Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 16
An Inhaled PI3Kδ Inhibitor Improves Recovery in Acutely Exacerbating COPD Patients: A Randomized Trial
Authors Cahn A, Hamblin JN, Robertson J, Begg M , Jarvis E , Wilson R, Dear G, Leemereise C, Cui Y, Mizuma M, Montembault M, Van Holsbeke C , Vos W, De Backer W , De Backer J, Hessel EM
Received 8 March 2021
Accepted for publication 4 May 2021
Published 3 June 2021 Volume 2021:16 Pages 1607—1619
DOI https://doi.org/10.2147/COPD.S309129
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Anthony Cahn,1 J Nicole Hamblin,2 Jon Robertson,3 Malcolm Begg,2 Emily Jarvis,3 Robert Wilson,1 Gordon Dear,4 Claudia Leemereise,5 Yi Cui,6 Maki Mizuma,7 Mickael Montembault,8 Cedric Van Holsbeke,9 Wim Vos,9 Wilfried De Backer,10 Jan De Backer,9 Edith M Hessel2
1Discovery Medicine, GlaxoSmithKline, Stevenage, UK; 2Refractory Respiratory Inflammation Discovery Performance Unit, GlaxoSmithKline, Stevenage, UK; 3Biostatistics, GlaxoSmithKline, Stevenage, UK; 4Mechanistic Safety & Disposition, GlaxoSmithKline, Ware, UK; 5Global Clinical Sciences & Delivery, GlaxoSmithKline, Amersfoort, the Netherlands; 6Pharma Safety, GlaxoSmithKline, Brentford, Middlesex, UK; 7Data Management & Strategy, GlaxoSmithKline, Tokyo, Japan; 8Global Clinical Sciences & Delivery, GlaxoSmithKline, Brentford, Middlesex, UK; 9FLUIDDA nv, Kontich, 2550, Belgium; 10Pulmonary Medicine & Pulmonary Rehabilitation, University of Antwerp, Antwerp, Belgium
Correspondence: Anthony Cahn
Discovery Medicine, GlaxoSmithKline, Stevenage, UK
Tel +44 1438766374
Email [email protected]
Purpose: This study evaluated the safety and efficacy of inhaled nemiralisib, a phosphoinositide 3-kinase δ (PI3Kδ) inhibitor, in patients with an acute exacerbation of chronic obstructive pulmonary disease (COPD).
Methods: In this double-blind, placebo-controlled study, 126 patients (40– 80 years with a post-bronchodilator forced expiratory volume in 1 sec (FEV1) ≤ 80% of predicted (previously documented)) were randomized 1:1 to once daily inhaled nemiralisib (1 mg) or placebo for 84 days, added to standard of care. The primary endpoint was specific imaging airway volume (siVaw) after 28 treatment days and was analyzed using a Bayesian repeated measures model (clintrials.gov: NCT02294734).
Results: A total of 126 patients were randomized to treatment; 55 on active treatment and 49 on placebo completed the study. When comparing nemiralisib and placebo-treated patients, an 18% placebo-corrected increase from baseline in distal siVaw (95% credible intervals (Cr I) (− 1%, 42%)) was observed on Day 28. The probability that the true treatment ratio was > 0% (Pr(θ> 0)) was 96%, suggestive of a real treatment effect. Improvements were observed across all lung lobes. Patients treated with nemiralisib experienced a 107.3 mL improvement in posterior median FEV1 (change from baseline, 95% Cr I (− 2.1, 215.5)) at day 84, compared with placebo. Adverse events were reported by 41 patients on placebo and 49 on nemiralisib, the most common being post-inhalation cough on nemiralisib (35%) vs placebo (3%).
Conclusion: These data show that addition of nemiralisib to usual care delivers more effective recovery from an acute exacerbation and improves lung function parameters including siVaw and FEV1. Although post-inhalation cough was identified, nemiralisib was otherwise well tolerated, providing a promising novel therapy for this acutely ill patient group.
Keywords: acute exacerbation, COPD, nemiralisib
Introduction
Chronic obstructive pulmonary disease (COPD) is caused by exposure to tobacco smoke, biomass fuel, bacterial colonization, small airway remodeling and fibrosis, chronic asthma and genetic causes such as alpha-1-antitrypsin deficiency.1 Exacerbations in COPD are episodes of acute inflammation, commonly following a viral and/or bacterial infection,1–3 but can have other causes such as air pollution and irritants, allergens and changes in air temperature. Exacerbations often lead to reduced physical activity, poor quality of life, hospitalization and repeated courses of oral corticosteroids and/or antibiotics.2,4–8 Standard of care treatment is not always fully effective, and the time course of recovery can be variable and lengthy, with lung function and symptoms improving at different rates. Importantly, a significant proportion of patients who experience a COPD exacerbation are more susceptible to a recurrent exacerbation event,9,10 and subsequent events are often more severe and associated with worse outcomes.11 Therefore, there is an unmet need for novel therapies that can effectively treat and improve recovery from acute exacerbations.
