Back to Journals » Journal of Pain Research » Volume 16
Analgesic Effect of Esketamine Combined with Tramadol for Patient-Controlled Intravenous Analgesia After Cesarean Section: A Randomized Controlled Trial
Authors Guo Y , Ding X , Wang S, Wang F , Zheng Z , Zou L
Received 27 June 2023
Accepted for publication 26 September 2023
Published 20 October 2023 Volume 2023:16 Pages 3519—3528
DOI https://doi.org/10.2147/JPR.S427702
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Ellen M Soffin
Yihui Guo,1,2 Xue Ding,1,2 Sheng Wang,1,2 Fei Wang,1,2 Zhongyi Zheng,1,2 Lifeng Zou1,2
1Department of Anesthesiology, The People’s Hospital of Pizhou, Xuzhou, Jiangsu, People’s Republic of China; 2Department of Anesthesiology, Pizhou Hospital Affiliated to Xuzhou Medical University, Xuzhou, Jiangsu, People’s Republic of China
Correspondence: Lifeng Zou, Department of Anesthesiology, The People’s Hospital of Pizhou, Pizhou Hospital affiliated to Xuzhou Medical University, 93 Renmin South Road, Pizhou, Xuzhou, Jiangsu, People’s Republic of China, Tel +8615380118786, Fax +86-25-86239959, Email [email protected]
Purpose: High rate of cesarean section (CS) bring challenges to analgesic management after CS. Previous studies state that adjuvant treatment with a low dose of esketamine intraoperatively could reduce postoperative pain and opioid consumption, and even prevent postpartum depression. However, few researches involve in patient-controlled intravenous analgesia (PCIA) with esketamine after CS. In this trial, we explored a new combination of esketamine with tramadol for PCIA after CS with the aim to provide a better analgesic regimen for use in the clinic.
Patients and Methods: 170 puerperae undergoing CS were recruited for this trial and randomly assigned into 2 groups (1:1): The control group received a formula of PCIA with butorphanol 0.1mg/kg and tramadol 400mg postoperatively, while the intervention group received a formula of PCIA with esketamine 1mg/kg and tramadol 400mg. The primary outcome was the mean numerical rating scale (NRS) scores at rest, sitting, and uterine contraction at 6 hours postoperatively. The second outcomes included the mean NRS scores at rest, sitting, and uterine contraction at 12, 24, and 48 hours postoperatively. The incidence of adverse events, postoperative sedation, postoperative sleep quality, maternal satisfaction regarding postoperative analgesia and the Edinburgh postnatal depression scale (EPDS) score were also be evaluated.
Results: The mean (SD) of the mean NRS scores at rest, sitting, and during uterine contraction at 6 hours postoperatively were 4.8 (0.7) points in the intervention group and 5.3 (0.5) points in the control group. The estimated mean difference between the two groups at 6 hours postoperatively was − 0.5 points (95% confidence interval [CI], − 0.7 to − 0.3; P < 0.001). Compared with the control group, the patients in the intervention group had a significantly lower mean pain intensity at 12 and 24 hours postoperatively (− 0.5 points [95% CI, − 0.6 to − 0.3]; P < 0.001 and − 0.2 points [95% CI, − 0.4 to 0]; P = 0.019 respectively). Otherwise, differences at 48 hours after surgery between the two groups were nonsignificant (0 points [95% CI, − 0.2 to 0.2]; P = 0.802). The incidence of adverse events in the intervention group (11.8%) was significantly lower than in the control group (24.7%) (ratio difference − 12.9, [95% CI, − 24.3 to − 1.5]; P = 0.029). No difference was found in postoperative sleep quality (P = 0.765), analgesic satisfaction (P= 0.818) and EPDS scores (P = 0.154) between the two groups.
Conclusion: In this trial, among patients undergoing CS, esketamine combined with tramadol by PCIA improved pain intensity 6 hours postoperatively compared with butorphanol combined with tramadol.
Plain Language Summary: Pain management after cesarean section is a clinical issue worthy of concern. Good postoperative pain management can reduce maternal fear of surgery and promote early postoperative breastfeeding and getting out of bed. Strong opioids are widely used for postoperative analgesia, but their many intolerable side effects plague mothers and medical staff.
