second hospital of Dalian medical university complying with the Declaration of Helsinki. Written informed consent was obtained from all participants. The inclusion criteria were as follows: (1) Patients with SCLC newly confirmed by pathology from February 2017 to September 2022; (2) Patients with baseline data, such as serum lipid, ki67 expression and TNM stage; (3) patients who received first-line treatment with platinum-containing drugs as primary and only treatment before advancement: 4–6 cycles of platinum-based chemotherapy was routinely performed (Chemorefractory SCLC was classified as patients develop resistance to platinum-based chemotherapy within 3 months10); (4) Patients with adequate imaging evidence that could be used to determine the SCLC stage before treatment (TNM stages in this study were assigned according to the 8th American Joint Committee on Cancer); and (5) Patients with complete follow-up data. Exclusion criteria were as follows: (1) Patients with composite SCLC, which contained the components of other pathological types; (2) Patients ever received lower lipid therapy; (3) Patients receiving dose reduction or drug withdrawal due to side effects of chemotherapy; and (3) Patients with other blood systems and solid tumors.
Clinical Information Collection
All data were obtained from the electronic medical record system of the second Hospital of Dalian Medical University. Patients’ clinical information included gender, age at diagnosis, treatment characteristics and progression-free survival (PFS), which is identified from the date of therapy to the date of relapse which was confirmed by imaging evidence. Tumor condition included TNM stage according to the 8th edition of the American Joint Committee on Cancer TNM staging system, histological subtype and ki67 expression. Laboratory indicators included serum lipid indexes, including total cholesterol (TC), triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), apolipoprotein A1 (apoA1), apolipoprotein B (apoB), and their four derivatives, HDL/LDL, TG/HDL, apoB/apoA1 and HDL/TC.
Establishment and Validation of a Predicting Nomogram for ES-SCLC Relapse
In this study, 158 patients were randomly assigned to training set of 112 samples (~7/10), and internal validation set of 46 samples (~3/10). The R package “survival” was used to integrate data on PFS and the clinicopathological characteristics. The univariate and multivariate Cox regression analysis was used to determine the prognostic significance of these characteristics in the training set. On the basis of multivariate Cox proportional hazards analysis, nomographs predicting rates of disease relapse at 3- (Chemorefractory), 6- and 9-months were created using the “RMS” software. The nomogram presents graphical data for these factors, and from the points linked with each risk factor, the prognosis risk of an individual patient may be computed. During the validation of the nomogram, the total points of each patient in the validation cohort were calculated according to the established nomogram. The concordance index (C-index) and the receiver operating characteristic (ROC) curve were used to evaluate the discriminating ability of the nomogram. The calibration plots and decision curve analysis (DCA) were used to assess the predictive power and clinical utility of the nomogram.
Survival Analysis of Patients with High-Risk and Low-Risk Score Based on Nomogram
The best risk score cut-off value was determined for the entire cohort using the R software program maxstat (Maximally selected rank statistics with multiple p-value approximations, version: 0.7–25). The minimum and maximum numbers of samples in each category were set at more than 25% and less than 75%, respectively. Based on available information, patients were divided into high-risk and low-risk groups. The prognosis difference between the two groups was examined using the Survfit function of the R software package, and the significance of the prognostic difference between the several groups of samples was assessed using the Log rank test.
Statistical Analysis
The ideal cutoff values for age, lipid index and their derivatives were determined using X-tile software v3.6.1 (Yale University) and all statistical analyses were performed using SPSS v24.0 (SPSS Inc., USA). Frequencies and proportions are given for categorical variables which were analyzed using chi-square test or Mann–Whitney U-test, as appropriate. The corresponding hazard ratios (HRs) and 95% confidence intervals were determined using multivariate Cox regression analysis (CIs). For DCA, the “ggDCA” R package was utilized. ROC analysis was performed using the R software package pROC to determine the area under the curve (AUC) (version 1.17.0.1). In particular, we gathered the patients’ follow-up duration and risk score and performed ROC analysis using the ROC function of pROC at 3, 6 and 9 months. For all analyses, a P value of < 0.05 was considered statistically significant.
