Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 18
Airway and Systemic Immune Responses Following the Third COVID-19 Vaccination in COPD Patients
Authors Southworth T, Jackson N, Singh D
Received 31 July 2023
Accepted for publication 30 November 2023
Published 20 December 2023 Volume 2023:18 Pages 3027—3036
DOI https://doi.org/10.2147/COPD.S433269
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Thomas Southworth,1,2 Natalie Jackson,2 Dave Singh1– 3
1Division of Infection, Immunity and Respiratory Medicine, University of Manchester, Manchester, UK; 2Medicines Evaluation Unit, Manchester, UK; 3Manchester University NHS Foundation Trust, Manchester, UK
Correspondence: Thomas Southworth, Medicines Evaluation Unit, The Langley Building, Southmoor Road, Manchester, M23 9QZ, UK, Tel + 44 161 946 4066, Email [email protected]
Introduction: Booster vaccinations are required to maintain protection against COVID-19. COPD patients are at higher risk of developing severe illness following SARS-CoV-2 infection. Previous cross-sectional analysis after the second COVID-19 booster showed similar immune responses in COPD patients and controls, but pre-vaccination samples were not available. This longitudinal study evaluated systemic and airway immune responses in COPD patients using samples obtained pre- and post-third COVID-19 vaccination.
Methods: Twelve COPD patients were recruited, with plasma, nasal and sputum (n = 10) samples collected pre-vaccination and 4- and 14-weeks post vaccination. Samples were analyzed for anti-spike IgA and IgG and cellular immunity. The ability of plasma and nasal samples to block ACE2-spike protein interaction was assessed for Wild type, Delta, and Omicron spike variants.
Results: Vaccinations increased anti-spike IgG in plasma (p < 0.001), nasal (IgG p < 0.001) and sputum (p = 0.002) samples, IgA in plasma (p < 0.001) and blood cellular immunity (p = 0.001). Plasma and nasal anti-spike IgA levels correlated (rho: 0.6, p = 0.02), with similar results for IgG (rho: 0.79, p = 0.003). Post-vaccination nasal (p = 0.002) and plasma (p < 0.001) samples were less effective at blocking Omicron spike binding to ACE2 compared to the Wild type spike variant.
Discussion: Airway and systemic immune responses against SARS-CoV-2 increased in COPD patients following a third COVID-19 vaccination. Nasal and systemic responses in COPD patients were less effective against Omicron variant compared to previous variants.
Keywords: COVID-19, vaccination, chronic obstructive pulmonary disease, omicron, airway, immunoglobulin
Introduction
World-wide vaccination programs against severe acute respiratory coronavirus 2 (SARS-CoV-2) have reduced rates of severe COVID-19 and mortality.1 These vaccines provide protection by stimulating systemic humoral and cellular immunity,2,3 with booster doses used to ensure maintained host immunity.4,5 Chronic obstructive pulmonary disease (COPD) patients are at higher risk of severe illness and mortality following SARS-CoV-2 infection.6 Sub-optimal immune responses to vaccination may occur in COPD patients as humoral responses, including immune memory, are altered in COPD patients compared to healthy subjects.7,8 Pre-pandemic, coronaviruses were associated with approximately 4% of exacerbations in COPD patients,9 but this may increase as COVID-19 becomes endemic within the population. The ability of vaccines to inhibit SARS-CoV-2 airway infections by inducing robust airway responses may prevent exacerbations.
We previously evaluated systemic and airway immune responses in COPD patients and healthy subjects following the second dose of SARS-CoV-2 vaccination;10 vaccination responses were similar between the two groups. However, the study only measured post-vaccination responses without the availability of paired pre-vaccination samples from the same subject, limiting the interpretation of the results.
