Back to Journals » Journal of Pain Research » Volume 15
Analgesic Efficacy of Intravenous Dexamethasone as an Adjunct to Ultrasound-Guided Paravertebral Block with Bupivacaine in Video-Assisted Thoracoscopic Surgery
Authors Termpornlert S , Vijitpavan A, Ngodngamthaweesuk M, Sangkum L , Saeaeh L, Pipatpongsa B, Leurcharusmee P , Wanishpongpan S, Sakura S
Received 14 May 2022
Accepted for publication 29 July 2022
Published 15 August 2022 Volume 2022:15 Pages 2351—2361
DOI https://doi.org/10.2147/JPR.S372780
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Ellen M Soffin
Sivaporn Termpornlert,1 Amorn Vijitpavan,1 Montien Ngodngamthaweesuk,2 Lisa Sangkum,1 Lalisa Saeaeh,1 Benjaporn Pipatpongsa,1 Prangmalee Leurcharusmee,3 Samon Wanishpongpan,3 Shinichi Sakura4
1Department of Anesthesiology, Faculty of Medicine Ramathibodi Hospital, Mahidol University, Bangkok, Thailand; 2Department of Cardiovascular Thoracic Surgery, Faculty of Medicine Ramathibodi Hospital, Mahidol University, Bangkok, Thailand; 3Department of Anesthesiology, Faculty of Medicine Maharaj Nakorn Chiang Mai Hospital, Chiang Mai University, Chiang Mai, Thailand; 4Department of Anesthesiology, University Hospital Shimane University Faculty of Medicine, Izumo, Japan
Correspondence: Sivaporn Termpornlert; Amorn Vijitpavan, Department of Anesthesiology, Faculty of Medicine Ramathibodi Hospital, Mahidol University, 270 Rama VI Road, Phaya Thai, Ratchathewi, Bangkok, 10400, Thailand, Tel +66-2-2011513, Fax +66-2-2011569, Email [email protected]; [email protected]
Purpose: Thoracic paravertebral block (TPVB) is a recommended regional analgesia during video-assisted thoracoscopic surgery (VATS). However, single-injection TPVB does not last long enough to provide sufficient acute postoperative pain relief. Continuous TPVB through a catheter is technically challenging and often unreliable. Intravenous dexamethasone extends the analgesic duration with some peripheral nerve blocks. However, data on the effect of intravenous dexamethasone on pain relief with TPVB are limited. This study aimed to assess the analgesic efficacy of intravenous dexamethasone in patients who received TPVB for VATS.
Patients and Methods: In this multicenter prospective randomized controlled trial, we recruited patients aged between 18 and 80 years with the American Society of Anesthesiologists of physical status class 1– 3 and underwent elective VATS. Patients under general anesthesia randomly received 8 mg of intravenous dexamethasone (group D) or normal saline (group C). Ultrasound-guided TPVB (USG-TPVB) was performed at the T4–T5 and T6-T7 spaces. Multimodal analgesia was achieved via paracetamol, tramadol and intravenous morphine for both study groups. The primary outcome was time for the first analgesic requirement. Postoperative pain in terms of numeric rating score (NRS), total morphine consumption and postoperative nausea and vomiting (PONV) were assessed.
Results: After excluding one patient, 59 patients were analyzed. There were no intergroup differences in baseline characteristics. The time to first analgesic requirement was longer in group D (305 [240, 510] minutes) than in group C (270 [180, 300] minutes) (P value = 0.02). The NRS at rest and on movement was lower in group D than in group C at 12 hours but did not differ at other time points. Postoperative morphine consumption was significantly lower in group D than in group C at 6,12,24 and 48 hours. Incidences of PONV were comparable between the groups.
Conclusion: Intravenous dexamethasone, used as an adjunct to a single-injection USG-TPVB prolonged analgesic duration, had an opioid-sparing effect and provided better postoperative pain relief after VATS.