Results
Baseline Clinicopathological Characteristics
We performed a retrospective analysis of the clinical data of 269 ES-SCLC patients. A total of 158 patients who fulfilled the criteria for lipid and survival information was enrolled and separated into the training (n = 112) and validating (n = 46) cohorts. Overall, the median age at diagnosis was 66 (40, 83) years. One hundred and twenty-five patients (79.1%) were male and 33 (20.9%) were female. There were 9 (5.7%), 57 (36.1%), 46 (29.1%) and 46 (29.1%) patients with T stages I, II, III and IIII. Four (2.6%), 5 (3.1%), 64 (40.5%) and 85 (53.8%) patients were diagnosed with N0, N1, N2 and N3. Thirty-two (20.3%), 54 (34.2%), 36 (22.8%) and 36 (22.8%) patients were diagnosed as M0, M1a, M1b and M1c stage, respectively. The median PFS was 6 months, ranged from 3 to 18 months. We divided normal distribution continuous variables, including the ki67 expression, lipid index and their derivatives, into categorical variables based on the optimum cut-off values. Detailed characteristics in the cohorts are summarized in Table 1.
 |
Table 1 Representativeness of Study Participants |
Nomogram Construction for Individualized Prediction of PFS in ES-SCLC Patients
By univariate analysis in the training cohort, ki67 expression and all lipid index were associated with the PFS (Table 2). With further selection by multivariate analysis, TC, HDL-C, TG/HDL and ki67 expression were determined as independent prognostic factors of PFS (Table 2). Then the nomogram combined with the TC, HDL-C, TG/HDL and ki67 expression was constructed based on the multivariate Cox regression analysis (Figure 1). The C-index was 0.758 (95% CI, 0.700–0.816) in the training cohort. The calibration curve adjusted by 1000 times bootstrap resampling also indicated that the prediction probability of the nomograms for 3- (Chemorefractory), 6- and 9-month PFS were consistent with the actual observation (Figure 2A). The predicted area under the curve (AUC) values for 3- (Chemorefractory), 6- and 9-months PFS in the training cohort utilizing the nomograms were 0.749, 0.841 and 0.905 (Figure 2B), respectively, all of which were superior to the AUC values predicted by the ki67 expression. Finally, we draw decision curves to illustrate the clinical applicability of the nomograms. The decision curves showed that the clinical effectiveness of the nomograms was better than that of ki67 expression within the actual threshold probability range (Figure 2C).
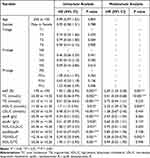 |
Table 2 Univariate and Multivariate Analyses of Factors Associated with ES-SCLC Relapse |
Validation of Predictive Accuracy of the Nomogram
In the validation cohort, the C-index of the nomogram for predicting ES-SCLC relapse was 0.792 (0.710–0.874). The calibration curve indicated good agreements between prediction and observation in the probability of 3- (Chemorefractory), 6- and 9-months PFS (Figure 3A). The AUC values for predicting 3- (Chemorefractory), 6- and 9-months PFS using the nomograms were 0.810, 0.886 and 0.991 (Figure 3B), respectively, all of which were also higher than those of ki67. DCA curve also demonstrated more positive net benefits in guiding clinical decisions than ki67 expression (Figure 3C).
Through nomogram modeling, all patients in this study were turned into a single risk score. Furthermore, the best risk score cutoff value was calculated. The minimum sample size was set at greater than 25%, while the maximum sample size was set at less than 75%. According to this information, patients were separated into high-risk and low-risk groups, with 32.6% (n = 72) in the high-risk group and 67.4% (n = 86) in the low-risk group. The K-M survival curves revealed that the PFS of patients in the low-risk group (median = 8 months) was significantly better compared with that in high-risk group (median = 3 months, P < 0.0001, Figure 4).
 |
Figure 4 Validation of predictive value of the nomogram. The progression free survival curves based on nomogram correlated risk score in the whole cohort. |
Discussion
In this study, we focused on the relationship between serum lipid and clinical outcome in ES-SCLC patients, who received platinum chemotherapy (carboplatin or cisplatin) combined with etoposide. Through multivariate cox analysis, novel nomograms were established containing TC, HDL-C, TG/HDL and ki67 expression, which could effectively predict the 3- (Chemorefractory), 6- and 9-months PFS. Compared with traditional ki67 expression, our model showed better differentiation, accuracy and clinical applicability.
According to current studies, ES-SCLC progressed quickly, on average, six months after the first treatment has been administered, and platinum-sensitive (PS) patients have a 15% to 20% better response rate to conventional second-line platinum chemotherapy than platinum-resistant (PR) patients, and their overall survival can be increased by 2–3 months.2 Platinum reactivation is recommended for PS patients as second-line treatment, while PR patients are recommended to try clinical trial medication such as topotecan or lurbinectedin because they would gain little from a platinum chemotherapy.3,4 It has been reported that DLL3-antibodies or combinations of PARPi and immunotherapy could be very promising treatment approach.11 The lack of strong prognostic and predictive biomarkers has hampered progress in therapy efficacy. This is most likely the primary reason of failure for the substances examined thus far. To increase the overall survival rate, it is crucial to predict the tumor’s response to platinum chemotherapy, so as to formulate individualized therapeutic regimens and follow-up strategies in time.