Esketamine, an anesthetic with powerful analgesic effects, has also been approved in recent years for treatment-resistant depression. In this current study, we used esketamine with weak opioid tramadol for patient-controlled intravenous analgesia after cesarean section. We found that, compared to traditional analgesic formulations (butorphanol combined with tramadol), esketamine combined with tramadol significantly improved early postoperative pain and reduced opioid-related adverse events. At the same time, esketamine used in patient-controlled intravenous analgesia after cesarean section did not increase central nervous system adverse reactions such as hallucinations.
Our findings provide a new strategy for post-operative analgesia after cesarean section and we believe that this strategy will be beneficial for cesarean patients.
Keywords: esketamine, postoperative pain, cesarean section, patient-controlled intravenous analgesia, postpartum depression, butorphanol
Introduction
Cesarean section (CS) is one of the most common surgical operations.1 In 2012, 23 million CSs were performed worldwide.2 High rate of CS is a concern globally and creates a significant challenge for postpartum pain management.3,4 Opioids are effective for acute postoperative pain but have numerous adverse effects such as postoperative nausea and vomiting (PONV) or respiratory depression.5,6 In recent years, since opioid-sparing pain management strategies have been advocated,7 multimodal analgesic therapies are increasingly used in clinical practice and are designed to alleviate postoperative pain and analgesic consumption.5,8
Ketamine inhibits N-methyl-D-aspartate (NMDA) receptor activation and attenuates wind-up and central sensitization associated with hyperalgesia, opioid tolerance, and chronic pain.5,9 Emerging researches show that adjuvant ketamine reduces pain and opioid consumption postoperatively.6,10–12 Esketamine is an S+ isomer of ketamine that is twice as potent as ketamine.13 It produces sedation, analgesia, and sympathetic activation with a relatively low risk of side effects to the mothers and infants,14,15 making it an excellent choice as an adjuvant for spinal anesthesia during CS.16 In addition, because of its powerful antidepressant effects, esketamine is approved for the treatment of treatment-resistant depression.17,18 Furthermore, perioperative use of esketamine has been documented to prevent postpartum depression.19,20
There are few studies on esketamine for postoperative analgesia and most studies have focused on combining low-dose esketamine as an adjunct with strong opioids, with the aim of reducing opioid consumption.20–23 In the current study, we explored a new combination of higher doses of esketamine with weak opioid tramadol for patient-controlled intravenous analgesia (PCIA) after CS. We hypothesized that it might further reduce opioid induced adverse effects and prevent postpartum depression while providing an excellent analgesic effect.
Subjects and Methods
Study Design
This single-center, prospective, randomized controlled trial was approved by the Medical Ethics Committee of Pizhou People’s Hospital of Jiangsu Province (20,220,727–01) and registered before patient enrollment at the Chinese Clinical Trial Registry (ChiCTR2200062848) on August 20, 2022. Study participants were recruited in the Department of Anesthesiology at the People’s Hospital of Pizhou from September 2022 to January 2023. The trial fulfilled the Helsinki Declarations and the reporting of this trial complied with the Consolidated Standards of Reporting Clinical Trials statement (CONSORT).24,25 All patients gave written informed consent before participating in the trial.
Inclusion and Exclusion Criteria
The following pregnant women would be included in this study: 1. Older than 18 years, 2. Had an American Society of Anesthesiologists class II- III, 3. A body mass index (BMI) between 18 to 40 kg/m2, 4. Would receive spinal anesthesia, 5. Would undergo an elective or emergency CS, and 6. Would receive PCIA.
The following pregnant women would be excluded in this study: 1. Were allergic to or addicted to any drug in this study, 2. Diagnosed with pregnancy-induced hypertension, preeclampsia, eclampsia, or prenatal depression (Edinburgh postnatal depression scale [EPDS] scores ≥10)26,27 diagnosed by a psychiatrist before enrollment and other pregnancy complications or other underlying diseases, 3. Could not accurately understand the numerical rating scale (NRS) pain and EPDS scores, and 4. Had been involved with other investigators before the start of the trial.