Keywords: thoracic paravertebral block, analgesic duration, postoperative pain, opioid-sparing effect
Introduction
Video-assisted thoracoscopic surgery (VATS), which is less invasive compared with thoracotomy, has been increasingly conducted. However, moderate-to-severe postoperative pain remains an unresolved issue to date.1–3 In paravertebral block (PVB), a local anesthetic is injected into the wedge-shaped paravertebral space (PVS) located lateral to where the spinal nerves emerge from the intervertebral foramina.4,5 This results in ipsilateral somatosensory and sympathetic nerve blockade. A recent guideline recommends using thoracic PVB (TPVB) as the regional analgesic of choice for VATS.6 It provides postoperative analgesia comparable to thoracic epidural analgesia with fewer adverse effects.7–11 Nevertheless, single-injection TPVB does not last long enough to cover acute postoperative pain, even with a long-acting local anesthetic. However, continuous TPVB using a catheter is technically challenging and often unreliable. Previous studies have shown that even experienced physicians often place catheter tips distant from the target position, leading to inadequate analgesia.12–14
Local anesthetics have been supplemented with various adjuvants, including clonidine, neostigmine, epinephrine, buprenorphine, dexmedetomidine, and dexamethasone, as a single injection to enhance postoperative analgesia of peripheral nerve blocks. Dexamethasone is a potent anti-inflammatory drug with a long half-life. Moreover, it is an antiemetic15 and has an opioid-sparing effect16–18 with minimal hemodynamic side-effects when administered intravenously at a dose of 0.11–0.2 mg/kg.17 When administered perineurally or intravenously, dexamethasone has been shown to increase the analgesic duration of some peripheral nerve blocks.19–25 The hypothesized mechanisms include the inhibition of nociceptive C fibers, suppression of ectopic neural discharge, peripheral or central anti-inflammatory effects, and suppression of neuropeptide immune response to injury.26 However, the use of perineural dexamethasone as an adjunct to PVB is off-label, and available data on the analgesic effect of intravenous dexamethasone with PVB are limited. This study aimed to assess the analgesic effect of 8 mg of intravenous dexamethasone in patients who received TPVB for VATS.
Materials and Methods
This multicenter prospective randomized controlled trial was registered in the Thai Clinical Trials Registry (www.thaiclinicaltrials.org) on October 10, 2019 (ID, TCTR20191010002). The study was complied with the Declaration of Helsinki and approved by the Ethics Committees of Ramathibodi Hospital, Mahidol University, Bangkok, Thailand and Faculty of Medicine, Chiang Mai University, Chiang Mai, Thailand, on November 25, 2019 (ethical-approval number, COA. MURA2019/1205). We recruited patients aged between 18 and 80 years with the American Society of Anesthesiologists physical status (ASA PS) class 1–3 and scheduled for elective VATS for lung surgery. Informed consent was obtained from all patients by a physician on the day before the operation. The exclusion criteria included a body mass index (BMI) of >35 kg/m2, pre-existing neuropathy, history of thoracic surgery, significant thoracic kyphoscoliosis, coagulopathy, allergy or contraindications to medications used in the study protocol, suspected newly diagnosed diabetes mellitus on initial screening (preoperative fasting glucose levels >126 mg/dl), uncontrolled diabetes mellitus (HbA1C levels >7%), chronic opioid use, chronic systemic corticosteroid use, active infection, and refusal to participate in the study. A central randomization using computer-generated random numbers with block sizes of 4 with a 1:1 allocation ratio was performed by a statistician from Ramathibodi Hospital. The randomized numbers were sent to anesthetic assistants from each participating center who were not involved in the study. Patients in the dexamethasone group (group D) received 8 mg of intravenous dexamethasone and those in the control group (group C) received normal saline intravenously. The patients were recruited from December 20, 2019 to March 31, 2021. The anesthetic assistants sequentially concealed allocation and prepared 8 mg of dexamethasone (1.6 mL) or normal saline (1.6 mL) in a syringe labeled as the study drug. Patients, surgeons, anesthesiologists, and outcome assessors were blinded to group allocation.