The SARS-CoV-2 Spike glycoprotein interacts with the angiotensin-converting enzyme 2 (ACE2) receptor allowing for cellular attachment and internalization.11 The vaccinations used for the first, second and third doses in the UK, and elsewhere, were based on the Spike protein of the original wild-type SARS-CoV-2 strain that was first reported in Wuhan (China) in 2019.12–14 Since late 2021, the Omicron variant has become a variant of concern worldwide,15 with at least 30 amino acid changes to the Spike protein compared to the wild-type strain.16 While there is a lower risk of severe disease and death following infection with Omicron than previous SARS-CoV-2 variants, the very high levels of transmission continue to pose demands on healthcare systems and may lead to significant morbidity, particularly in vulnerable populations.17
In this study, we prospectively evaluated the immune responses to the third COVID-19 vaccination, with a longitudinal sample collection including pre- and post-booster airway and systemic sampling. The aims were to (1) evaluate in COPD patients the immune response to the third vaccination in different anatomical locations, namely the blood, nose, and lungs and (2) evaluate protection against the Omicron variant, which was becoming the dominant variant of concern in the United Kingdom (UK) during the role out of the third vaccine dose.
Methods
Subjects
COPD patients (n = 12) were recruited prior to receiving the third dose of SARS-CoV-2 vaccination; all the patients had been enrolled in our previous study investigating immune responses following a second COVID vaccination.10 The design of the study is summarised in Figure 1. Blood, plasma, nasal and sputum samples were collected up to 6 weeks prior to vaccination and 4- and 14-weeks post vaccination. Three COPD patients developed COVID-19 after donating the 4-week samples, so 14-week samples were not collected. COPD was diagnosed according to GOLD criteria,18 including a post bronchodilator first second of forced expiration/forced vital capacity (FEV1 / FVC) ratio of <0.7 and had a smoking history of >10 pack years. All patients were over 40 years of age and reported no history of SARS-CoV-2 infection, which was supported by the exclusion of patients who had a positive result for serum antibodies against SARS-CoV-2 nucleocapsid protein (Roche anti-SARS-CoV-2 assay, performed by The Doctors Lab, London, UK). Subjects all provided written informed consent using a protocol that complied with the Declaration of Helsinki and was approved by the local ethics committee (10/H1003/108).
 |
Figure 1 Study design schematic. |
Antibody Assessments
Plasma, nasal epithelial lining fluid and sputum supernatants were collected, and anti-spike immunoglobulin (Ig) A and IgG levels measured, as previously described.10 For plasma, the ELISAs (AESKU Diagnostics, Wendelsheim, Germany) were performed as per the manufacturer’s instructions using the supplied IgA and IgG standards, which were pre-prepared in a plasma-like buffer, for quantification. As this plasma-like buffer did not reflect the matrix composition of nasal or sputum samples, the Aesku ELISAs were modified using recombinant anti-spike IgA and IgG standards (Native Antigen Company, Oxford, UK) made up in PBS containing 1% bovine serum albumin and 0.05% Tween-20 (Sigma-Aldrich, Poole, UK). Initially, a 12-point immunoglobulin standard curve, with a top concentration of 1000 ng/mL, was used with the modified assays to identify the exponential component of the standard curve, with 7 concentrations of standards being chosen to cover this region in future assays. The limit of detection (LOD) for the assays was identified using multiple blanks, with the lower level of quantification (LLOQ) being the standard point above the LOD plus three standard deviations. To assess the performance of the modified assays, parallelism experiments were performed using multiple dilutions of samples collected from subjects with a recent history of COVID-19. Results suggested matrix interference with the assay when nasal and sputum samples were used neat, with an acceptable minimal required dilution of 2-fold for both sample types.