Phosphoinositide 3-kinase δ (PI3Kδ), is a lipid kinase expressed exclusively in leukocytes where it regulates activation, proliferation and function of multiple cell types, through phosphorylation of AKT.12 Nemiralisib (GSK2269557) is a potent and highly selective inhaled PI3Kδ inhibitor.13,14 The PI3Kδ pathway is upregulated in blood neutrophils and lung tissue derived from COPD patients.15,16 Moreover, individuals with inherited activating mutations in PI3Kδ display profound immune defects including recurrent respiratory infections.17–19 As COPD exacerbations are often triggered by lung infections, PI3Kδ inhibitors may provide therapeutic benefit through their unique potential to improve neutrophil immune function by correcting the aberrant directionality of chemotaxis observed in COPD neutrophils,20 and by inhibiting inflammatory mediator release.21 Both of these aspects of neutrophil function are not known to be addressed by corticosteroids.
Functional respiratory imaging (FRI) is an emerging technique for the assessment of alterations in lung function.22 The core feature of FRI is that local measurements of airway volumes and resistance can be performed, while conventional lung function incorporates the whole respiratory system into a single number. Of the parameters derived, specific imaging airways volume (siVaw) has shown significant correlation with forced expiratory volume in 1 sec (FEV1) and patient reported outcomes in both stable and acutely exacerbating patients.23,24 The siVaw has also shown promise in demonstrating drug-induced improvements in lung function using sample sizes far smaller than typically required in FEV1-focused trials,25 and was recently used to predict the onset of an exacerbation,26 hence it was selected as the primary endpoint for the current trial.
This study evaluated the effects of inhaled nemiralisib, given in addition to standard of care treatment, in COPD patients presenting with a moderate or severe acute exacerbation using a combination of FRI and conventional lung function techniques. To our knowledge, this study is the first time a PI3Kδ inhibitor has been evaluated in this patient population.
Methods
Study Design and Patients
This was a randomized, double-blind, placebo-controlled, parallel group study that evaluated the effects of nemiralisib (1 mg) in addition to standard of care in COPD patients recruited from secondary care in five European countries between March 2015 and January 2016 (Figure 1A).
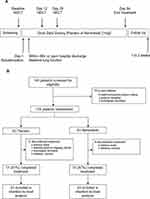 |
Figure 1 Study schematic (Panel A) and trial profile (Panel B). Abbreviation: HRCT, high resolution computed tomography. |
Eligible patients were male or female, aged 40–80 years with an established 6-month history of COPD,1 a previously documented post-bronchodilator FEV1/forced vital capacity (FVC) ratio of <0.70, an FEV1 ≤80% of predicted values,27 a smoking history of ≥10 pack years and a diagnosed acute exacerbation of COPD requiring escalation in therapy including both antibiotics and oral corticosteroids. An acute exacerbation was defined as described by Seemungal et al,9 as a recent change in at least two major and one minor symptom, one major and two minor symptoms, or all 3 major symptoms. Exclusion criteria included patients with invasive mechanical ventilation or hemodynamic instability, significant heart failure or pneumonia (detailed in NCT02294734).
Written informed consent was obtained from each patient. The study (GSK protocol: PII116678) was conducted in accordance with the Declaration of Helsinki and relevant ethics committee/institutional review boards and regulatory authorities reviewed and approved the study, detailed in Supplementary Materials, Appendix 1.
Procedures
Patients presenting with an acute exacerbation of COPD at an outpatient clinic, emergency department, or who had been hospitalized for their exacerbation were invited to participate. The “baseline” measurements for this study were performed at the presentation/onset of the exacerbation.
Over a short screening period (up to 3 days), baseline assessments were conducted including medical history, safety assessments, lung function tests and FRI using low dose high resolution computed tomography (LD-HRCT).
Standard of care treatment (a combination of antibiotics and systemic corticosteroids to all subjects) was started after diagnosis, though could have been started shortly before formal diagnosis. The LD-HRCT scan was conducted at the earliest opportunity, within 48 h of diagnosis. Patients were then randomized 1:1 to receive 84 days treatment with nemiralisib (1 mg) or placebo. Patients continued to use their normal maintenance therapy throughout the study. First dose administration took place within 24 h of completing the LD-HRCT scan. Both active and placebo treatments were administered via the DISKUS dry powder inhaler, containing nemiralisib as the hemisuccinate salt, GSK2269557H, in a lactose monohydrate blend and placebo containing lactose monohydrate (GSK, Ware, UK). During treatment, patients attended study visits as outlined in Figure 1A.