Study Protocol
In the operating room, blood pressure is continuously monitored via an arterial line. General anesthesia was induced using propofol, fentanyl, and cisatracurium. A left-sided double-lumen tube was inserted, and the proper position was confirmed via fiberoptic bronchoscopy. The study drug was administered immediately before placing the patient in the lateral position with the operative side up. Ultrasound-guided TPVB (USG-TPVB) was performed at the T4–T5 and T6–T7 PVS by an anesthesiology resident or an anesthesiologist under the supervision of an anesthesiologists with experience in TPVB (ST, AV, and PL) using a high-frequency linear transducer (12 MHz, 9L-RS Probe; GE Vivid iq, Wuxi, China, or 13–6 MHz, HFL38xp Probe; Sonosite X-Porte, Washington, United States). The target PVS was determined by counting the transverse processes in the cranial-to-caudal direction. The transducer was then placed in the transverse plane at the desired PVS (Figure 1) to obtain a transverse view of the PVS, the transverse process, the internal intercostal membrane, and the pleura (Figure 2). An echogenic needle (Pajunk; Medizintechnologie GmbH, Geisingen, Germany) was inserted in-plane in the lateral-to-medial direction. The correct position of the needle tip was confirmed by injecting 0.5–1 mL of normal saline. If an anterior displacement of the pleura and PVS widening were visible, 15 mL of 0.33% bupivacaine was slowly injected through the needle. Anesthesia was maintained using air, oxygen, and sevoflurane. The ventilation mode was switched to one-lung ventilation before the surgical procedure, and respiratory rate and tidal volume were adjusted to maintain adequate oxygenation and ventilation. Fentanyl and cisatracurium were administered as required. For the main operation port, an incision of 2–4 cm was made at the fourth or fifth intercostal space along the anterior axillary line. For the camera port, an incision of 1.5–2 cm was made at the seventh or eighth intercostal space on the anterior or middle axillary line. In some patients, an additional operation port was made as an incision of 1.5–2 cm at the fourth, fifth, or sixth intercostal space depending on the operation and the surgeon’s preference. An intrathoracic drainage tube was inserted in all patients via the camera port incision at the end of the operation.
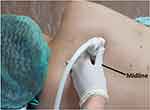 |
Figure 1 Anatomical landmark and a linear transducer position for transverse plane of paravertebral space at the level of T4-5. |
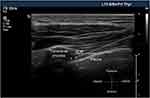 |
Figure 2 Ultrasound image of thoracic paravertebral space. The asterisk represents paravertebral space (PVS). The arrow represents needle direction. Abbreviation: IICM, internal intercostal membrane. |
During anesthesia, mean arterial pressure (MAP) was maintained between ± 20% of the baseline value throughout the operation. Hypotension was defined as a 20% decrease below the baseline MAP or a MAP of <60 mmHg. Bradycardia was defined as a heart rate of <60 beats per minute. Hypotension was managed by controlling volume status and administering 3–6 mg of ephedrine or 5–10 mcg of norepinephrine. In case of bradycardia, 0.6 mg of atropine was administered. Point-of-care (POC) glucose levels (ACCU-CHEK-Inform II, Instrumental error 5%) were checked every 2 hours after the induction and maintained between 80 and 200 mg/dl. Patients with POC glucose levels of 200–250 or >250 mg/dl received 4 or 6 units of intravenous insulin, respectively. POC glucose testing was repeated at 1 hour after insulin administration. Acetaminophen at a dose of 15 mg/kg and ondansetron at 0.15–0.2 mg/kg were intravenously injected during skin suturing. Neostigmine at 2.5 mg and glycopyrrolate at 0.4 mg were administered to reverse residual muscle relaxation before extubation.
At the postanesthetic care unit (PACU), dermatomal blockade was examined using a pinprick test bilaterally at the midclavicular line. Unsuccessful block was defined as failure when less than three levels of dermatomal blockade were verified. Multimodal analgesia was achieved via intravenous patient-controlled analgesia (PCA) with morphine as a rescue analgesia for 48 hours. The PCA setting was PCA only; PCA dose was 1 mg of morphine with a 5-minute lockout interval. Oral analgesic drugs were administered on postoperative day 1 as follows: 37.5 mg of tramadol and 325 mg of paracetamol as one tablet three times per day. One tablet of paracetamol (500 mg) was given every 6 hours as per the patient’s request. Data on postoperative nausea and vomiting (PONV) and adverse events of USG-TPVB, including hypotension, pneumothorax, hematoma, local anesthetic systemic toxicity, epidural blockade, and total spinal block, were recorded.
Measurements
The primary outcome was the time to first analgesic requirement, defined as duration from the block time until the first time the patient requested morphine PCA. The secondary outcomes were intraoperative fentanyl consumption, hypotension, postoperative pain intensity at rest and on movement as reported on the numeric rating scale (NRS) from 0 (no pain at all) to 10 (worst imaginable pain) at 6, 12, 24, 48 hours; total morphine consumption at 6, 12, 24, 48 hours; PONV; other complications; and patient satisfaction score from 0 (worst) to 10 (best).
Sample Size Calculation
This is the first study to test the effect of adding intravenous dexamethasone to enhance the analgesic effect of TPVB in VATS. Thus, we calculated the required sample size based on our pilot study using intravenous dexamethasone as an adjunct to bupivacaine for TPVB in VATS. According to the study, the time to first analgesic dose in the dexamethasone group and control group was 303 ± 42 minutes and 269 ± 47 minutes, respectively. Furthermore, 27 patients were required per group to detect a longer analgesic duration based on a type I error of 0.05 and 80% power. Expecting a 10% dropout rate, we included 30 patients in each group.