Disturbance of lipid metabolism in cancer cells can lead to structural changes of tumor cell membrane, turbulence of intracellular energy metabolism, dysregulation of cell signal transduction and gene expression.12 As the source and transforming component of lipid metabolism in tumor microenvironment, serum lipids play an important role in tumorigenesis.13 However, there has been no in-depth study of association between serum lipids and prognosis of SCLC.14 It is therefore necessary to further explore the association between dyslipidemia and the clinical characteristics of SCLC.
Using multiple regression analysis, we concluded that their lipid index, TC, HDL-C and TG/HDL-C were independent risk factors for chemoresistance and FPS in ES-SCLC patients. Cholesterol is an important component of the cell membrane and is essential for maintaining lipid raft stability which promotes cell proliferation.15 In the literature, patients of prostate cancer and cervical cancer with elevated total serum TC levels have a poor prognosis.16 However, patients of gastric cancer, and primary liver cancer with lower total serum TC levels suggest a poor prognosis.17,18 Consistently, our research found that lower serum TC levels were an independent risk factor for chemorefractory and relapse of ES-SCLC. The hypothesis was that the rapid growth of cancer cells, especially in the late stage, requires the involvement of large amounts of cholesterol, which in turn promotes total cholesterol depletion.19
HDL-C is known as a vascular scavenger, which reduces cholesterol levels in cancer cells, peripheral tissues and tumor lessens biofilm raw materials by transporting cholesterol from surrounding tissues and converting it into bile acids for excretion from the intestine, which may antagonize the function of intracellular cholesterol in cancer cells to some extent. Some studies have suggested that reduced HDL-C level is a consequential or causal factor in the development and progression of cancer.20,21
However, an inverse correlation between HDL-C and cancer risk has been shown by some epidemiological studies. Yang et al reported that dyslipidemia, including low level of HDL-C, was independently associated with the improvements of overall survival and recurrence-free survival in patients with colon.22 Our studies also found that high-level HDL-C in SCLC patients was associated with a poor prognosis. Though immunomodulatory, anti-oxidative, anti-apoptotic, and anti-inflammatory properties were postulated for HDLs, which might influence proliferative and inflammatory pathways in cancer development,23 HDL-C does not invariably reflect the functionality of HDLs. On the other hand, the controversies regarding the association between HDL-C and tumors also reside in the face of the different studied populations and sample sizes.24,25
TG/HDL-C is one of the derivatives of blood lipids. Previous studies proposed that its high level is associated with a poor postoperative prognosis of breast cancer and gastric cancer.26,27 In contrast, Huang et al demonstrated that a higher TG/HDL-C level is associated with a better prognosis of colorectal cancer.28 As far as we know, this study is the first time to propose TG/HDL-C as a meaningful prognostic marker for SCLC patients, which indicated that low level of TG/HDL-C was independently associated with the improvements of chemotherapy respondence and PFS. The contradictory assessment of the prognostic role of TG/HDL-C may be caused by different tumor types, study populations, and cut-off values. Although it remains unclear why a higher TG/HDL-C level is associated with a better prognosis of SCLC, two speculations may explain this phenomenon. Firstly, it is speculated that such association is related to the corresponding lower HDL-C concentration. Secondly, since TG is one of the prime lipid metabolites involved in energy supply,29 our findings support the hypothesis that an elevated level of TG/HDL-C may represent a better nutritional status and is related to a good prognosis of SCLC.
The ki67 is a well-known proliferation marker for the evaluation of cell proliferation. It has been reported30 that ki67 is an important predictor of SCLC prognosis, the lower expression of ki67 in SCLC is better for chemotherapy efficacy, and longer PFS. In particular, 70% ki67 expression index is an important critical index for the progression of small cell lung cancer, which is in good agreement with the findings of our analysis.
Above all, with the aid of multivariate Cox regression analysis, a novel SCLC prognostic nomogram integrating ki67 expression, TC, HDL and TG/HDL-C was established and suggested more favorable discriminative and predictive ability compared with the ki67 expression alone, which were identified by ROC curves and DCA analysis. Although newer methods like fluid biopsy and genetic testing seem to increase the accuracy of cancer prognosis predictions, they have drawbacks including a hefty price tag and labor-intensive analysis.31 The majority of them are also still through clinical studies. In clinical practice, a prostate biopsy can be used to determine the Gleason sum, and laboratory investigations can be used to calculate the HALP. Total points generated by the nomogram could therefore aid us in making informed choices regarding adjuvant therapy and follow-up plans.