Randomization and Blinding
An anesthesia nurse who was not involved in this trial randomly assigned the subjects to either the control (group C) or the intervention group (group E) (1: 1) using a computer-generated random sequence via SPSS version 25.0. The maternal allocation was concealed in sealed, opaque envelopes marked with consecutive numbers. The anesthesia nurse who was not involved in this trial prepared the study medication in a postoperative analgesia pump according to the group allocation and labelled it with the study identification number. All the parturients, anesthesiologists, surgeons, nurses providing postoperative care, treatment physicians, and the investigators assessing the clinical outcomes were blinded to the group assignment. In the event of acute complications postoperatively, the anesthesiologist would unblind and manage them appropriately.
Procedures
Spinal anesthesia was administered to all parturients at the L3-L4 or L2-L3 lumbar regions using 7.5–9 mg of bupivacaine. At the end of surgery, the subjects received PCIA with different drug combinations according to the group allocation. PCIA included either butorphanol 0.1mg/kg + tramadol 400mg in group C or esketamine 1mg/kg + tramadol 400mg in group E, both were diluted to 100 mL with normal saline. All patients received continuous infusion (basal rate of 2 mL/h) and a 0.5mL on-demand bolus with a lockout interval of 15 min. The infusion started immediately after suturing the skin incision and lasted for 48 h. If the patient was in severe pain after the CS, flurbiprofen axetil 50 mg was administered at the discretion of the anesthesiologist. Postoperative nausea and vomiting (PONV) were treated using tropisetron 4 mg.
Primary Outcome
The primary outcome of this trial was the mean numerical rating scale (NRS) scores at rest, sitting, and uterine contraction at 6 hours postoperatively (NRS; 0 = no pain, 10 = pain as bad as you can imagine28). Pain is evaluated at 6, 12, 24, and 48 hours postoperatively.
Second Outcomes
The second outcomes were the mean NRS scores at rest, sitting, and uterine contraction at 12, 24, and 48 hours postoperatively, the incidence of adverse events, postoperative sedation, postoperative sleep quality, maternal satisfaction regarding postoperative analgesia and the EPDS score.
The adverse effects included PONV, constipation, urinary retention, skin pruritus, respiratory depression, and central nervous system (CNS) adverse reactions such as hallucinations, confusion and disorientation, dizziness, and diplopia. The incidence of adverse effects was recorded as dichotomous data (yes/no) using an investigators’ inquiry up to 48 hours.
Postoperative sedation was evaluated using the Richmond Agitation Sedation Scale (RASS). RASS is a 10-point scale with four levels of anxiety or agitation from −5 to +4.29 −5 devotes a state of no response to voice or physical stimulation, 0 means a calm and alert state and +4 indicates a patient who is overtly combative or violent and endangers medical staff.29 Maternal sleep quality was rated on a scale of 1–4 (1 - slept well; 2 - slept uneasy; 3 - nightmare; 4 - sleepless).5 Puerpera’s satisfaction with postoperative analgesic effect was assessed using a scale of 0–3 (0 - dissatisfied; 1 - somewhat satisfied; 2 - moderately satisfied; 3 - highly satisfied).4 The EPDS score (ranging from 0 to 30) was used to screen perinatal depression and a threshold of 10 or more is defined as perinatal depression.30 Postoperative sedation, maternal sleep quality, and puerpera’s satisfaction with postoperative analgesic effect was recorded during the investigators’ inquiry up to 48 hours. The EPDS scores were recorded on postoperative day 5 during the investigators’ bedside inquiry and on postoperative day 42 by telephone follow-up call.
Sample Size Calculation
Based on previous observations, the standard deviation of the mean NRS scores at rest, sitting, and uterine contraction at 6 hours postoperatively was 2. The authors hypothesized that esketamine could reduce the mean NRS scores by 1 point compared with butorphanol. Eighty-five patients in each group were required using a two-tailed α of 0.05 and a power of 90%. To allow a 10% dropout, 188 patients were enrolled in this trial (94 patients in each group). We used PASS 15.0 software (NCSS, LLC) to perform sample size calculation.