Statistical Analyses
Statistical analyses were conducted using StataCorp, version14 (College Station, Texas, United States: StataCorp LP; 2015). Numerical data were expressed as mean ± SD or median (range) as appropriate. Qualitative data were expressed as frequency and percentage. The χ2 test or Fisher's exact test was used to examine the relationship between qualitative variables. For quantitative data, a comparison between the two groups was made using the independent sample t-test or the Mann–Whitney U-test as appropriate. Repeated measures were compared using the Friedman test or analysis of variance. P value of <0.05 was considered significant.
Results
The eligibility of 66 patients was assessed, and 6 were excluded: 2 with previous thoracic surgery, 1 with chronic steroid use, 1 with chronic opioid use, 1 with active pulmonary infection, and 1 with coagulopathy. Finally, 60 patients were included who were randomly divided into two groups of 30 patients. Furthermore, one patient in the dexamethasone group was excluded because of conversion to thoracotomy; finally, 29 patients were included in group D and 30 patients in group C (Figure 3). There were no intergroup differences in terms of demographics (Table 1). The type of surgery, surgical technique, operative site, operative time, and amount of blood loss were comparable between the two groups (Table 1). Intraoperative hypotension occurred in 18% of patients in both groups (Table 2). Intraoperative fentanyl consumption was similar in both groups (Table 2). POC glucose levels at 2 hours after induction were comparable in both groups (Table 2). There was no incidence of block failure. The number of dermatomal sensory decrease by pinprick at the PACU was 3–9 dermatomes, and no significant difference was observed between the groups (Table 3). The time to first analgesic requirement was significantly longer in group D (Table 3). Postoperative NRS at rest and on movement in group D was lower than that in group C at 12 hours but not different at 6, 24, and 48 hours (Figures 4 and 5). Total morphine consumption at 6, 12, 24, and 48 hours was lower in group D than in group C (Figure 6). Incidence of PONV was comparable in both groups at 6, 12, 24, and 48 hours (Table 2). Patient satisfaction score was similar in both groups (Table 2).
 |
Table 1 Demographic and Clinical Characteristics |
 |
Table 2 Secondary Outcomes |
 |
Table 3 Analgesic Profile During the Postoperative Period |
 |
Figure 3 CONSORT flow diagram. Notes: Adapted from: Schulz KF, Altman DG, Moher D, CONSORT Group. CONSORT 2010 Statement: Updated Guidelines for Reporting Parallel Group Randomised Trials. PLoS Med. 2010;7(3):e1000251.27 Copyright: © 2010 Schulz et al. Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0/legalcode). https://journals.plos.org/plosmedicine/article?id=10.1371/journal.pmed.1000251. |
Discussion
VATS is painful, and inadequate pain control during the perioperative period leads to postoperative complications, such as pneumonia, atelectasis, or respiratory failure. TPVB is recommended as the first-choice regional anesthetic modality.6 However, single-injection TPVB does not last for a sufficient duration to cover acute postoperative pain, even with long-acting local anesthetic, and placement of a catheter into PVS is challenging. This prospective randomized control trial determined that 8 mg of intravenous dexamethasone enhanced analgesia of USG-TPVB in patients undergoing VATS, as demonstrated by the longer time from the TPVB to the first analgesic requirement in patients given dexamethasone compared with those receiving normal saline. In this study, we focused on the analgesic effect of intravenous dexamethasone with TPVB after VATS. Thus, the primary outcome was the duration from the block time to the first PCA requirement as it represents the analgesic duration of the block. However, we did not exactly assess the duration of sensorimotor blockade. This was because the assessment of motor blockade following PVB was complicated and an hourly sensory evaluation period was impractical. Consistent with our findings, Bakeer et al found that adding intravenous dexamethasone to the single-shot multilevel TPVB in breast cancer surgery improved the analgesic efficacy and prolonged the time to the first analgesic requirement.25 Given the difference in techniques used for TPVB, the dose of local anesthetic, and the surgical procedure itself, postoperative pain after these two types of surgery might differ in intensity and duration. In our study, the time to the first analgesic requirement (activation of a PCA with morphine) was longer by 35 minutes, which was statistically significant. This study also proved that intravenous dexamethasone enhanced the analgesic outcomes of TPVB in VATS by reducing postoperative morphine consumption at 6, 12, 24, and 48 hours and pain scores at rest and on movement at 12 hours. However, there were no differences in pain scores at 6, 24, and 48 hours between the study groups. This could be because the pain score was our secondary outcome and the sample size might have been underpowered to detect such differences. In addition, pain could have been controlled by intravenous PCA by the patients themselves at the time points of assessment. In our study, the ratio of male to female for group D is 1:2, while it is 1:4 for group C. There is evidence for sex differences in pain sensitivity and analgesic response. Men and women differ in their responses to pain, with increased pain sensitivity and risk for clinical pain commonly being observed among women.28,29 Manuel et al found that women are at higher risk of developing postoperative pain after thoracic and cardiac procedures.30 We think this is not going to affect the main findings because there are more females in the control group. However, if the ratio of gender is comparable, it might provide more clinical difference of analgesic prolongation between the groups.