LD-HRCT scans (1–2 mSv per scan) were conducted at both total lung capacity (TLC) and functional residual capacity (FRC) at baseline and after 12 and 28 days of treatment. During LD-HRCT measurements, patients wore a nose clip and TLC and FRC lung volumes were determined with a handheld spirometer (Spirostik™ by Geratherm® Respiratory). Anonymized scans were sent to FLUIDDA nv, (Kontich, Belgium) for analysis. All airways present in each scan were analyzed unless otherwise stated. Further details of the methodology can be found in Supplementary Methods.
Full lung function including spirometry, plethysmography and diffusion capacity were measured at the clinic within 48 h of starting treatment for outpatients or prior to discharge for hospitalized patients, and subsequently on Days 28 and 84 of treatment. The following assessments were also made during the study: daily FEV1 and peak expiratory flow (PEF), the COPD Assessment Test (CAT),28 and the modified Medical Research Council Dyspnea Scale (mMRC),29 vital signs, electrocardiograph (ECG) and routine laboratory tests, and blood sampling for PK analysis. See the Supplementary Methods for further methodology details.
Outcomes
The primary endpoint was the change from baseline in siVaw after 28 days of treatment. Other LD-HRCT endpoints were secondary endpoints (details in Supplementary Methods). Further clinical endpoints were change from baseline in lung function parameters, CAT and mMRC scores, reliever medication use, number of treatment failures and exacerbation rates (see Supplementary Methods for further details), safety and PK parameters.
 |
Table 3 Placebo Corrected Changes in Secondary LD-HRCT Endpoints |
Statistical Analyses
We utilized data from a now published data set from a similar population which describes a between-subject standard deviation of 0.145 (natural logarithmic scale) in siVaw; the primary endpoint of this study.30 A sample size of approximately 50 subjects completing both post dose HRCT scans per arm was estimated to be sufficient to declare success at the end of the study; precise assumptions and calculations are described in the Supplementary Methods.
The study was not designed to formally test any hypothesis, this was an exploratory study to estimate the effects of nemiralisib on a number of functional imaging endpoints; therefore, the primary endpoint, siVaw, was analyzed using a Bayesian repeated measures model. The siVaw values were loge transformed prior to analysis. The pre-dose siVaw values were grouped into a separate treatment arm and were fitted as a time point in the model, allowing modelling of missing data points. Treatment by time by lobe/region was fitted as a three-way interaction. Age, gender and BMI were fitted as covariates in all models. Adjusted posterior medians and 95% credible intervals (Cr I) were constructed for each of the treatment arms at both time points, in addition to the comparison of nemiralisib/placebo. Specific posterior probabilities that the true treatment difference is greater than specified quantities were produced. Further details on the statistical analysis of other endpoints are available in the Supplementary Methods.
Results
Patient Characteristics and Safety
In total, 126 patients were recruited and randomized to treatment as depicted in Figure 1B. “Baseline” characteristics at the point of index exacerbation were similar between groups (Table 1).
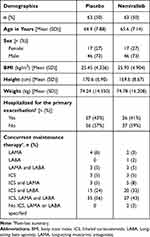 |
Table 1 Baseline Characteristics |
Adverse events (AEs) were reported by 41 (65%) patients in the placebo group and 49 (78%) patients in the nemiralisib group (Table 2). The most commonly reported AEs were cough, worsening COPD and headache. Cough was reported more commonly with nemiralisib (22 (35%)) than with placebo (2 (3%)), and in 20 (out of the 22) patients cough was assessed as related to treatment. There were no clinically significant abnormal laboratory results, vital signs or ECGs when compared between groups.
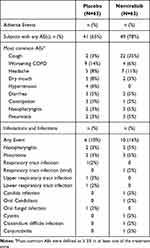 |
Table 2 Summary of Adverse Events |
Primary Endpoint: Specific Airway Volume (siVaw) as Measured by LD-HRCT
When comparing nemiralisib and placebo-treated patients, an 18% increase from baseline in distal siVaw measured at FRC on Day 28 was observed (posterior median ratio from baseline, 95% Cr I (−1%, 42%); Figure 2). The probability that the true treatment ratio was >0% (Pr(θ>0)) was 96%, suggestive of a real treatment effect. This increase in siVaw represented a 10% increase in the nemiralisib group (95% Cr I (−4%, 27%)) and a 7% decline in the placebo group (95% Cr I (−19%, 8%)). Day 28 siVaw values at FRC in the nemiralisib-treated group indicated greater improvements across all lung lobes compared with placebo. From Day 12 to Day 28 results showed continued improvement over time in this parameter (Figure 3).
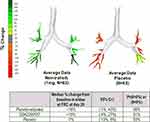 |
Figure 2 Median % change from baseline in distal region siVaw at FRC after 28 days. Abbreviations: siVaw, specific imaging airway volume; FRC, functional residual capacity. |
 |
Figure 3 Placebo-corrected change in siVaw at FRC after 12 and 28 days of treatment with nemiralisib. |
There were no notable changes in siVaw measured at TLC in any regions of the lung, possibly reflective of COPD patients suffering from difficulty with expiration rather than inspiration.
