Back to Journals » Journal of Pain Research » Volume 12
Altered amplitude of low-frequency fluctuation and regional cerebral blood flow in females with primary dysmenorrhea: a resting-state fMRI and arterial spin labeling study
Authors Zhang YN , Huo JW , Huang YR, Hao Y, Chen ZY
Received 17 June 2018
Accepted for publication 14 February 2019
Published 16 April 2019 Volume 2019:12 Pages 1243—1250
DOI https://doi.org/10.2147/JPR.S177502
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Michael A Ueberall
Ya-Nan Zhang,1 Jian-Wei Huo,1 Yi-Ran Huang,2 Ying Hao,3 Zi-Yue Chen4
1Department of Radiology, Beijing Hospital of Traditional Chinese Medicine Affiliated to Capital Medical University, Beijing 100010, People’s Republic of China; 2School of Acupuncture-Moxibustion & Tuina, Beijing University of Chinese Medicine, Beijing, 100029, People’s Republic of China; 3Beijing International Center for Mathematical Research, Peking University, Beijing 100871, People’s Republic of China; 4Department of Acupuncture and Moxibustion, Yanshan Hospital, Beijing 102500, People’s Republic of China
Purpose: The current study aimed to explore the central mechanism of primary dysmenorrhea (PD) by investigating the alterations in resting state amplitude of low-frequency fluctuation (ALFF) and regional cerebral blood flow (CBF) between PD patients and healthy controls (HCs).
Patients and methods: A total of 34 female subjects including 20 PD patients and 14 HCs underwent resting-state functional magnetic resonance imaging (rs-fMRI) and arterial spin labeling technique (ASL) MRI during menstrual phase. Subsequently, the differences in ALFF and CBF were compared in the two groups. The visual analog scores for pain (VAS-P) and for anxiety (VAS-A) were applied to assess cramping pain and related symptoms in PD patients. Finally, Pearson’s correlation analysis was performed to analyze relationships between the neuroimaging findings and clinical characteristics.
Results: Compared to HCs, PD patients had decreased ALFF in the right cerebellum posterior lobe, right middle temporal gyrus, right parahippocampal gyrus, right hippocampus, right brainstem and left parietal lobe. In addition, elevated CBF values were observed in the right inferior frontal gyrus, right precentral gyrus, and right superior temporal gyrus. There was no significant correlation between ALFF, CBF values and clinical characteristics including onset age of dysmenorrhea, VAS-A, and VAS-P in PD patients.
Conclusion: The preliminary alterations of ALFF and CBF values in PD patients were observed in different pain-related brain regions, which were involved in multiple dimensions of pain and pain modulation. The combination of rs-fMRI and ASL MRI might provide complementary information for a better understanding of the central mechanism in PD.
Keywords: primary dysmenorrhea, resting state functional magnetic resonance imaging, amplitude of low frequency fluctuation, arterial spin labeling, cerebral blood flow
Introduction
Primary dysmenorrhea (PD) is one of the most frequent gynecological diseases, manifested by spasmodic cramping in the lower abdomen during menstrual period without any organic pelvic lesions.1 Many epidemiological surveys of dysmenorrhea prevalence in recent years have been shown at least 70% of menstruating women worldwide have suffered from different degrees of dysmenorrhea. Of patients 56.3–80.34% describe their menstrual pain as moderate or severe pain,2–4 which has negative effects on individual daily and academic activities, quality of life and so on.3–6 At present, the most widely accepted pathogenesis for PD is the overproduction of uterine prostaglandins that stimulate myometrial contractions and cause ischemia during endometrial sloughing.7 However, definite pathophysiology remains to be investigated. Some brain imaging studies show recurrent menstrual pain can cause changes in brain structure, cerebral perfusion and neuronal activity.8–12
The resting-state functional magnetic resonance imaging (rs-fMRI) has been extensively applied and it has been suggested that it provides a better understanding of the pathophysiology with PD subjects on spontaneous neuronal activities.11,13–16 The rs-fMRI analysis methods include amplitude of low-frequency fluctuation (ALFF),11 regional homogeneity (ReHo)13,14 and functional connectivity (FC) and similar.11,15,17 Among many rs-fMRI metrics, ALFF indirectly reflects spontaneous neuronal activities through examining the amplitude of low-frequency oscillations of the blood oxygen level-dependent (BOLD) signal,18 which reaches the best balance between test–retest reliability and replicability.19 Using both ALFF and FC methods, previous study provided evidence that the default mode network (DMN) dysfunction has been involved in menstrual pain. In addition, the ALFF values in the left medial prefrontal cortex (mPFC) in PD patients positively correlated with illness duration, indicating that mPFC may be a more characteristic brain region.11,20