Dexamethasone has also been administered perineurally with various peripheral nerve blocks. Mao et al31 and Saleh et al32 showed that perineural dexamethasone in TPVB prolongs the duration of analgesics and improves postoperative analgesia in thoracotomy. Previous studies have compared the analgesic duration of peripheral nerve blocks when dexamethasone was administered via various routes. Heesen et al33 and Chong et al34 suggested that perineural dexamethasone provided longer analgesic duration compared with intravenous administration of the drug. However, the meta-analysis of 14 randomized controlled trials by Zhao et al concluded that both routes of administration provided equivalent analgesia when used as adjuncts for nerve blocks in the upper and lower extremities.35 There is limited evidence comparing analgesic effects between intravenous and perineural dexamethasone as an adjunct to TPVB. In the present study, the analgesic effect of perineural dexamethasone was not included because it would be of off-label use. There is a concern regarding possible neurotoxic effects of dexamethasone when administered close to the nerves.36 During PVB, the injected solution could spread into the epidural space which could lead to an unintentional epidural steroid injection. In this study, we used only a single dose (8 mg) of intravenous dexamethasone. It is possible that different intravenous dexamethasone doses would provide different results which could be of clinical significance. Thus, we suggest that further research on the analgesic effects of intravenous dexamethasone with TPVB in VATS is warranted.
Complications of corticosteroids, such as hyperglycemia, infection, and adrenal insufficiency, depend on the dose and duration of treatment. A risk–benefit decision must be made for each individual. As for methylprednisolone, Bjerregaard et al found that high-dose preoperative intravenous methylprednisolone significantly reduced pain but increased blood glucose levels on the day of surgery in patients undergoing VATS to lobectomy under general anesthesia combined with TPVB and an intercostal catheter.37 As for dexamethasone, Murphy et al demonstrated that the peak serum glucose concentration occurred about 2 hours after the administration of dexamethasone and serum glucose levels did not significantly differ among the groups receiving dexamethasone at a dose of 4 mg and 8 mg as well as saline at any measurement time point.38 In our study, even though we excluded patients with contraindications to corticosteroid therapy and used a single intermediate dose of dexamethasone, two patients without diabetes mellitus developed intraoperative hyperglycemia after the administration of the drug. The POC glucose levels at 2 hours after receiving dexamethasone of these two patients were 225 mg/dL and 215 mg/dL. Both patients received four units of intravenous insulin after that blood glucose was well controlled. This result stresses the importance of careful use of intravenous dexamethasone to enhance analgesia in surgical patients.
Performing the block procedure under general anesthesia is not common. Zengin et al39 showed that USG-TPVB was safely performed after general anesthesia in order to prevent patient’s anxiety and ensure comfort so as this study. Various techniques of TPVB have been studied, but there is no standard.40,41 The selection of the technique remains at the discretion of the operator. In our setting, we chose the ultrasound-guided transverse approach from the transverse process view. The needle was introduced using the in-plane technique from the lateral side of the probe and punctured the internal intercostal membrane to the PVS. In the transverse process view, the target of needle tip is distal to the neuraxial structure compared to a articular process view, which the spinal nerve root emerges from the intervertebral foramina. We think that performing the PVB under ultrasound guidance by an experienced person using transverse process view provides an extra margin of safety from nerve injury, especially in anesthetized patients. The failure rates with ultrasound guidance are low (0–2.3%).42,43 Reported complication rates in ultrasound-guided techniques are also low. The incidences of vascular puncture, pneumothorax, and nerve damage were less than 1%.11 Epidural spread occurred in 2.1%.42 The incidence of vascular puncture increased with multi-injection techniques. However, there was no block failure or complication from the block in our study. This could be explained by a good needle visualization and the ability to see the spread of local anesthetic in the appropriate and intended location.