Notably, the improvement in distal airway volumes was also accompanied by a reduction in specific imaging airway resistance (siRaw) and specific imaging airway wall thickness (siVaww) in the distal regions of the lung, as well as a reduction in the low attenuation score (LAS), a marker of hyperinflation, and an increased airflow to the lower lobes, as indicated by internal airflow lobar distribution (IALD) (Table 3). Therefore, improvement in multiple imaging parameters in nemiralisib-treated patients compared with placebo was demonstrated.
Secondary Endpoints: Lung Function Parameters as Measured by Spirometry
A 107.3 mL (95% Cr I (−2.1, 215.5)) improvement in posterior median FEV1 (change from baseline) was observed at the end of 84 days’ treatment with nemiralisib, compared with placebo (Figure 4, Panel A), suggesting that exacerbating patients on active treatment demonstrated an improved recovery in lung function. This was further supported by an improvement in the median ratio from baseline in specific airway conductance (sGaw) in nemiralisib-treated patients over 84 days, with the largest placebo-corrected ratio observed at Day 28 (1.215 (95% Cr I (1.011, 1.458))) (Figure 4, Panel B); the ratio at Day 84 was 1.068 (95% Cr I (0.902, 1.262)). Interestingly, a post-hoc analysis split by index exacerbation severity, showed that patients hospitalized for their acute exacerbation improved most from treatment with nemiralisib. This was consistently shown for both FEV1 and sGaw (placebo-corrected posterior median change from baseline at Day 84 for hospitalized subjects: FEV1=305.8 mL (95% Cr I (130.4, 476.9)); sGaw=1.45 1/kPa*s (95% Cr I (1.05, 2.02))) (Figure 4, Panels A and B). Rescue medication use was higher with nemiralisib than placebo (post-hoc summary; Table S1, Supplementary Materials).
Secondary Endpoints: Patient Reported Outcomes and Re-Exacerbations
Mean CAT score decreased from baseline by an average of 3 points (improved health status) at Day 84 of treatment with both nemiralisib and placebo (Figure S1A, Supplementary Materials). Similarly, patients on either treatment showed improvements in breathlessness as assessed by mMRC (Figure S1B, Supplementary Materials), reflective of recovery from exacerbation.
There was a numerical reduction in both treatment failures and the number of patients who developed new exacerbation events in the nemiralisib-treated group compared to placebo (Figure 5). In placebo-treated patients, a greater proportion of re-exacerbations were severe (46%) when compared with patients treated with nemiralisib (33%) (severe defined by the need for hospitalization and investigator-reported, see Figure 5). Over the 12-week treatment period, the rate of investigator reported exacerbations that required hospitalization was 0.25 in the placebo group (95% CI (0.10, 0.62)) compared with 0.15 on nemiralisib (95% CI (0.05, 0.44)). Post-hoc summary on overall patient oral corticosteroid burden showed that there was less oral corticosteroid taken during treatment with nemiralisib versus placebo (12.4 vs 15.6 days, respectively).
Secondary Endpoints: Pharmacokinetics
Plasma concentrations of nemiralisib were at steady-state by Day 12, with levels maintained through to Day 84 following repeated daily dosing. Similar geometric mean pre-dose levels were observed on Day 12 (821 pg/mL (95% CI (670, 1006))) and Day 84 (835 pg/mL (95% CI (676, 1033))). Between subject variability (% CVb) was high, ranging from 72% to 96% at pre-dose time points from Day 12 to Day 84. Notably, no clear differences were observed in plasma concentrations between those patients reporting post-inhalation cough and those who did not.
Discussion
This proof of concept study explored whether nemiralisib, a highly selective and potent inhaled inhibitor of PI3Kδ, could deliver more effective recovery from a COPD exacerbation when added to standard of care in patients presenting with an acute exacerbation. Nemiralisib-treated patients demonstrated an 18% placebo-corrected increase from baseline in distal siVaw measured at FRC on Day 28, with a consistent improvement observed across all lung lobes, thus meeting the primary endpoint of the study. In addition, placebo-corrected FEV1 and sGaw at Day 84 improved, respectively, by 107.3 mL (difference) and 1.068 (ratio) compared to baseline. During the exacerbation recovery period, results compared to placebo show continued improvement over time in lung function-related parameters including siVaw, sGaw and FEV1. In summary, our study shows that nemiralisib could potentially deliver improved recovery from exacerbation, as demonstrated by changed lung function parameters, which would be of valuable therapeutic benefit for acutely exacerbating COPD patients.