Functional activation of brain regions is thought to be reflected by increases in the regional cerebral blood flow (CBF) and in the BOLD signal.21 The BOLD signal is influenced by not only CBF but also blood volume or oxygen metabolism.22 Because of this, ALFF does not incompletely explain the neural mechanisms of pain. CBF can accurately and indirectly reflect cerebral metabolism and widely be applied in pain studies. A few studies have explored perfusion abnormalities of the whole brain with 18F-fluorodeoxyglucose positron emission tomography (18F-FDG PET) in PD patients. They proposed that activation of thalamo-orbitofrontal-prefrontal networks may contribute to the generation of pain and mechanisms of central sensitization in PD.9,23 PET can be expensive and involves exposure to radiation; however, arterial spin labeling (ASL) MRI can detect functional activation associated with pain perception by measuring regional CBF as a noninvasive, radiationless and non-contrast technique.21,24 To our knowledge, ASL MRI has not yet been applied in PD patients. In recent years, research on the combination of rs-fMRI and ASL MRI has been increasing gradually.25,26 The combination of these two techniques may provide more complementary information for understanding pathophysiology with PD.
In this study, we collected rs-fMRI and ASL MRI data from PD patients and HCs to systematically investigate alterations of resting-state ALFF and CBF in whole brain and possible relationships between ALFF and CBF. We also evaluated whether clinical characteristics were correlated with altered ALFF and CBF in PD patients. Therefore, we hypothesized that aberrant regions of ALFF and CBF values might be found in PD patients in various pain-related brain regions, which were involved in multiple dimensions of pain and pain modulation.
Patients and methods
Ethics statement
The study was approved by the Medical and Experimental Animal Ethics committee of Beijing University of Chinese Medicine (reference: 2015BZHYLL0112), which was conducted in accordance with the Declaration of Helsinki.
Subjects
From December 2016 to January 2018, right-handed women with PD were recruited from a university. All subjects in this study were university students or graduate students at school. All subjects were screened and diagnosed by a doctor, who was familiar with the details of menstrual questionnaires and pelvic ultrasonography. All subjects gave their written informed consents.
The eligible PD patients met the following criteria: (1) satisfied the definition of PD in line with the No. 345 – Primary Dysmenorrhea Consensus Guideline; (2) 16–30-year-old females without child; (3) a regular and almost normal menstrual cycle of 21–35 days; (4) the course of menstrual pain was present for longer than 6 months; (5) no drugs and other methods had been used to treat dysmenorrhea in the last month; (6) the average visual analog score (VAS) of menstrual pain≥40 mm (0=no pain sensation, 100=the worst pain sensation) in the three recent menstrual cycles. For HCs, the inclusion criteria were similar to those for PD patients, except that the controls had no menstrual pain symptoms.
Exclusion criteria for all participants were as follows: (1) secondary dysmenorrhea caused by pelvic organic diseases, such as endometriosis, pelvic inflammation and so on; (2) having severe life-threatening disease, asthma, or psychiatric disorder, etc.; (3) any oral contraceptives, analgesics and antidepressant having been used within 6 months; (4) having any contraindication of MRI examination.
In this study, there were 20 PD patients and 14 HCs who fulfilled the inclusion criteria and completed MRI scans. Among these subjects, six subjects with excessive head motion were excluded from the study. The remaining rs-fMRI and ASL MRI data collected from 16 PD patients and 12 HCs were analyzed. The demographic data were recorded before the MRI scan. The VAS for pain (VAS-P) was completed to assess the cramping pain during menstrual period and VAS for anxiety (VAS-A) was used to evaluate related symptoms experienced by PD patients retrospectively in the last three months.