Statistical Analysis
All endpoints in this trial were assessed in the intention-to-treat population of patients who were randomized and no interim analyses were undertaken. We did not use the multiple imputation method to account for the missing data (The EPDS scores on postoperative day 42) because they were few (account for 8%). The Kolmogorov–Smirnov test was performed to assess the normality of the data’s distribution. For numerical data with a normal distribution (weight, duration of operation and preoperative EPDS score), we used mean (standard deviation, SD) for description and an independent sample t-test for analysis. For numerical data with skewed distribution (age, height, BMI, intraoperative blood loss, and RASS score) which were shown as median (interquartile range, IQR), we used the Mann–Whitney U-test for analysis. Categorical data (ASA classification, preoperative and postoperative sleep quality, the incidence of adverse events, level of maternal satisfaction regarding postoperative analgesia) which were presented as numbers and percentages were analyzed by χ2 test or Fisher’s exact test (at least one cell in a four-fold table has a frequency less than five). For the repeated measurement data (NRS score and EPDS score), we used the linear mixed models, which incorporated group, time and the treatment × time interaction term. Considering that the secondary outcomes in this trial are exploratory, we did not adjust for multiple comparisons while analyzing the secondary outcomes. IBM SPSS Statistics Version 25.0 was used to perform all statistical analyses. The P-value was two-sided and P < 0.05 was considered statistically significant.
Results
A total of 170 subjects were randomly assigned to this study and all participants received treatment regimens according to their randomization. Figure 1 is a flow chart that illustrates the patients’ path through the trial. Eight patients in the control group and six patients in the intervention group were lost to follow-up 42 days after surgery; therefore, their last EPDS scores were not provided.
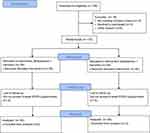 |
Figure 1 CONSORT flow diagram. |
Table 1 shows the baseline characteristics of all participants. The p-value indicated an imbalance of the duration of operation between the two groups (P = 0.045). The operation time of the control group was slightly longer than that of the intervention group.
 |
Table 1 Participants Baseline Characteristics |
After the analysis of linear mixed effects model, the differences in the NRS scores postoperatively between the intervention and the control groups were statistically significant (P < 0.001). There were also statistical differences in NRS scores at different time points after the operation (P < 0.001). The interaction effects between time and group in the NRS scores were statistically different (P < 0.001).
Primary Outcome
The mean (SD) of the mean NRS scores at rest, sitting, and uterine contraction at 6 hours postoperatively were 4.8 (0.7) points in the intervention group and 5.3 (0.5) points in the control group. The mean difference between the two groups at 6 hours postoperatively was −0.5 points (95% confidence interval [CI], −0.7 to −0.3; P < 0.001) (Table 2). There were significant differences between the two groups.
 |
Table 2 NRS Scores of the Two Groups at Different Time Points Postoperatively |
Second Outcomes
Compared with the control group, the patients in the intervention group had a significantly lower mean pain intensity at 12 and 24 hours postoperatively (−0.5 points [95% CI, −0.6 to −0.3]; P < 0.001 and −0.2 points [95% CI, −0.4 to 0]; P = 0.019 respectively). Otherwise, differences at 48 hours after surgery between the two groups were nonsignificant (0 points [95% CI, −0.2 to 0.2]; P = 0.802) (Table 2).
In terms of adverse events, the incidence of adverse events in the intervention group (11.8%) was significantly lower than in the control group (24.7%) (ratio difference −12.9, [95% CI, −24.3 to -1.5]; P = 0.029). Thirteen patients experienced postoperative nausea and vomiting in the control group. Three patients had constipation, four patients had urinary retention, one patient felt dizziness and three patients had skin pruritus in the control group. In the intervention group, five patients experienced postoperative nausea and vomiting, two patients had constipation, two patients had urinary retention, three patients felt dizziness, one patient had skin pruritus and one patient reported confusion and disorientation (Table 3).
 |
Table 3 Adverse Events, Postoperative Sleep Quality and Analgesic Satisfaction of the Two Groups |
No difference was found in postoperative sleep quality (P = 0.765) and analgesic satisfaction (P= 0.818). Most patients in this trial had good sleep quality after surgery and were highly satisfied with the analgesic effect (Table 3). All patients remained calm and alert postoperatively and all patients had a RASS score of 0.