To capture the time course of recovery, LD-HRCT was performed at diagnosis, Days 12 and 28. Nemiralisib treatment showed an increase in effectiveness between Day 12 and Day 28 in most LD-HRCT endpoints. A possible explanation for this may be that by Day 12, the majority of patients would have only recently stopped treatment with antibiotics and systemic corticosteroids, and the beneficial effects of these treatments may have influenced Day 12 LD-HRCT endpoints. While the relationship between LD-HRCT and traditional lung function endpoints is not fully understood, and the clinical translation of the improvement in siVaw in nemiralisib-treated patients is unknown, the siVaw improvement observed in this study was of similar magnitude to the siVaw improvement seen following recovery from exacerbation in an earlier study using FRI.30 The approach used in the earlier study differs slightly from the current study where the primary endpoint was conducted at Day 28 irrespective of if the subject had fully recovered or not. Furthermore, a similar improvement in siVaw (~25%) was observed with acute ipratropium bromide treatment in stable COPD,25 which is similar to our study. In the current study, the observed effects were greatest in the distal airways, with a corresponding drop in airway resistance, compared with the central airways where little change was seen. This suggests a reproducibility in observations in both stable and exacerbating patients across different studies. Although the improvement was observed across all lung lobes, the subtle differences observed between each lobe requires further research.
The use of LD-HRCT endpoints is gaining traction as a sensitive alternative to traditional lung function measures, providing anatomical, regional and modelled functional information that can be obtained for a multitude of parameters down to the lobar level that cannot be obtained by conventional spirometry. Furthermore, smaller numbers of patients are required to detect changes in FRI, building confidence in the efficacy of novel mechanisms thereby not exposing significant numbers of acutely ill patients to an unproven new treatment. The differences shown between treatments using FRI technology is an encouraging observation and future studies will explore the clinical relevance of these findings. Beyond studies in COPD, FRI has shown promise in early detection of bronchiolitis obliterans syndrome in lung transplant patients,31 and loss of FEV1 following lobectomy/pneumonectomy,32 and most recently in redistribution of pulmonary blood flow in COVID-19 patients.33,34
The role of nemiralisib in improving the recovery from exacerbation is further supported by other LD-HRCT parameters measured on Day 28. These include a nemiralisib-induced reduction in siRaw, which may have occurred as a result of the increase in airway volume. A similar pattern was seen with plethysmographic sGaw. SiVaww was also reduced by nemiralisib, potentially reflecting a reduction in airway inflammation and mucus. In addition, a greater reduction in the LAS was observed in nemiralisib-treated patients compared with placebo, suggesting a greater reduction in pulmonary hyperinflation, and hyperinflation is a well-recognized contributor to symptoms in COPD patients. Assessing the lobar volume changes between TLC and FRC allows modelling of regional ventilation. The change in IALD observed in nemiralisib but not placebo-treated patients suggests a relative increase in airflow to the lower lobes on nemiralisib, perhaps reflecting reduced hyperinflation in the upper lobes. An increase in IALD to a particular lung region as a consequence of treatment also has the potential to improve deposition of other inhaled treatments, including bronchodilators and inhaled steroids.
LD-HRCT was not performed at day 84 to focus the analysis on the effect of nemiralisib on the acute recovery phase following exacerbation; however, treatment continued for 84 days to explore the impact on additional secondary endpoints and future exacerbations.
In this study, more traditional lung function endpoints were included to allow for correlation between LD-HRCT and standard measures. Unexpectedly, in exacerbating patients FEV1 declined from baseline to day 28 in both treatment arms; however, nemiralisib treatment improved the FEV1 over time, whereas the FEV1 in patients on placebo had not fully recovered to baseline by day 84. The FEV1 in patients on nemiralisib recovered to 27 mL above baseline, with an average difference of 107 mL compared with placebo at day 84. It should be noted that “baseline” FEV1 was not measured at the start of exacerbation, where the priority was to obtain LD-HRCT endpoints but the FEV1 was performed after randomization, and after the start of standard of care treatment. It was considered that lung function maneuvers might be difficult to complete for exacerbating patients and consequently highly variable. In addition, FEV1 was being used to track longer-term effects of nemiralisib out to day 84 once the exacerbation had resolved. Thus, baseline FEV1 also reflects the activity of systemic corticosteroids and the increased bronchodilator use early in exacerbation, perhaps explaining its initial decline during recovery. Spirometry performed at later time points was not required to be post-bronchodilator, which might also have resulted in lower values compared to the first measurement and may explain differences from other studies which observed recovery in FEV1 post exacerbation.35 In general, there are few interventional or imaging studies,22 interrogating responses immediately after an acute exacerbation.