MRI data acquisition
MRI data were acquired using a 3.0 Tesla scanner (Magnetom Skyra, Siemens Medical Solutions, Erlangen, Germany) at the Department of Radiology of the Beijing Hospital of Traditional Chinese Medicine Affiliated to Capital Medical University. The MRI scan was performed during the first–third day of all subjects' menstrual cycles. Sponge cushions and earmuffs were provided to limit head motion and reduce scanner noise, respectively. During the scan, subjects were required to relax, close their eyes, remain awake and think about nothing in particular.
A high resolution T1-weighted structural image was obtained using a magnetization-prepared rapid-acquired gradient echo sequence (MPRAGE) (repetition time (TR)=2300 ms; echo time (TE) =2.32 ms; slices=192; flip angle =8°; field of view (FOV)=240 mm×240 mm; data matrix=256×256; voxel size =0.9 mm×0.9 mm×0.9 mm). Rs-fMRI was acquired by mean of a gradient-echo echo planar imaging (EPI) sequence using the following settings: TR=3000 ms; TE=30 ms; slices=40; flip angle=90°; FOV=220 mm×220 mm; matrix resolution=94×94; voxel size=2.3 mm×2.3 mm×3.0 mm. The parameters of two-dimensional pulsed ASL (2D PASL) sequence were as follows: TR=2700 ms; TE=13 ms; slices=14; dist factor=25%;inversion time=1800 ms; bolus duration=700 ms; flip angle=90°; FOV=230×230 mm; matrix size=64×64; voxel size=3.6 mm×3.6 mm×5.0 mm.
ALFF preprocessing and analysis
Imaging data preprocessing was conducted by the toolbox for Data Processing & Analysis of Brain Imaging (DPABI, Yan et al 2016,
The ALFF maps were calculated using the DPABI toolbox. Firstly, the preprocessing time series was transformed to the frequency domain by fast Fourier transform, and the square root of the power spectrum was calculated at each frequency. Then, temporally bandpass filtering (0.01–0.1 Hz) was applied to remove low-frequency drift and physiological high-frequency noise. Finally, ALFF values of each voxel were normalized by Fisher z-transformation.
CBF preprocessing and analysis
CBF maps were calculated automatically by a Siemens scanner. The subsequent preprocess was performed using Statistical Parametric Mapping (SPM) 12 (Wellcome Department of Imaging Neuroscience, London, UK,
Statistical analysis
A two-sample t-test was used to analyze demographic data and clinical characteristics including age, the onset age of dysmenorrhea, menstrual cycle and phase between PD patients and HCs by using the SPSS 22 (IBM Corporation, Armonk, NY, USA). The significance level was set at a threshold of p<0.05.
The group analysis was conducted using two-sample t-test in the DPARSF software to examine the differences between ALFF and CBF maps in whole brain adjusting for age. The Gaussian Random Field (GRF) correction was applied to perform the multiple comparison correction. The ALFF and CBF values of abnormal brain regions were extracted. Pearson’s correlation analysis was performed to investigate underlying relationships between abnormal ALFF or CBF values and onset age of dysmenorrhea, VAS-A, and VAS-P in PD patients.
Results
Demographics and clinical symptoms
As shown in Table 1, 16 PD patients and 12 HCs were included in the final analysis. There was no statistically significant difference between the two groups in age, onset age of dysmenorrhea, menstrual cycle and phase (p>0.05). All PD patients suffered from painful cramps, other symptoms included nausea, vomiting, headache, back pain, leg pain, dizziness, fatigue, and so on.
 | Table 1 Demographic and clinical characteristics of subjects |
ALFF and CBF differences between groups
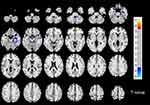
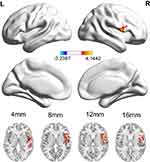
Compared to HCs, PD patients show decreased ALFF values in the right cerebellum posterior lobe (mainly lobule VIII,partly lobule VIIB), right middle temporal gyrus, right limbic lobe, right parahippocampal gyrus, right hippocampus, right brainstem, and left parietal lobe (includes left precuneus) (Figure 1 and Table 2), whereas PD patients had increased CBF in the right inferior frontal gyrus, right precentral gyrus and right superior temporal gyrus (Figure 2 and Table 2). In addition, we did not find any significant correlation between ALFF, CBF values and clinical characteristics including onset age of dysmenorrhea, VAS-A and VAS-P in PD patients (all p>0.05).