The LLOQ for anti-spike IgA and IgG in plasma were 53.1 and 1625 U/mL, respectively, and in nasal and sputum samples anti-spike IgA and IgG were both 0.8 ng/mL. To enable statistical analysis, samples with levels below the LLOQ were assigned the arbitrary value of half LLOQ. Using plasma samples collected prior to the COVID-19 pandemic, AESKU has defined the serological positivity threshold of 12U/mL for both anti-spike IgA and IgG in plasma. The manufacturer states that the sensitivity and specificity of the anti-spike IgA assay in plasma are 94.6% and >99%, respectively, and 98.6% and >99% for the IgG assay. Due to insufficient numbers of samples collected pre-COVID and from recently infected subjects, positivity thresholds, and diagnostic sensitivities and specificities, were not determined for nasal or sputum assays.
Cellular Immunity
Heparinized blood was collected for cellular immunity assessment using Euroimmun’s Quan-T-Cell assay and associated IFNγ ELISA (Lübeck, Germany), which measures SARS-CoV-2 spike protein-induced IFNγ from blood T-cells. Blood was treated with or without spike protein for 20 hours prior to collection of plasma for IFNγ analysis. The assays were performed as per the manufacturer’s instructions with an IFNγ limit of quantification of 31.07mIU/mL. Based on blood samples collected from healthy individuals at the start of the pandemic, with no history of COVID-19, the manufacturer-defined serological positivity threshold for the assay is 200 mIU/mL.
SARS-CoV-2 Variant Inhibitor Assay
The ability of 4-week post-vaccine plasma and nasal samples to block the interaction of human ACE2 with recombinant SARS-CoV-2 spike protein from Wild type, Delta and Omicron variants of the virus was assessed using an ELISA-based pseudo-neutralization assay (SARS-CoV-2 variant inhibitor screening kit, Biotechne, Abingdon, UK). Samples were assessed at 6 different dilutions (nasal: 4-fold dilutions from neat; plasma: 3-fold dilutions from an initial 27-fold dilution), with results reported as half-maximal inhibitory dilution (ID50). Samples where the most concentrated dilution did not inhibit ACE2/spike interaction by 50% were assigned the arbitrary value of 1 for nasal samples and 27 for plasma, which were the most concentrated dilutions assessed, to enable statistical analysis.
Statistics
Distribution of data was assessed using the D’Agostino and Pearson test. Comparisons between pre- and post-third vaccinations and between second and third vaccine response were by Wilcoxon test. Correlations were assessed by Spearman’s rank test. ID50s of ACE2-spike interaction were calculated from a four-parameter logistic sigmoidal curve for each sample. Comparisons in the inhibition of the various spike variants were assessed by Friedman Test, with Dunn’s multiple comparison test comparing Wild type with Delta and Omicron. All analyses were carried out using GraphPad Prism version 9.4.1 (San Diego, California, USA).
Results
Subjects
The clinical characteristics of the patients are summarized in Table 1 and Supplementary Table 1. A mixture of COPD patients with GOLD grades 1–3 were included. The majority of patients received Comirnaty as the third vaccine dose, 83.3% (10/12). The median time interval between the second and third vaccine doses was 201 days. Baseline sputum samples were successfully collected from 10 patients.
 |
Table 1 Clinical Characteristics of Patients |
Humoral and Cellular Immunity Responses Post Third Vaccination
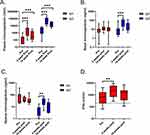
Levels of anti-spike IgA and IgG in plasma were above the kit manufacturers-defined positivity threshold (12 IU/mL) for all pre- and post-vaccination samples tested (Figure 2A). For cellular immunity, 9 of the 12 pre-vaccination samples were above the positivity threshold (200 mIU/mL), with all 4-week post-vaccination samples, and 8 of the 14-week post-vaccination samples, being positive (Figure 2D). There were increases in anti-spike IgG levels in plasma (p < 0.0001), nasal (p = 0.0005) and sputum (p = 0.0019) samples, and cellular immunity (p = 0.0010) responses in samples collected 4-weeks post vaccination compared to pre-vaccine samples (Figure 2). For anti-spike IgA levels, there were increases in plasma IgA levels at 4 weeks (p = 0.0004), but no increase in nasal (p = 0.95) or sputum (p = 0.69) anti-spike IgA. Vaccine-induced immunity declined between 4- and 14-weeks post vaccination, with only plasma anti-spike IgA (p = 0.0489) and IgG (p = 0.0368) levels and nasal IgG levels remaining higher than pre-vaccination after 14-weeks. There were no significant differences between the 4-week and 14-week levels for any of the immune responses measured.