After the analysis of linear mixed effects models, we found no difference of EPDS scores postoperatively between the intervention and the control group (P = 0.154). There were statistical differences in EPDS scores at different time points after the CS (P = 0.009). The interaction effect between time and group regarding EPDS scores was statistically different (P = 0.003). The EPDS scores of patients in the intervention group were significantly lower than those in the control group at 5 days after surgery (−0.6 points [95% CI, −1.0 to −0.2]; P = 0.004). There was no significant difference in EPDS scores at 42 days after surgery between the two groups (−0.1 points [95% CI, -0.5 to 0.3]; P = 0.704) (Table 4).
 |
Table 4 EPDS Scores of the Two Groups at Different Time Points Preoperatively or Postoperatively |
Discussion
In this current trial, we found that esketamine at 1 mg/kg combined with tramadol for PCIA in patients undergoing CS significantly reduced the NRS scores by 0.5 points at 6 hours postoperatively compared to butorphanol combined with tramadol. Although the difference of 0.5 points is low, the control group in our study is butorphanol combined with tramadol, which is widely used in China for postoperative analgesia and has a better analgesic effect and lower adverse effects compared to traditional strong opioids.31 Therefore, the lower the NRS scores obtained in the esketamine group at 6 hours postoperatively compared to butorphanol is evidence of its strong analgesic effect.
Previous studies have shown that severe pain early after CS is associated with chronic postoperative pain and postpartum depression.32 In our clinical trial, we found that there were significant differences in NRS scores between the two groups at 6, 12 and 24 hours postoperatively while no difference at 48 hours after CS was observed. These results revealed that 1mg/kg esketamine combined with tramadol in PCIA significantly reduced early postoperative pain after CS.
Furthermore, the incidence of adverse events was significantly lower in the esketamine group compared to butorphanol. Although the incidence of adverse reactions was lower with butorphanol and tramadol compared to traditional strong opioids,31 we observed from this trial that a significant number of subjects in the butorphanol group (24.7%) still had adverse events such as nausea, vomiting, and skin pruritus. The administration of esketamine in postoperative PCIA reduced the incidence of adverse effects by approximately 10%. Among them, the worrying adverse effects associated with esketamine such as hallucinations and diplopia did not occur, except for three subjects who felt mild vertigo and one who reported temporary disorientation.
Studies have investigated ketamine’s role during cesarean delivery.10,12,33 A systematic review and meta-analysis in 2020 by Wang et al evaluated ketamine for pain control after CS and found that adding ketamine during the perioperative CS period reduced the degree of pain after CS as well as opioid consumption.33 However, few studies have been conducted on the role of esketamine, the S-enantiomer of ketamine,17 in pain management after CS. The majority of these studies focused on low doses (0.2–0.5 mg/kg) of esketamine in combination with strong opioids.19,20,34,35 In this current study, we tried using a higher dose of esketamine (1 mg/kg) in combination with the weak opioid analgesic tramadol in PCIA after CS, instead of using a strong opioid. Our results showed that the higher dose of esketamine (1 mg/kg) significantly reduced pain intensity and the incidence of opioid-related adverse reactions after CS compared to the positive control group. Additionally, the combination did not increase esketamine-related adverse reactions, suggesting that the higher dose of esketamine (1 mg/kg) can be safely used in opioid-sparing pain management strategies after CS.
Clinical trials in recent years have shown that esketamine has a rapid antidepressant effect.17,18 Due to changes in hormone levels, women who have had a CS are more likely to experience depression or depression-like symptoms after surgery compared to the average postoperative patients. Low dose esketamine combined with sufentanil in PCIA after CS has been shown to reduce the incidence of postpartum depression.19 Our trial confirmed significantly lower EPDS scores in the esketamine group compared to the control group at 5 days postoperatively. However, because none of the participants met the diagnostic criteria for postpartum depression36,37 confirmed via the postoperative follow-up EPDS scores, we cannot yet conclude that esketamine used for postoperative PCIA improved the occurrence of postpartum depression in this study. There was no significant difference in EPDS scores between the two groups at 42 days postoperatively, which may be related to the short-term administration of esketamine.