 | Table 2 Brain regions with changed ALFF and CBF values in PD patients compared with HCs |
Discussion
The main finding of the current study was that the menstrual pain caused not only altered ALFF, but also activation of CBF in the brain by using functional MRI. Comparing to HCs, the ALFF values decreased in the right brainstem, left precuneus, right limbic lobe including the hippocampus and parahippocampal gyrus and right cerebellum posterior lobe. The increase of regional CBF was mainly observed in the right inferior frontal gyrus, right precentral gyrus, and right superior temporal gyrus. Those altered brain regions were involved in many aspects of pain modulation, such as emotion, cognition, sensory function and so on, which might contribute to the pathophysiology of PD.13
As is known, the brainstem has been identified as associated with menstrual pain, especially periaqueductal gray (PAG). Accumulating evidence suggested that PAG matter, a key structure in the neuraxis of pain modulatory systems, was widely recognized as playing an essential role in PD.15,17 A previous study showed decreased FC between PAG and DMN in PD patients, which might indicate maladaptive neuroplasticity of the descending pain modulatory systems. The authors considered that dysfunction might make PD females in the later life more vulnerable to many functional disorders and chronic pain conditions.17 The current research found decreased ALFF values in right brainstem although not in PAG. It possibly implied that PD patients should be treated aggressively as early as possible.
The DMN is recognized as an essential network composed of several brain regions that preferentially activates in the resting state. The precuneus as a major region of the DMN played a key role in a wide range of advanced cognitive functions, such as self-related processing, episodic memory, and aspects of consciousness.20 Another study reported precuneus was also associated with the perception of pain, and endogenous pain modulation.27 An fMRI study demonstrated alterations in pain process and modulation regions by examining ALFF and FC changes in DMN including the precuneus, dorsomedial prefrontal cortex, and anterior cingulate cortex and thalamus.11 Conversely, the value of ALFF was decreased in the precuneus in the present study. We believed that this might be attributed to, firstly, that the fMRI was performed during the subjects' pain-free condition in the previous study, while the patients of this study were in the menstrual phase. Another reason was different sample sizes, as the number of patients in this study was less than in the previous research.
Apart from the above-mentioned areas, the hippocampus and adjacent regions are also often reported as part of the DMN. The hippocampus and parahippocampal gyrus had been a great contribution to central processing of anxiety-related behaviors and long-term memory.28 However, previous evidence from animal and human studies suggested that the hippocampus was also significantly associated with pain processing. In addition, the persistent or chronic pain states could produce great effects on hippocampal morphology, metabolism and function.29 The work on the hippocampal slices of rats demonstrated that peripheral persistent nociception tends to result in various forms of central neural plasticity in the pain-related brain areas,29,30 which probably results in long-term dysfunction of synaptic transmission and modulation at different levels of the central nervous system. Our findings revealed decreased ALFF in the hippocampus during menstrual period in PD patients, which suggested that the hippocampus might participate in pain processing. In addition, a PET study demonstrated that hypometabolic areas mostly located in the limbic system were closely related to emotion in PD patients.23 Therefore, our findings might suggest that limbic lobe dysfunction in the processing of pain or uncomfortable sensations may be related to the generating mechanism of PD.