Anti-spike IgA and IgG levels in plasma and nasal samples collected 4-weeks post vaccination correlated (Figure 3A and B. IgA: rho = 0.657, p = 0.024; IgG; rho = 0.790, p = 0.003). Sputum anti-spike IgG levels correlated with plasma IgG levels (rho = 0.76, p = 0.015; Figure 3C), while sputum and plasma anti-spike IgA levels did not correlate. Baseline plasma levels of anti-spike IgA and IgG did not correlate with levels in baseline nasal or sputum samples.
Neutralization Antibody Responses Against SARS-CoV-2 Variants
To investigate the effectiveness of the third vaccination against different SARS-CoV-2 variants of concern, we performed an ELISA-based binding assay to assess the ability of 4-week post-vaccination plasma and nasal samples to block the interaction between the human ACE2 receptor and spike proteins from Wild type, Delta, and Omicron variants. For plasma and nasal samples, inhibition of Omicron spike binding was lower compared to Wild type and Delta spike variants (Figure 4; median ID50 values: Plasma: Wild type 986; Delta 1322 and Omicron 164; Nasal: Wild type 3.4; Delta 2.8 and Omicron 1.0).
Discussion
In COPD patients, airway and systemic immune responses against SARS-Cov-2 increased following a third COVID-19 vaccination. This is an important finding as COPD patients are at increased risk of severe illness following SARS-CoV-2 infection.6 Plasma and nasal samples collected post-vaccination had lower ability to block Omicron variant Spike protein interaction with ACE2 compared to Spike proteins from earlier SARS-CoV-2 variants.
Our previous cross-sectional study examined systemic and airway immune responses following the second vaccination dose.10 We reported that vaccine-induced immune responses in COPD patients were similar to those in healthy subjects, but the interpretation of the results was influenced by the lack of paired pre-vaccination measurements. Here, we report a longitudinal study design with measurements pre- and post-third vaccination in COPD patients, with results demonstrating increases in anti-COVID immune responses in both systemic and airway samples. Chaiwong et al reported that systemic neutralizing antibody and T-cell responses were increased following ChAdOx-1 vaccinations and the levels were similar in COPD patients and healthy subjects.19 In this study, we also evaluated nasal and sputum responses; vaccine induced anti-spike IgG responses were observed across plasma, nasal and sputum samples, but the anti-spike IgA response was only observed in the plasma.
Nasal and plasma anti-spike IgG levels correlated, which was also shown in our previous study and by Havervall et al.10,20 These findings corroborate the spill-over effect of systemic IgG to the mucosa.21 Unlike our previous study, post-vaccination nasal IgA levels did correlate with plasma levels. In contrast, IgA levels in plasma and nasal samples prior to the third vaccination did not correlate (Rho: −0.2, p = 0.45). This post-vaccination association suggests that systemic “spill over” of anti-spike IgA may be occurring, but this “spill over” is likely to be relatively minor as nasal IgA levels remained low post-vaccination and without any significant upregulation at 4-weeks. In contrast to this minor change following vaccination, induction of nasal anti-spike IgA following infection with the SARS-CoV-2 omicron variant has been linked to local mucosal IgA production.22
While nasal anti-spike IgA levels are increased following COVID-19,20,22 levels were poorly boosted in the nasal mucosa of healthcare workers following a fourth dose, irrelevant of previous SARS-CoV-2 infection.23 We have shown similar results for nasal and sputum samples in COVID-19 naïve COPD patients following a third vaccination. Mucosal anti-spike IgA, but not IgG, is important for preventing SARS-CoV-2 Omicron variant infection.20 Future vaccines that expose the airways to COVID-19 antigens may reduce infection rates.