After dividing the patients into groups with low- and high-risk score, the group with a high-risk score developed rapidly and had a bad prognosis. Thus, the nomogram-based risk system provides a convenient and intuitive tool to initially classify ES-SCLC patients into the different prognostic stage. We hypothesized that patients in the high-risk score category could receive more potent medication, such as topotecan or lurbinectedin coupled with platinum chemotherapy. Large-scale clinical trials are necessary to validate the specific treatment.
This research is a single-center study with a relatively small sample size, so further multi-center, large-sample studies are needed to verify the relationship between serum lipids and clinical features and prognosis of SCLC. Furthermore, individuals with several treatment approaches will be able to be enrolled and compared to determine the benefits of various treatment strategies. We will conduct further molecular research on the relationship between lipid metabolism and biological behaviors of SCLC cells and closely link them to patients' serum lipid levels and prognosis, bringing new thinking to the treatment of SCLC.
Conclusion
A novel, serum-lipid-profile-based nomogram for ES-SCLC patients was successfully constructed, providing new insight into the crosstalk among serum lipids and SCLC prognosis, which will help clinicians to conveniently and accurately evaluate the prognosis of these patients and identify high-risk groups, so as to formulate individualized therapeutic regimens and follow-up strategies in time.
Acknowledgments
This work received technical supported from the Second Affiliated Hospital of Dalian Medical University, Dalian, Liaoning, China.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Lee J-H, Saxena A, Giaccone G. Advancements in small cell lung cancer. Semin Cancer Biol. 2023;93:123–128. doi:10.1016/j.semcancer.2023.05.008
2. L-L L, C-F Y, Xie H-T, et al. Biomarkers and factors in small cell lung cancer patients treated with immune checkpoint inhibitors: a meta-analysis. Cancer Med. 2023;12(10):11211–11233. doi:10.1002/cam4.5800
3. Lin G, Yao Z, Kang K, Wang H, Luo R, Lu Y. Efficacy and toxicity profile of first-line treatment for extensive-stage small cell lung cancer: a bayesian network meta-analysis. Cancer Med. 2023;12(9):10230–10242. doi:10.1002/cam4.5750
4. Cani M, Napoli VM, Garbo E, et al. Targeted therapies in small cell lung cancer: from old failures to novel therapeutic strategies. Int J Mol Sci. 2023;24(10):8883. doi:10.3390/ijms24108883
5. Demedts IK, Vermaelen KY, van Meerbeeck JP. Treatment of extensive-stage small cell lung carcinoma: current status and future prospects. Eur Respir J. 2010;35(1):202–215. doi:10.1183/09031936.00105009
6. Cheng H, Wang M, Su J, et al. Lipid metabolism and cancer. Life. 2022;12(6):784. doi:10.3390/life12060784
7. Li Y, Shang C, Liang H, Zhang K, Wu Y, Guo H. Associations of novel serum lipid index with epithelial ovarian cancer chemoresistance and prognosis. Front Oncol. 2023;13:1052760. doi:10.3389/fonc.2023.1052760
8. Bai S, Wang H, Shao R, et al. Lipid profile as a novel prognostic predictor for patients with acute myeloid leukemia. Front Oncol. 2023;13:950732. doi:10.3389/fonc.2023.950732
9. Pope ED 3rd, Kimbrough EO, Vemireddy LP, Surapaneni PK, Copland JA 3rd, Mody K. Aberrant lipid metabolism as a therapeutic target in liver cancer. Expert Opin Ther Targets. 2019;23:473–483. doi:10.1080/14728222.2019.1615883
10. Van Meerbeeck JP, Fennell DA, De Ruysscher DK. Small-cell lung cancer. Lancet. 2011;378(9804):1741–1755. doi:10.1016/S0140-6736(11)60165-7
11. Hendriks LEL, Menis J, Reck M. Prospects of targeted and immune therapies in SCLC. Expert Rev Anticancer Ther. 2019;19(2):151–167. doi:10.1080/14737140.2019.1559057
12. Bian X, Liu R, Meng Y, Xing D, Xu D, Lu Z. Lipid metabolism and cancer. J Exp Med. 2021;218(1):e20201606. doi:10.1084/jem.20201606
13. Tang X, Brindley DN. Lipid phosphate phosphatases and cancer. Biomolecules. 2020;10(9):1263. doi:10.3390/biom10091263
14. Chen Q, Tang F, Zhang H. Significance of the expression of TC and TG levels in the initial diagnosis and treatment of SCLC patients and their tie-in with prognosis. Contrast Media Mol Imaging. 2022;5:2022.