The cerebellum is traditionally thought to be a motor processing related brain structure, but it has been very limited in the research on the pain. A meta-analysis of neuroimaging studies has confirmed the cerebellum was involved in pain or pain modulation, and possible functional processes were associated with all dimensions in regards to pain including emotion, cognition, sensory function, and motor control.31,32 An fMRI study suggested that pain-related cerebellar activation induced by innocuous and noxious thermal stimuli was reported in hemispheric lobule VI and the anterior vermis varied with pain intensity; these brain regions could reflect pain perception and were assumed to be related to sensory-motor integration and the expression of emotional behavior.33 Another neuroimaging study showed different cerebellar activation patterns to brush and heat stimuli between healthy and neuropathic pain subjects, including areas involved in secondary sensory processing (lobule VIIIB), and cognition (lobules VIIB, Crus I, Crus II).34 The present study produced an interesting result that decreased ALFF values were found in the right cerebellum posterior lobe (mainly lobule VIII,and partly lobule VIIB). The results suggested the cerebellum might participate in secondary sensory processing and cognition during menstrual pain. The increased glycometabolism in the cerebellum was mentioned in a study that indicated acupuncture could relieve pain by activating the area involved in pain, but the specific role of the cerebellum was not detailed.35
In this study, altered ALFF values were found to be involved in pain-related brain regions, whereas increased CBF was observed in the right frontal lobe and temporal lobe. Previous investigations36 indicated that the frontal lobe almost integrated all the sensory perception and had a lot of association with the limbic system, especially the prefrontal cortex. This is the advanced part of the brain development, performing processing and integration for sensory and motorial information of the whole brain. Furthermore, a study suggested that the pain of PD during the menstrual phase could correlate with a dysfunctional pain modulatory system involving the motor cortex.15 It was explained why high perfusion occurred in the inferior frontal gyrus and precentral gyrus in our study. Besides, increased CBF values were observed in the superior temporal gyrus, which was involved in pain control. It might also be related to the noise of the MRI machine and the tension and anxiety caused by the test.
The present results demonstrated the regions of abnormal ALFF were not consistent with that of changed CBF in menstrual phase in PD patients. Due to the different theories of ALFF and CBF, they had different sensitivities in detecting changes in neuronal activity in PD. Besides, 2D PASL did not cover the whole brain, so it is not used to assess subtentorial brain tissue. Some regions of decreased ALFF were located in the subtentorial part. Though 2D PASL has been more widely applied because of its speed and sensitivity, it yielded fewer activation clusters than other labeling approaches, particularly 3D ASL.37,38 There were other several limitations in this study. First, the PD subjects were in the menstrual phase. We did not compare brain function changes in different menstrual cycles. Second, we only measured the changes of ALFF in given brain regions in rs-fMRI. Functional connectivity methods should be concerned to reflect the network integration effect in the follow-up study. Finally, the number of our cases was small, and we will recruit more patients in the future.
In conclusion, the current results demonstrated the ongoing menstrual pain in PD patients caused altered ALFF and CBF values, which were involved in various aspects of pain and pain modulation. We hoped that the combination of rs-fMRI and ASL MRI might provide complementary information to help improve understanding of the pathophysiology of PD.
Acknowledgments
This study was supported by National Natural Science Foundation of China (NSFC): The central analgesic mechanism of moxibustion in treating Primary Dysmenorrhea: a multimodal functional MRI study (Project Number: 81503652).
Author contributions