In a post-hoc analysis of both FEV1 and sGaw measures, data were separated by severity of the initial (index) exacerbation (ie, requiring hospitalization or not). This revealed a substantial improvement in both FEV1 and sGaw in nemiralisib but not placebo-treated patients who experienced a severe index exacerbation. This suggests either that the airways of patients with severe index exacerbations are more responsive to PI3Kδ inhibition, or alternatively, a nemiralisib-responder phenotype may be more prevalent among patients suffering from severe exacerbations. Interestingly, when siVaw was similarly examined, improvements were present in both severe and moderate index exacerbation groups treated with nemiralisib, with a slightly larger siVaw improvement in the moderate index exacerbation group on Day 28.
While our study was not powered to explore the effect of nemiralisib on re-exacerbations, numerically fewer subjects on nemiralisib than placebo experienced new exacerbation events, and subsequent exacerbations on nemiralisib were less severe. Reducing the risk of future new exacerbations is important for COPD patients, as these events are known to decrease life expectancy,6 and affect loss of lung function. As anticipated, no notable treatment difference between groups was observed in CAT scores measured at days 28 and 84. However, both CAT and mMRC scores in both groups improved and reflected clinical recovery from exacerbation.
In response to inhalation of nemiralisib, mild-to-moderate post-inhalation cough was reported in 35% of patients compared with 3% of patients on placebo. Overall, nemiralisib was otherwise generally well tolerated but three subjects discontinued study drug due to cough. Cough has been noted with other PI3Kδ inhibitors,36 possibly due to a direct effect on neuronal activity.37
Pharmacokinetic data demonstrated that systemic exposure in exacerbating patients was similar to that observed in stable patients,14 and importantly, was similar between patients who did and did not report cough. The exposure data confirm that the extrapolated target exposure in lungs of nemiralisib-treated patients was at levels expected to provide sufficient target inhibition for the treatment duration.38
As exacerbations of COPD are quite heterogeneous in clinical presentation,1–3 our study captured spontaneous sputum samples in an attempt to characterize the pathogens present at the point of exacerbation. Unfortunately, an insufficient number of samples were obtained for any meaningful analysis, and may have required a larger sample size. This is a limitation of the current study, particularly if only a proportion of exacerbations, such as bacterially driven, can be effectively treated by nemiralisib. However, the ability to accurately determine the drivers of an exacerbation at the point of presentation is currently not broadly feasible, and hence our study represents the current standard of patient management. We believe improvements in rapid point of care diagnostics will allow treatment with more precision in the future. The time between onset of symptoms and presentation for enrollment is not known, but represents the real-world situation of patients presenting after differing durations of symptomology.
To our knowledge, this is the first study exploring the therapeutic benefit of PI3Kδ inhibition in exacerbating COPD patients, using both novel and more traditional endpoints. Although immediate post-inhalation cough was identified as a drug-related adverse event, nemiralisib was otherwise generally well tolerated and demonstrated an acceptable safety profile in this acutely ill patient group. Based on the data presented here, we believe that nemiralisib given in addition to standard of care has the potential to deliver more effective recovery in patients experiencing an acute exacerbation.
Abbreviations
AE, adverse event; AT, air trapping; BVD, blood vessel density; CAT, COPD Assessment Test; COPD, chronic obstructive pulmonary disease; Cr I, credible interval; ECG, electrocardiograph; FEV1, forced expiratory volume in 1 sec; FRC, functional residual capacity; FRI, functional respiratory imaging; FVC, forced vital capacity; IALD, internal airflow lobar distribution; LAS, low attenuation score; LD-HRCT, low dose high resolution computed tomography; mMRC, modified Medical Research Council Dyspnea Scale; PEF, peak expiratory flow; PI3Kδ, phosphoinositide 3-kinase δ; SiRaw, specific imaging airway resistance; SiVaw, specific imaging airway volume; SiVaww, specific imaging airway wall thickness; TLC, total lung capacity.
Data Sharing Statement
Anonymized individual participant data and study documents can be requested for further research from www.clinicalstudydatarequest.com.
Acknowledgments
DISKUS is owned by/licensed to the GSK group of companies. Editorial support was provided by Kate Hollingworth of Continuous Improvement Ltd and funded by GSK.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was funded by GlaxoSmithKline (NCT02294734). The sponsor/funder, as described under author contributions, was involved in the study design, data analysis, interpretation of data and writing of the manuscript.
Disclosure
AC, JR, MB, GD, CL, YC, MMiz, MMont and EJ are GSK employees and all hold GSK shares. JNH, RW and EMH were GSK employees at the time of the study conduct, analysis and interpretation, and hold GSK shares. JNH and EMH are named on patents for compound GSK2269557 (nemiralisib). JNH reports patents WO2010125082A1 and WO2012055846A1 issued. EMH has a patent WO2015055691A1 issued. CVH, WV and JDB are employees of FLUIDDA, the company responsible for FRI analysis and interpretation. WV and JDB hold FLUIDDA shares. WDB reports costs from University Hospital Antwerp to perform the study. The current affiliation of JNH is Discovery, Charles River, Chesterford Research Park, Cambridge, UK. The current affiliation of RW is DLRC, Letchworth Garden City, UK. The current affiliation of WV is OncoRadiomics, Liège, Belgium. The current affiliation of EMH is Eligo Bioscience, Paris, France. The authors report no other conflicts of interest in this work.