Limitations of our study are as follows. We did not study the effect of perineural dexamethasone injection because it was for off-label use. We used only a single dose (8 mg) of intravenous dexamethasone. It is possible that different doses would be associated with different enhancement effects of analgesia. We assessed patients for 48 hours after the surgery without long-term follow-up. Further research and more comprehensive studies on the optimal dose of intravenous dexamethasone, long-term follow-up, and incidence of chronic postsurgical pain are warranted.
Conclusion
Intravenous dexamethasone, used as an adjunct to single-injection 2-level USG-TPVB using bupivacaine prolonged analgesic duration and provided an opioid-sparing effect. The addition of intravenous dexamethasone is recommended for TPVB with bupivacaine along with multimodal analgesia to provide better postoperative pain relief after VATS.
Data Sharing Statement
Whether the authors intend to share individual deidentified participant data: Yes.
What specific data they intend to share: Individual participant data that underlie the results reported in this article, after deidentification (text, tables, and figures). What other study documents will be made available: study protocol. How the data will be accessible: Data access should be directed to [email protected]. When and for how long they will be made available: beginning 9 months and ending 36 months following article publication.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Kavanagh BP, Katz J, Sandler AN. Pain control after thoracic surgery. Rev Curr Tech Anesthesiol. 1994;81(3):737–759.
2. Bendixen M, Jørgensen OD, Kronborg C, Andersen C, Licht PB. Postoperative pain and quality of life after lobectomy via video-assisted thoracoscopic surgery or anterolateral thoracotomy for early stage lung cancer: a randomised controlled trial. Lancet Oncol. 2016;17(6):836–844. doi:10.1016/S1470-2045(16)00173-X
3. Holbek BL, Horsleben Petersen R, Kehlet H, Hansen HJ. Fast-track video-assisted thoracoscopic surgery: future challenges. Scand Cardiovasc J. 2016;50(2):78–82. doi:10.3109/14017431.2015.1114665
4. Francine D, Harendra A, Priya AK. Paravertebral block for thoracic surgery. J Cardiothor VASC AN. 2018;32(2):915–927. doi:10.1053/j.jvca.2017.10.003
5. Karmakar MK. Thoracic paravertebral block. Anesthesiology. 2001;95(3):771–780. doi:10.1097/00000542-200109000-00033
6. Feray S, Lubach J, Joshi GP, Bonnet F, Van de Velde M; PROSPECT Working Group of the European Society of Regional Anaesthesia and Pain Therapy. PROSPECT guidelines for video-assisted thoracoscopic surgery: a systematic review and procedure-specific postoperative pain management recommendations. Anaesthesia. 2022;77(3):311–325. doi:10.1111/anae.15609
7. Ballantyne JC, Carr DB, deFerranti S, et al. The comparative effects of postoperative analgesic therapies on pulmonary outcome: cumulative meta-analyses of randomized, controlled trials. Anesth Analg. 1998;86(3):598–612. doi:10.1213/00000539-199803000-00032
8. Kaiser AM, Zollinger A, De Lorenzi D, Largiadèr F, Weder W. Prospective, randomized comparison of extrapleural versus epidural analgesia for postthoracotomy pain. Ann Thorac Surg. 1998;66(2):367–372. doi:10.1016/S0003-4975(98)00448-2
9. Furrer M, Rechsteiner R, Eigenmann V, Signer C, Althaus U, Ris HB. Thoracotomy and thoracoscopy: postoperative pulmonary function, pain and chest wall complaints. Eur J Cardiothorac Surg. 1997;12(1):82–87. doi:10.1016/S1010-7940(97)00105-X
10. Davies RG, Myles PS, Graham JM. A comparison of the analgesic efficacy and side-effects of paravertebral vs epidural blockade for thoracotomy-a systematic review and meta-analysis of randomized trials. Br J Anaesth. 2006;96(4):418–426. doi:10.1093/bja/ael020
11. Yeung JHY, Gates S, Naidu BV, Wilson MJA, Gao SF. Paravertebral block versus thoracic epidural for patients undergoing thoracotomy. Cochrane Database Syst Rev. 2016. doi:10.1002/14651858.CD009121.pub2
12. Luyet C, Herrmann G, Ross S, et al. Ultrasound-guided thoracic paravertebral puncture and placement of catheters in human cadavers: where do catheters go? Br J Anaesth. 2011;106(2):246–254. doi:10.1093/bja/aeq309
13. Huang QW, Li JB, Huang Y, Zhang WQ, Lu ZW. A comparison of analgesia after a thoracoscopic lung cancer operation with a sustained epidural block and a sustained paravertebral block: a randomized controlled study. Adv Ther. 2020;37(9):
14. Termpornlert S, Sakura S, Aoyama Y, Wittayapairoj A, Kishimoto K, Saito Y. Distribution of injectate administered through a catheter inserted by three different approaches to ultrasound-guided thoracic paravertebral block: a prospective observational study. Reg Anesth Pain Med. 2020;45:866–871.