A notable observation was the decline in spike-specific antibodies by week 14, also observed in other studies following a third vaccine dose.24–26 While protection against hospitalization due to COVID infections wanes following vaccination, protection levels are still 86% effective at 2–4 months following a 3rd dose of mRNA vaccine,27 suggesting that declining plasma antibodies within 14 weeks may not directly relate to clinical protection. Memory B-cells produce little secreted antibodies under normal conditions but respond rapidly when challenged.28 Muecksck et al showed that COVID booster vaccinations lead to an expansion and diversification of memory B-cells.29 This diversification led to an increase in memory B-cell antibody breadth and potency against variants that were not specifically targeted by the vaccine, with the proportion of Omicron neutralising antibodies increasing from 15% after the 2nd vaccine to 50% after a 3rd vaccine dose. Thus, an enhanced memory B-cell response is likely to be a key mechanism by which a 3rd vaccine dose offers protection against severe disease.
While causing less severe acute illness,30 the high transmission rates of the Omicron variant are a major healthcare concern. Vaccines developed using the wild-type spike antigen induce systemic neutralizing antibody responses that are less-effective against the Omicron variant compared to previous variants of concern.19,31,32 We used plasma and nasal samples to investigate this issue in COPD patients and also observed impaired neutralizing antibody responses against the Omicron variant. This impaired neutralization response to the Omicron variant is similar in plasma from COPD patients and age-matched healthy subjects.19 Bivalent vaccines, containing antigens against both the wild-type and Omicron spike proteins, have been approved by regulators.33,34 These vaccines induce greater systemic neutralizing antibody responses against the Omicron variants than the original monovalent vaccines.33 Further research is required to assess if these bivalent vaccines boost airway defenses against the Omicron variant.
A limitation of this study was the small sample size. One reason for the limited sample size was that study recruitment coincided with the lifting of COVID-19 restrictions in the UK and the emergence of the Omicron variant, causing increased infection rates. However, the results we have observed in this small COPD cohort align with those seen in larger general population cohorts, such as vaccine-induced increases in nasal anti-spike IgG, but minimal changes in nasal anti-spike IgA35,36 and lower anti-Omicron responses in blood compared to those against Wild-type and Delta spike variants,37,38 increasing confidence in their validity. While attempts were made to exclude subjects with a history of COVID-19, including screening for serum antibodies against the SARS-CoV-2 nucleocapsid protein, enrollment of subjects with a history of mild non-symptomatic infection may have occurred. As positivity thresholds for the nasal and sputum assays used in this study have not been defined, it was not possible to state if the immune responses measured were above pre-pandemic levels. While we have shown that anti-spike IgG levels are increased in airway samples from COPD patients following vaccination and shown that post-vaccine samples can inhibit interaction between the spike protein and the ACE2 receptor, further studies will be required to see if these vaccines reduce coronavirus associated exacerbations in COPD.
Conclusion
This study has provided further evidence that SARS-CoV-2 vaccines induce immune responses in COPD patients, which is an important finding as it is recommended that COPD patients continue to receive seasonal vaccinations against COVID-19. We have also demonstrated that vaccines based on the wild-type SARS-CoV-2 spike protein induce nasal, and systemic responses in COPD patients that are less effective against Omicron variant compared to previous variants of concern.