For all secondary outcomes present in this study, our conclusions should be considered exploratory due to the sample size calculations being based on the primary outcome. Therefore, there was insufficient power to detect differences in the secondary outcomes.
This study has some limitations. In our clinic trial, sixty-two of the 170 participants underwent an emergency cesarean section, accounting for 36.5% of all participants. In order to make this study more applicable to real-world clinical situations, we did not restrict the type of cesarean delivery. However, some studies have confirmed that emergency cesarean section patients are more likely to have postpartum depression and pain,38,39 which may have affected the outcomes. In addition, a portion of the EPDS score follow-up at 42 days postoperatively were missing, which may have an impact on the conclusions regarding postpartum depression. Furthermore, all outcomes in this study were self-reported by the participants and may lead to recall bias or be vulnerable to investigator interpretation.
Conclusion
In summary, our study demonstrates that esketamine combined with tramadol for PCIA after CS significantly improved pain intensity at 6 hours postoperatively compared to butorphanol combined with tramadol. Future research should focus on exploring more rational doses of esketamine in combination with opioids or other non-opioid medications to reduce pain after CS and evaluating the exact effect of esketamine on reducing the incidence of postpartum depression.
Data Sharing Statement
Six months after the main results are published, the individual participant data of this study can be accessed with the permission of the corresponding author.
Acknowledgments
The authors thank Lei Wang, Qinyan Wang, Qian Yuan, Zhi Dong for their valuable work in interviewing the subjects in this study and appreciate Professor Weijun Zheng (Zhejiang Chinese Medical University) for his guidance in the statistical issues in the manuscript. The authors also acknowledge AiMi Academic Services for English language editing and review services.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Eisenach JC, Pan PH, Smiley R, Lavand’homme P, Landau R, Houle TT. Severity of acute pain after childbirth, but not type of delivery, predicts persistent pain and postpartum depression. Pain. 2008;140(1):87–94. doi:10.1016/j.pain.2008.07.011
2. Molina G, Weiser TG, Lipsitz SR, et al. Relationship between cesarean delivery rate and maternal and neonatal mortality. JAMA. 2015;314(21):2263–2270. doi:10.1001/jama.2015.15553
3. Rosenstein MG, Chang SC, Sakowski C, et al. Hospital quality improvement interventions, statewide policy initiatives, and rates of cesarean delivery for nulliparous, term, singleton, vertex births in California. JAMA. 2021;325(16):1631–1639. doi:10.1001/jama.2021.3816
4. Sun S, Guo Y, Wang T, Huang S. Analgesic effect comparison between nalbuphine and sufentanil for patient-controlled intravenous analgesia after cesarean section. Front Pharmacol. 2020;11:574493. doi:10.3389/fphar.2020.574493
5. Brinck ECV, Maisniemi K, Kankare J, Tielinen L, Tarkkila P, Kontinen VK. Analgesic effect of intraoperative intravenous S-ketamine in opioid-naïve patients after major lumbar fusion surgery is temporary and not dose-dependent: a randomized, double-blind, placebo-controlled clinical trial. Anesth Analg. 2021;132(1):69–79. doi:10.1213/ANE.0000000000004729
6. Assouline B, Tramer MR, Kreienbuhl L, Elia N. Benefit and harm of adding ketamine to an opioid in a patient-controlled analgesia device for the control of postoperative pain: systematic review and meta-analyses of randomized controlled trials with trial sequential analyses. Pain. 2016;157(12):2854–2864. doi:10.1097/j.pain.0000000000000705