Ya-Nan Zhang performed MRI data acquisition, data analysis and manuscript writing. Jian-Wei Huo, Yi-Ran Huang and Ying Hao contributed to experimental design, financial support and revision of the manuscript. Zi-Yue Chen carried out clinical data collection and arrangement. All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Iacovides S, Avidon I, Baker FC. What we know about primary dysmenorrhea today: a critical review. Hum Reprod Update. 2015;21(6):762–778. doi:10.1093/humupd/dmv039
2. Potur DC, Bilgin NC, Komurcu N. Prevalence of dysmenorrhea in university students in Turkey: effect on daily activities and evaluation of different pain management methods. Pain Manag Nurs. 2014;15(4):768–777. doi:10.1016/j.pmn.2013.07.012
3. Abu HHA, Mitaeb AA, Al-Hamshri S, Sweileh WM. Prevalence of dysmenorrhea and predictors of its pain intensity among Palestinian female university students. BMC Womens Health. 2018;18(1):18. doi:10.1186/s12905-018-0516-1
4. Ameade EPK, Amalba A, Mohammed BS. Prevalence of dysmenorrhea among University students in Northern Ghana; its impact and management strategies. BMC Womens Health. 2018;18(1):39. doi:10.1186/s12905-018-0532-1
5. Yesuf TA, Eshete NA, Sisay EA. Dysmenorrhea among University Health Science Students, Northern Ethiopia: impact and associated factors. Int J Reprod Med. 2018;2018:9730328. doi:10.1155/2018/9730328
6. Chiu MH, Hsieh HF, Yang YH, Chen HM, Hsu SC, Wang HH. Influencing factors of dysmenorrhoea among hospital nurses: a questionnaire survey in Taiwan. BMJ Open. 2017;7(12):e017615. doi:10.1136/bmjopen-2017-017615
7. Bernardi M, Lazzeri L, Perelli F, Reis FM, Petraglia F. Dysmenorrhea and related disorders. F1000Res. 2017;6(F1000 Faculty Rev):1645. doi:10.12688/f1000research.10493.2
8. Fang L, Gu C, Liu X, et al. Metabolomics study on primary dysmenorrhea patients during the luteal regression stage based on ultra performance liquid chromatography coupled with quadrupole‑time‑of‑flight mass spectrometry. Mol Med Rep. 2017;15(3):1043–1050. doi:10.3892/mmr.2017.6116
9. Tu CH, Niddam DM, Chao HT, et al. Abnormal cerebral metabolism during menstrual pain in primary dysmenorrhea. Neuroimage. 2009;47(1):28–35. doi:10.1016/j.neuroimage.2009.03.080
10. Liu P, Yang J, Wang G, et al. Altered regional cortical thickness and subcortical volume in women with primary dysmenorrhoea. Eur J Pain. 2016;20(4):512–520. doi:10.1002/ejp.753
11. Liu P, Liu Y, Wang G, et al. Aberrant default mode network in patients with primary dysmenorrhea: a fMRI study. Brain Imaging Behav. 2017;11(5):1479–1485. doi:10.1007/s11682-016-9627-1
12. Liu J, Liu H, Mu J, et al. Altered white matter microarchitecture in the cingulum bundle in women with primary dysmenorrhea: A tract-based analysis study. Hum Brain Mapp. 2017;38(9):4430–4443. doi:10.1002/hbm.23670
13. Jin L, Yang X, Liu P, et al. Dynamic abnormalities of spontaneous brain activity in women with primary dysmenorrhea. J Pain Res. 2017;10:699–707. doi:10.2147/JPR.S121286
14. Wu TH, Tu CH, Chao HT, et al. Dynamic changes of functional pain connectome in women with primary dysmenorrhea. Sci Rep. 2016;6:24543. doi:10.1038/srep24543
15. Kutch JJ, Tu FF. Altered brain connectivity in dysmenorrhea: pain modulation and the motor cortex. Pain. 2016;157(1):5–6. doi:10.1097/j.pain.0000000000000364
16. Liu P, Liu Y, Wang G, et al. Changes of functional connectivity of the anterior cingulate cortex in women with primary dysmenorrhea. Brain Imaging Behav. 2017;11(5):1479–1485. doi:10.1007/s11682-016-9627-1
17. Wei SY, Chao HT, Tu CH, et al. Changes in functional connectivity of pain modulatory systems in women with primary dysmenorrhea. Pain. 2016;157(1):92–102. doi:10.1097/j.pain.0000000000000340
18. Zou QH, Zhu CZ, Yang Y, et al. An improved approach to detection of amplitude of low-frequency fluctuation (ALFF) for resting-state fMRI: fractional ALFF. J Neurosci Methods. 2008;172(1):137–141. doi:10.1016/j.jneumeth.2008.04.012
19. Chen X, Lu B, Yan CG. Reproducibility of R-fMRI metrics on the impact of different strategies for multiple comparison correction and sample sizes. Hum Brain Mapp. 2018;39(1):300–318. doi:10.1002/hbm.23843
20. Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129(Pt 3):564–583. doi:10.1093/brain/awl004
21. Peyron R, Laurent B, García-Larrea L. Functional imaging of brain responses to pain. A review and meta-analysis (2000). Neurophysiol Clin. 2000;30(5):263–288.