15. Xu H, Zhou S, Tang Q, Xia H, Bi F. Cholesterol metabolism: new functions and therapeutic approaches in cancer. Biochim Biophys Acta Rev Cancer. 2020;1874(1):188394. doi:10.1016/j.bbcan.2020.188394
16. YuPeng L, YuXue Z, PengFei L, et al. Cholesterol levels in blood and the risk of prostate cancer: a meta-analysis of 14 prospective studies. Cancer Epidemiol Biomarkers Prev. 2015;24(7):1086–1093. doi:10.1158/1055-9965.EPI-14-1329
17. Na C, Wang X, Guo J, Liu P. Prognostic significance of preoperative serum triglycerides and high-density lipoproteins cholesterol in patients with non-small cell lung cancer: a retrospective study. Lipids Health Dis. 2021;20(1):69. doi:10.1186/s12944-021-01492-y
18. Wen Y, Wang G, Chen HD, et al. Total cholesterol and the risk of primary liver cancer in Chinese males: a prospective cohort study. Zhonghua Yu Fang Yi Xue Za Zhi. 2020;54(7):753–759. doi:10.3760/cma.j.cn112150-20190809-00646
19. Kitson SJ, Lindsay J, Sivalingam VN, et al. The unrecognized burden of cardiovascular risk factors in women newly diagnosed with endometrial cancer: a prospective case control study. Gynecol Oncol. 2018;148(1):154–160. doi:10.1016/j.ygyno.2017.11.019
20. Bobin-Dubigeon C, Nazih H, Blanchard V, Croyal M, Bard JM. Circulating HDL and non-HDL associated apolipoproteins and breast cancer severity. J Clin Med. 2022;11(5):1345. doi:10.3390/jcm11051345
21. Fan Y, Ding X, Wang J, et al. Decreased serum HDL at initial diagnosis correlates with worse outcomes for triple-negative breast cancer but not non-TNBCs. Int J Biol Markers. 2015;30(2):e200–e207. doi:10.5301/jbm.5000143
22. Yang Y, Mauldin PD, Ebeling M, et al. Effect of metabolic syndrome and its components on recurrence and survival in colon cancer patients. Cancer. 2013;119(8):1512–1520. doi:10.1002/cncr.27923
23. Onwuka JU, Okekunle AP, Olutola OM, et al. Lipid profile and risk of ovarian tumours: a meta-analysis. BMC Cancer. 2020;20(1):200. doi:10.1186/s12885-020-6679-9
24. Pirro M, Ricciuti B, Rader DJ, Catapano AL, Sahebkar A, Banach M. High density lipoprotein cholesterol and cancer: marker or causative? Prog Lipid Res. 2018;71:54–69. doi:10.1016/j.plipres.2018.06.001
25. Ganjali S, Banach M, Pirro M, Fras Z, Sahebkar A. HDL and cancer - causality still needs to be confirmed? Update 2020. Semin Cancer Biol. 2021;73:169–177. doi:10.1016/j.semcancer.2020.10.007
26. Dai D, Chen B, Wang B, et al. Pretreatment TG/HDL-C ratio is superior to triacylglycerol level as an independent prognostic factor for the survival of triple negative breast cancer patients. J Cancer. 2016;7(12):1747–1754. doi:10.7150/jca.15776
27. Hu D, Peng F, Lin X, et al. Prediction of three lipid derivatives for postoperative gastric cancer mortality: the Fujian prospective investigation of cancer (Fiesta) study. BMC Cancer. 2018;18(1):785. doi:10.1186/s12885-018-4596-y
28. Huang D, Zheng S, Huang F, et al. Prognostic nomograms integrating preoperative serum lipid derivative and systemic inflammatory marker of patients with non-metastatic colorectal cancer undergoing curative resection. Front Oncol. 2023;13:1100820. doi:10.3389/fonc.2023.1100820
29. Tcheng M, Roma A, Ahmed N, et al. Very long chain fatty acid metabolism is required in acute myeloid leukemia. Blood. 2021;137(25):3518–3532. doi:10.1182/blood.2020008551
30. Zhang X, Ma L, Hao Y, Zuo X, Cheng Y. P112 significance of low expression of tumor associated Gene ki67 in recurrence of small cell lung cancer. J Thorac Oncol. 2018;12(12):S1089. doi:10.1016/j.jtho.2018.10.138
31. Yao X, Wang T, Yang Sun M, Yuming Y, Guixin D, Liu J. Diagnostic value of lncRNA HOTAIR as a biomarker for detecting and staging of non-small cell lung cancer. Biomarkers. 2022;27(6):526–533. doi:10.1080/1354750X.2022.2085799
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