15. Numazaki M, Fujii Y. Reduction of postoperative emetic episodes and analgesic requirements with dexamethasone in patients scheduled for dental surgery. J Clin Anesth. 2005;17(3):182–186. doi:10.1016/j.jclinane.2004.06.018
16. Worni M, Schudel HH, Seifert E, et al. Randomized controlled trial on single dose steroid before thyroidectomy for benign disease to improve postoperative nausea, pain, and vocal function. Ann Surg. 2008;248(6):1060–1066. doi:10.1097/SLA.0b013e31818c709a
17. De Oliveira GSJ, Almeida MD, Benzon HT, McCarthy RJ. Perioperative single dose systemic dexamethasone for postoperative pain: a meta-analysis of randomized controlled trials. Anesthesiology. 2011;115(3):575–588. doi:10.1097/ALN.0b013e31822a24c2
18. Nielsen RV, Siegel H, Fomsgaard JS, et al. Preoperative dexamethasone reduces acute but not sustained pain after lumbar disk surgery: a randomized, blinded, placebo-controlled trial. Pain. 2015;156(12):2538–2544. doi:10.1097/j.pain.0000000000000326
19. Movafegh A, Razazian M, Hajimaohamadi F, Meysamie A. Dexamethasone added to lidocaine prolongs axillary brachial plexus blockade. Anesth Analg. 2006;102(1):263–267. doi:10.1213/01.ane.0000189055.06729.0a
20. Vieira PA, Pulai I, Tsao GC, Manikantan P, Keller B, Connelly NR. Dexamethasone with bupivacaine increases duration of analgesia in ultrasound-guided interscalene brachial plexus blockade. Eur J Anaesthesiol. 2010;27(3):285–288. doi:10.1097/EJA.0b013e3283350c38
21. Parrington SJ, O’Donnell D, Chan VW, et al. Dexamethasone added to mepivacaine prolongs the duration of analgesia after supraclavicular brachial plexus blockade. Reg Anesth Pain Med. 2010;35(5):422–426. doi:10.1097/AAP.0b013e3181e85eb9
22. Knezevic NN, Anantamongkol U, Candido KD. Perineural dexamethasone added to local anesthesia for brachial plexus block improves pain but delays block onset and motor blockade recovery. Pain Physician. 2015;18(1):1–14.
23. Chalifoux F, Colin F, St-Pierre P, Godin N, Brulotte V. Low dose intravenous dexamethasone (4 mg and 10 mg) significantly prolongs the analgesic duration of single-shot interscalene block after arthroscopic shoulder surgery: a prospective randomized placebo-controlled study. Can J Anaesth. 2017;64(3):280–289. doi:10.1007/s12630-016-0796-6
24. Clement JC, Besch G, Puyraveau M, et al. Clinical effectiveness of single dose of intravenous dexamethasone on the duration of ropivacaine axillary brachial plexus block: the randomized placebo-controlled ADEXA trial. Reg Anesth Pain Med. 2019;44(3):371–374. doi:10.1136/rapm-2018-100035
25. Bakeer AH, Abdallah NM, Kamel MA, Abbas DN, Ragab AS. The impact of intravenous dexamethasone on the efficacy and duration of analgesia of paravertebral block in breast cancer surgery: a randomized controlled trial. J Pain Res. 2019;12:61–67. doi:10.2147/JPR.S181788
26. Hargreaves KM, Costello A. Glucocorticoids suppress levels of immunoreactive bradykinin in inflamed tissue as evaluated by microdialysis probes. Clin Pharmacol Ther. 1990;48(2):168–178. doi:10.1038/clpt.1990.132
27. Schulz KF, Altman DG, Moher D; CONSORT Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. PLoS Med. 2010;7(3):e1000251. doi:10.1371/journal.pmed.1000251
28. Bartley EJ, Fillingim RB. Sex differences in pain: a brief review of clinical and experimental findings. BJA. 2013;111(1):52–58. doi:10.1093/bja/aet127