22. Simon AB, Buxton RB. Understanding the dynamic relationship between cerebral blood flow and the BOLD signal: implications for quantitative functional MRI. Neuroimage. 2015;116:158–167. doi:10.1016/j.neuroimage.2015.03.080
23. Gong P, Zhang MM, Jiang LM, et al. 18F-FDG PET brain imaging research in primary dysmenorrhea patients. Chin J Integr Med. 2006;26(2):114.
24. Owen DG, Bureau Y, Thomas AW, Prato FS, St. Lawrence KS. Quantification of pain-induced changes in cerebral blood flow by perfusion MRI. Pain. 2008;136(1–2):85–96. doi:10.1016/j.pain.2007.06.021
25. Ma X, Wang D, Zhou Y, et al. Sex-dependent alterations in resting-state cerebral blood flow, amplitude of low-frequency fluctuations and their coupling relationship in schizophrenia. Aust N Z J Psychiatry. 2016;50(4):334–344. doi:10.1177/0004867415601728
26. Zhu J, Zhuo C, Xu L, Liu F, Qin W, Yu C. Altered coupling between resting-state cerebral blood flow and functional connectivity in schizophrenia. Schizophr Bull. 2017;43(6):1363–1374. doi:10.1093/schbul/sbx051
27. Zyloney CE, Jensen K, Polich G, et al. Imaging the functional connectivity of the periaqueductal gray during genuine and sham electroacupuncture treatment. Mol Pain. 2010;6:80. doi:10.1186/1744-8069-6-80
28. Strange BA, Witter MP, Lein ES, Moser EI. Functional organization of the hippocampal longitudinal axis. Nat Rev Neurosci. 2014;15(10):655–669. doi:10.1038/nrn3785
29. Liu MG, Chen J. Roles of the hippocampal formation in pain information processing. Neurosci Bull. 2009;25(5):237–266. doi:10.1007/s12264-009-0905-4
30. Zhao XY, Liu MG, Yuan DL, et al. Nociception-induced spatial and temporal plasticity of synaptic connection and function in the hippocampal formation of rats: a multi-electrode array recording. Mol Pain. 2009;5:55.
31. Moulton EA, Schmahmann JD, Becerra L, Borsook D. The cerebellum and pain: passive integrator or active participator. Brain Res Rev. 2010;65(1):14–27. doi:10.1016/j.brainresrev.2010.05.005
32. Allen G, McColl R, Barnard H, Ringe WK, Fleckenstein J, Cullum CM. Magnetic resonance imaging of cerebellar-prefrontal and cerebellar-parietal functional connectivity. Neuroimage. 2005;28(1):39–48. doi:10.1016/j.neuroimage.2005.06.013
33. Helmchen C, Mohr C, Erdmann C, Binkofski F. Cerebellar neural responses related to actively and passively applied noxious thermal stimulation in human subjects: a parametric fMRI study. Neurosci Lett. 2004;361(1–3):237–240. doi:10.1016/j.neulet.2003.12.017
34. Borsook D, Moulton EA, Tully S, Schmahmann JD, Becerra L. Human cerebellar responses to brush and heat stimuli in healthy and neuropathic pain subjects. Cerebellum. 2008;7(3):252–272. doi:10.1007/s12311-008-0011-6
35. Gong P, Zhang MM, Wang Q, et al. [Effect of acupuncture at Sanyinjiao (SP 6) on glucose metabolism in the patient of dysmenorrhea]. Zhongguo Zhen Jiu. 2006;26(1):51–55.
36. Casey KL. Forebrain mechanisms of nociception and pain: analysis through imaging. Proc Natl Acad Sci U S A. 1999;96(14):7668–7674.
37. Vidorreta M, Wang Z, Rodríguez I, Pastor MA, Detre JA, Fernández-Seara MA. Comparison of 2D and 3D single-shot ASL perfusion fMRI sequences. Neuroimage. 2013;66:662–671. doi:10.1016/j.neuroimage.2012.10.087
38. Dolui S, Vidorreta M, Wang Z, et al. Comparison of PASL, PCASL, and background-suppressed 3D PCASL in mild cognitive impairment. Hum Brain Mapp. 2017;38(10):5260–5273. doi:10.1002/hbm.23732
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.