7. Grosu I, De Kock M. New concepts in acute pain management: strategies to prevent chronic postsurgical pain, opioid-induced hyperalgesia, and outcome measures. Anesthesiol Clin. 2011;29(2):311–327. doi:10.1016/j.anclin.2011.04.001
8. Fay EE, Hitti JE, Delgado CM, et al. An enhanced recovery after surgery pathway for cesarean delivery decreases hospital stay and cost. Am J Obstet Gynecol. 2019;221(4):349 e1–349 e9. doi:10.1016/j.ajog.2019.06.041
9. Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 2009;139(2):267–284. doi:10.1016/j.cell.2009.09.028
10. Adhikari P, Subedi A, Sah B, Pokharel K. Analgesic effects of intravenous ketamine after spinal anaesthesia for non-elective caesarean delivery: a randomised controlled trial. BMJ open. 2021;11(6):e044168. doi:10.1136/bmjopen-2020-044168
11. Nielsen RV, Fomsgaard JS, Siegel H, et al. Intraoperative ketamine reduces immediate postoperative opioid consumption after spinal fusion surgery in chronic pain patients with opioid dependency: a randomized, blinded trial. Pain. 2017;158(3):463–470. doi:10.1097/j.pain.0000000000000782
12. Haliloglu M, Ozdemir M, Uzture N, Cenksoy PO, Bakan N. Perioperative low-dose ketamine improves postoperative analgesia following Cesarean delivery with general anesthesia. J Matern Fetal Neonatal Med. 2016;29(6):962–966. doi:10.3109/14767058.2015.1027190
13. Hempelmann G, Kuhn DF. Klinischer Stellenwert des S-(+)-Ketamin. Anaesthesist. 1997;46(1):S3–7. German. doi:10.1007/pl00002461
14. Timm C, Linstedt U, Weiss T, Zenz M, Maier C. Sympathomimetische Effekte auch bei niedriger Dosierung von Esketamin. Anaesthesist. 2008;57(4):338–346. German. doi:10.1007/s00101-008-1331-0
15. Pees C, Haas NA, Ewert P, Berger F, Lange PE. Comparison of analgesic/sedative effect of racemic ketamine and S(+)-ketamine during cardiac catheterization in newborns and children. Pediatr Cardiol. 2003;24(5):424–429. doi:10.1007/s00246-002-0356-4
16. Zhang X, Wang J, An X, et al. Optimum dose of spinal ropivacaine with or without single intravenous bolus of S-ketamine during elective cesarean delivery: a randomized, double-blind, sequential dose-finding study. BMC Pregnancy Childb. 2021;21(1):746. doi:10.1186/s12884-021-04229-y
17. Kim J, Farchione T, Potter A, Chen Q, Temple R. Esketamine for treatment-resistant depression - first FDA-approved antidepressant in a new class. New Engl J Med. 2019;381(1):1–4. doi:10.1056/NEJMp1903305
18. Mahase E. Depression: EU approves expanded use of esketamine for rapid reduction of symptoms. BMJ. 2021;372(398). doi:10.1136/bmj.m3479
19. Wang Y, Zhang Q, Dai X, Xiao G, Luo H. Effect of low-dose esketamine on pain control and postpartum depression after cesarean section: a retrospective cohort study. Ann Palliat Med. 2022;11(1):45–57. doi:10.21037/apm-21-3343
20. Han Y, Li P, Miao M, Tao Y, Kang X, Zhang J. S-ketamine as an adjuvant in patient-controlled intravenous analgesia for preventing postpartum depression: a randomized controlled trial. BMC Anesthesiol. 2022;22(1):49. doi:10.1186/s12871-022-01588-7
21. Nielsen RV, Fomsgaard JS, Nikolajsen L, Dahl JB, Mathiesen O. Intraoperative S-ketamine for the reduction of opioid consumption and pain one year after spine surgery: a randomized clinical trial of opioid-dependent patients. Eur J Pain. 2019;23(3):455–460. doi:10.1002/ejp.1317
22. Wang X, Lin C, Lan L, Liu J. Perioperative intravenous S-ketamine for acute postoperative pain in adults: a systematic review and meta-analysis. J Clin Anesth. 2021;68:110071. doi:10.1016/j.jclinane.2020.110071