29. Yang MMH, Hartley RL, Leung AA, et al. Preoperative predictors of poor acute postoperative pain control: a systematic review and meta-analysis. BMJ Open. 2019;9(4):1–9. doi:10.1136/bmjopen-2019-030833
30. Pereira MP, Pogatzki-Zahn E. Gender aspects in postoperative pain. Curr Opin Anesthesiol. 2015;28:546–558. doi:10.1097/ACO.0000000000000226
31. Mao Y, Zuo Y, Mei B, et al. Efficacy of perineural dexamethasone with ropivacaine in thoracic paravertebral block for postoperative analgesia in elective thoracotomy: a randomized, double-blind, placebo-controlled trial. J Pain Res. 2018;11:1811–1819. doi:10.2147/JPR.S164225
32. Saleh AH, Hassan PF, Elayashy M, et al. Role of dexamethasone in the paravertebral block for pediatric patients undergoing aortic coarctation repair. BMC Anesthesiol. 2018;18(1):178. doi:10.1186/s12871-018-0637-y
33. Heesen M, Klimek M, Imberger G, Hoeks SE, Rossaint R, Straube S. Co-administration of dexamethasone with peripheral nerve block: intravenous vs perineural application: systematic review, meta-analysis, meta-regression and trial-sequential analysis. BJA Br J Anaesth: 212–27. 2018;120(2):212–227. doi:10.1016/j.bja.2017.11.062
34. Chong MA, Berbenetz NM, Lin C, Singh S. Perineural versus intravenous dexamethasone as an adjuvant for peripheral nerve blocks: a systematic review and meta-analysis. Reg Anesth Pain Med. 2017;42(3):319–326. doi:10.1097/AAP.0000000000000571
35. Zhao WL, Ou XF, Liu J, Zhang WS. Perineural versus intravenous dexamethasone as an adjuvant in regional anesthesia: a systematic review and meta-analysis. J Pain Res. 2017;10:1529–1543. doi:10.2147/JPR.S138212
36. Williams BA, Hough KA, Tsui BY, Ibinson JW, Gold MS, Gebhart GF. Neurotoxicity of adjuvants used in perineural anesthesia and analgesia in comparison with ropivacaine. Reg Anesth Pain Med. 2011;36(3):225–230. doi:10.1097/AAP.0b013e3182176f70
37. Bjerregaard LS, Jensen PF, Bigler DR, et al. High-dose methylprednisolone in video-assisted thoracoscopic surgery lobectomy: a randomized controlled trial. Eur J Cardiothorac Surg. 2018;53(1):209–215. doi:10.1093/ejcts/ezx248
38. Murphy GS, Szokol JW, Avram MJ, et al. The effect of single low-dose dexamethasone on blood glucose concentrations in the perioperative period: a randomized, placebo-controlled investigation in gynecologic surgical patients. Anesth Analg. 2014;118(6):1204–1212. doi:10.1213/ANE.0b013e3182a53981
39. Zengin M, Sazak H, Baldemir R, Ulger G, Alagoz A. The effect of erector spinae plane block and combined deep and superficial serratus anterior plane block on acute pain after video-assisted thoracoscopic surgery: a randomized controlled study. J Cardiothorac Vasc Anesth. 2022;13(6):e15614.
40. Pawa A, Wojcikiewicz T, Barron A, El-Boghdadly K. Paravertebral blocks: anatomical, practical, and future concepts. Curr Anesthesiol Rep. 2019;9(3):263–270. doi:10.1007/s40140-019-00328-x
41. Nair S, Gallagher H, Conlon N. Paravertebral blocks and novel alternatives. BJA Educ. 2020;20(5):158–165. doi:10.1016/j.bjae.2020.01.006
42. Terkawi AS, Tsang S, Sessler DI, et al. Improving analgesic efficacy and safety of thoracic paravertebral block for breast surgery: a mixed-effects meta-analysis. Pain Phys. 2015;18(5):E757–E780. doi:10.36076/ppj.2015/18/E757
43. Sekandarzad MW, van Zundert AAJ, Lirk PB, Doornebal CW, Hollmann MW. Perioperative anesthesia care and tumor progression. Anesth Analg. 2017;124(5):1697–1708. doi:10.1213/ANE.0000000000001652
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