References
1. From the Global Strategy for the Diagnosis, Management and Prevention of COPD. Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2020. Available from: http://goldcopd.org/.
2. Wedzicha JA, Seemungal TAR. COPD exacerbations: defining their cause and prevention. Lancet. 2007;370(9589):786–796. doi:10.1016/S0140-6736(07)61382-8
3. Bafadhel M, McKenna S, Terry S, et al. Acute exacerbations of chronic obstructive pulmonary disease: identification of biologic clusters and their biomarkers. Am J Respir Crit Care Med. 2011;184:662–671. doi:10.1164/rccm.201104-0597OC
4. Miravitlles M, Ferrer M, Pont À, et al.; IMPAC Study Group. Exacerbations impair quality of life in patients with chronic obstructive pulmonary disease. A two-year follow-up study. Thorax. 2004;59:387–395. doi:10.1136/thx.2003.008730
5. Niewoehner DE, Lokhnygina Y, Rice K, et al. Risk indexes for exacerbations and hospitalizations due to COPD. Chest. 2007;131:20–28. doi:10.1378/chest.06-1316
6. Soler-Cataluña JJ, Martínez-García MA, Román Sánchez P, Salcedo E, Navarro M, Ochando R. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax. 2005;60:925–931. doi:10.1136/thx.2005.040527
7. Wilson R. Treatment of COPD exacerbations: antibiotics. Eur Respir Rev. 2005;14:32–38. doi:10.1183/09058180.05.00009404
8. Hunter MH, King DE. COPD: management of acute exacerbations and chronic stable disease. Am Fam Physician. 2001;64:603–613.
9. Seemungal TA, Donaldson GC, Bhowmik A, Jeffries DJ, Wedzicha JA. Time course and recovery of exacerbations in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2000;16:1608–1613. doi:10.1164/ajrccm.161.5.9908022
10. Hurst JR, Donaldson GC, Quint JK, Goldring JJ, Baghai-Ravary R, Wedzicha J. Temporal clustering of exacerbations in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2009;179:369–374. doi:10.1164/rccm.200807-1067OC
11. Suissa S, Dell’Aniello S, Ernst P. Long-term natural history of chronic obstructive pulmonary disease: severe exacerbations and mortality. Thorax. 2012;67:957–963. doi:10.1136/thoraxjnl-2011-201518
12. Fruman DA, Chiu H, Hopkins BD, Bagrodia S, Cantley LC, Abraham RT. The PI3K pathway in human disease. Cell. 2017;170:605–635.
13. Down K, Amour A, Baldwin IR, et al. Optimization of novel indazoles as highly potent and selective inhibitors of phosphoinositide 3-kinase δ for the treatment of respiratory disease. J Med Chem. 2015;58(18):7381–7399. doi:10.1021/acs.jmedchem.5b00767
14. Cahn A, Hamblin JN, Begg M, et al. Safety, pharmacokinetics and dose-response characteristics of GSK2269557, an inhaled PI3Kδ inhibitor under development for the treatment of COPD. Pulm Pharmacol Ther. 2017;46:69–77. doi:10.1016/j.pupt.2017.08.008
15. Milara J, Lluch J, Almudever P, Freire J, Xiaozhong Q, Cortijo J. Roflumilast N-oxide reverses corticosteroid resistance in neutrophils from patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2014;134(2):314–322. doi:10.1016/j.jaci.2014.02.001
16. To Y, Ito K, Kizawa Y, et al. Targeting phosphoinositide-3-kinase-delta with theophylline reverses corticosteroid insensitivity in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2010;182:897–904. doi:10.1164/rccm.200906-0937OC
17. Angulo I, Vadas O, Garçon F, et al. Phosphoinositide 3-kinase gene mutation predisposes to respiratory infection and airway damage. Science. 2013;342(6160):866–871. doi:10.1126/science.1243292
18. Stark AK, Chandra A, Chakraborty K, et al. PI3Kδ hyper-activation promotes development of B cells that exacerbate Streptococcus pneumoniae infection in an antibody-independent manner. Nat Commun. 2018;9:3174. doi:10.1038/s41467-018-05674-8
19. Lucas CL, Kuehn HS, Zhao F, et al. Dominant-activating germline mutations in the gene encoding the PI(3)K catalytic subunit p110δ result in T cell senescence and human immunodeficiency. Nat Immunol. 2014;15(1):88–97. doi:10.1038/ni.2771
20. Sapey E, Stockley JA, Greenwood H, et al. Behavioral and structural differences in migrating peripheral neutrophils from patients with chronic obstructive pulmonary disease. AM J Respir Crit Care Med. 2011;183:1176–1186. doi:10.1164/rccm.201008-1285OC
21. Stark A-K, Sriskantharajah S, Hessel EM, Okkenhaug K. PI3K inhibitors in inflammation, autoimmunity and cancer. Curr Opin Pharmacol. 2015;23:82–91.