23. Brinck ECV, Virtanen T, Mäkelä S, et al. S-ketamine in patient-controlled analgesia reduces opioid consumption in a dose-dependent manner after major lumbar fusion surgery: a randomized, double-blind, placebo-controlled clinical trial. PLoS One. 2021;16(6):e0252626. doi:10.1371/journal.pone.0252626
24. Schulz KF, Altman DG, Moher D, Group C. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c332. doi:10.1136/bmj.c332
25. Moher D, Schulz KF, Altman DG. The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomised trials. Lancet. 2001;357(9263):1191–1194. doi:10.1016/S0140-6736(00)04337-3
26. Figueiredo B, Pinto TM, Costa R. Exclusive breastfeeding moderates the association between prenatal and postpartum depression. J hum lact. 2021;37(4):784–794. doi:10.1177/0890334421991051
27. Fang Q, Tu Y, Fan X, et al. Inflammatory cytokines and prenatal depression: is there a mediating role of maternal gut microbiota? J Psychiatry Res. 2023;164:458–467. doi:10.1016/j.jpsychires.2023.06.034
28. Giudice LC, As-Sanie S, Arjona Ferreira JC, et al. Once daily oral relugolix combination therapy versus placebo in patients with endometriosis-associated pain: two replicate Phase 3, randomised, double-blind, studies (SPIRIT 1 and 2). Lancet. 2022;399(10343):2267–2279. doi:10.1016/s0140-6736(22)00622-5
29. Sessler CN, Gosnell MS, Grap MJ, et al. The Richmond agitation-sedation scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166(10):1338–1344. doi:10.1164/rccm.2107138
30. Patton GC, Romaniuk H, Spry E, et al. Prediction of perinatal depression from adolescence and before conception (VIHCS): 20-year prospective cohort study. Lancet. 2015;386(9996):875–883. doi:10.1016/s0140-6736(14)62248-0
31. Cai Q, Gong H, Fan M, Chen W, Cai L. The analgesic effect of tramadol combined with butorphanol on uterine cramping pain after repeat caesarean section: a randomized, controlled, double-blind study. J Anesth. 2020;34(6):825–833. doi:10.1007/s00540-020-02820-9
32. Kainu JP, Sarvela J, Tiippana E, Halmesmäki E, Korttila KT. Persistent pain after caesarean section and vaginal birth: a cohort study. Int J Obstet Anesth. 2010;19(1):4–9. doi:10.1016/j.ijoa.2009.03.013
33. Wang J, Xu Z, Feng Z, Ma R, Zhang X. Impact of ketamine on pain management in cesarean section: a systematic review and meta-analysis. Pain Physician. 2020;23(2):135–148.
34. Suppa E, Valente A, Catarci S, Zanfini BA, Draisci G. A study of low-dose S-ketamine infusion as “preventive” pain treatment for cesarean section with spinal anesthesia: benefits and side effects. Minerva Anestesiol. 2012;78(7):774–781.
35. Shen J, Song C, Lu X, et al. The effect of low-dose esketamine on pain and post-partum depression after cesarean section: a prospective, randomized, double-blind clinical trial. Front Psychiatry. 2022;13:1038379. doi:10.3389/fpsyt.2022.1038379
36. Stewart DE, Vigod S. Postpartum depression. N Engl J Med. 2016;375(22):2177–2186. doi:10.1056/NEJMcp1607649
37. Levis B, Negeri Z, Sun Y, Benedetti A, Thombs BD. Accuracy of the Edinburgh Postnatal Depression Scale (EPDS) for screening to detect major depression among pregnant and postpartum women: systematic review and meta-analysis of individual participant data. BMJ. 2020;371:m4022. doi:10.1136/bmj.m4022
38. Sun L, Wang S, Li X-Q. Association between mode of delivery and postpartum depression: a systematic review and network meta-analysis. Aust N Z J Psychiatry. 2020;55(6):588–601. doi:10.1177/0004867420954284
39. Skov SK, Hjorth S, Kirkegaard H, Olsen J, Nohr Ellen A. Mode of delivery and short‐term maternal mental health: a follow‐up study in the Danish national birth cohort. Inter J Gynecol Obstet. 2022;159(2):457–465. doi:10.1002/ijgo.14155
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