22. Rangelov BA, Young AL, Jacob J, et al. Thoracic imaging at exacerbation of chronic obstructive pulmonary disease: a systematic review. Int J Chron Obstruct Pulmon Dis. 2020;15:1751–1787. doi:10.2147/COPD.S250746
23. Vos W, Van Holsbeke C, Van Geffen W, et al. Changes in functional respiratory imaging (FRI) endpoints correlate with changes in patient reported outcomes (PRO) after recovering from acute COPD exacerbation. Eur Respir J. 2015;46(suppl 59):OA2948.
24. Vos W, Van Holsbeke C, De Backer J, De Backer WA. Correlation between changes in FEV1 and functional respiratory imaging biomarkers in a large population of asthma and COPD patients. Am J Respir Crit Care Med. 2015;191:A2266.
25. De Backer LA, Vos WG, Salgado R, et al. Functional imaging using computer methods to compare the effect of salbutamol and ipratropium bromide in patient-specific airway models of COPD. Int J Chron Obstruct Pulmon Dis. 2011;6:637–646. doi:10.2147/COPD.S21917
26. Lanclus M, Clukers J, Van Holsbeke C, et al. Machine learning algorithms utilizing functional respiratory imaging may predict COPD exacerbations. Acad Radiol. 2019;26:1191–1199. doi:10.1016/j.acra.2018.10.022
27. Quanjer PH, Tammeling GJ, Cotes JE, Pedersen OF, Peslin R, Yernault JC. Lung volumes and forced ventilatory flows. Report working party standardization of lung function tests, European Community for steel and coal. Official Statement of the European Respiratory Society. Eur Respir J Suppl. 1993;6(suppl 16):5–40. doi:10.1183/09041950.005s1693
28. Jones PW, Harding G, Berry P, Wiklund I, Chen W-H, Kline Leidy N. Development and first validation of the COPD assessment test. Eur Respir J. 2009;34:648–654. doi:10.1183/09031936.00102509
29. Bestall JC, Paul EA, Garrod R, Garnham R, Jones PW, Wedzicha JA. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax. 1999;54:581–586. doi:10.1136/thx.54.7.581
30. van Geffen WH, Hajian B, Vos W, et al. Functional respiratory imaging: heterogeneity of acute exacerbations of COPD. Int J Chron Obstruct Pulmon Dis. 2018;13:1783–1792. doi:10.2147/COPD.S152463
31. Barbosa E, Ferreira F, Vos W, et al. Quantitative functional respiratory imaging may predict the mechanism of FEV1 decline after lung transplantation. Eur Respir J. 2017;50:PA2465.
32. Vos W, Janssens A, Van Holsbeke C, et al. Functional respiratory imaging to predict post-operative FEV1 after lobectomy/pneumonectomy. Eur Respir J. 2014;44:P342. doi:10.1183/09031936.00011714
33. Lins M, Vandevenne J, Thillai M, et al. Assessment of small pulmonary blood vessels in COVID-19 patients using HRCT. Acad Radiol. 2020;27:1449–1455. doi:10.1016/j.acra.2020.07.019
34. Thillai M, Patvardhan C, Swietlik EM, et al. Functional respiratory imaging identifies redistribution of pulmonary blood flow in patients with COVID-19. Thorax. 2020. doi:10.1136/thoraxjnl-2020-215395
35. Watz H, Tetzlaff K, Magnussen H, et al. Spirometric changes during exacerbations of COPD: a post hoc analysis of the WISDOM trial. Respir Res. 2018;19:251–266. doi:10.1186/s12931-018-0944-3
36. Coutré SE, Barrientos JC, Brown JR, et al. Management of adverse events associated with idelalisib treatment: expert panel opinion. Leuk Lymphoma. 2015;56:2779–2786. doi:10.3109/10428194.2015.1022770
37. Sánchez-Alegría K, Flores-León M, Avila-Muñoz E, Rodríguez-Corona N, Arias C. PI3K Signaling in neurons: a central node for the control of multiple functions. Int J Mol Sci. 2018;19:3725. doi:10.3390/ijms19123725
38. Wilson R, Cahn A, Montembault M, et al. Safety, tolerability, pharmacokinetics (PK) and pharmacodynamics (PD) of single (SD) and repeat (RD) inhaled doses of a novel phosphoinositide 3-kinase δ inhibitor (PI3Kδ), GSK2269557, administered to healthy smokers. Eur Respir J. 2014;44(Suppl 58):3411.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