Abbreviations
ACE2, angiotensin-converting enzyme 2; BMI, Body mass index; CAT, Chronic obstructive pulmonary disease assessment test; COPD, Chronic obstructive pulmonary disease; ELISA, Enzyme-linked immunosorbent assay; FEV1, First second of forced expiration; FVC, Forced vital capacity; GOLD, Global Initiative for Chronic Obstructive Pulmonary Disease; ID50, Half maximal inhibitory dilution; IFNγ, Interferon gamma; Ig A/G, Immunoglobulin A/G; mRNA, Messenger ribonucleic acid; ng/mL, Nanogram per millilitre; SARS-CoV-2, Severe acute respiratory coronavirus 2; U/mL, Units per millilitre; UK, United Kingdom.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Ethics Approval and Informed Consent
Subjects all provided written informed consent using a protocol that complied with the Declaration of Helsinki and was approved by the local ethics committee (North West Research Ethic Committee; reference: 10/H1003/108).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research was supported by the Medicines Evaluation Unit and the North West Lung Centre Charity, Manchester, UK. DS receives support from the NIHR Manchester Biomedical Research Centre. This report is independent research, and the views expressed in this publication are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
Disclosure
DS reports personal consulting fees from Aerogen, AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, CSL Behring, Epiendo, Genentech, GlaxoSmithKline, Glenmark, Gossamerbio, Kinaset, Menarini, Novartis, Orion, Pulmatrix, Sanofi, Synairgen, Teva, Theravance Biopharm and Verona Pharma. TS and NJ declare no competing interests for this work.
References
1. Watson OJ, Barnsley G, Toor J, et al. Global impact of the first year of COVID-19 vaccination: a mathematical modelling study. Lancet Infect Dis. 2022;22(9):1293–1302. doi:10.1016/S1473-3099(22)00320-6
2. Gross R, Zanoni M, Seidel A, et al. Heterologous ChAdOx1 nCoV-19 and BNT162b2 prime-boost vaccination elicits potent neutralizing antibody responses and T cell reactivity against prevalent SARS-CoV-2 variants. EBioMedicine. 2022;75:103761. doi:10.1016/j.ebiom.2021.103761
3. Munro APS, Feng S, Janani L, et al. Safety, immunogenicity, and reactogenicity of BNT162b2 and mRNA-1273 COVID-19 vaccines given as fourth-dose boosters following two doses of ChAdOx1 nCoV-19 or BNT162b2 and a third dose of BNT162b2 (COV-BOOST): a multicentre, blinded, Phase 2, randomised trial. Lancet Infect Dis. 2022;22(8):1131–1141. doi:10.1016/S1473-3099(22)00271-7
4. Kherabi Y, Launay O, Luong Nguyen LB. COVID-19 vaccines against omicron variant: real-world data on effectiveness. Viruses. 2022;14(10):2086. doi:10.3390/v14102086
5. Shao W, Chen X, Zheng C, et al. Effectiveness of COVID-19 vaccines against SARS-CoV-2 variants of concern in real-world: a literature review and meta-analysis. Emerg Microbes Infect. 2022;11(1):2383–2392. doi:10.1080/22221751.2022.2122582
6. Singh D, Mathioudakis AG, Higham A. Chronic obstructive pulmonary disease and COVID-19: interrelationships. Curr Opin Pulm Med. 2022;28(2):76–83. doi:10.1097/MCP.0000000000000834
7. Parpaleix A, Boyer L, Wiedemann A, et al. Impaired humoral and cellular immune responses to influenza vaccination in chronic obstructive pulmonary disease patients. J Allergy Clin Immunol. 2017;140(6):1754–1757 e6. doi:10.1016/j.jaci.2017.07.038
8. Di Stefano A, Dossena F, Gnemmi I, et al. Decreased humoral immune response in the bronchi of rapid decliners with chronic obstructive pulmonary disease. Respir Res. 2022;23(1):200. doi:10.1186/s12931-022-02125-3
9. Zwaans WA, Mallia P, van Winden MEC, et al. The relevance of respiratory viral infections in the exacerbations of chronic obstructive pulmonary disease—a systematic review. J Clin Virol. 2014;61(2):181–188. doi:10.1016/j.jcv.2014.06.025
10. Southworth T, Jackson N, Singh D. Airway immune responses to COVID-19 vaccination in COPD patients and healthy subjects. Eur Respir J. 2022;60(2):2200497. doi:10.1183/13993003.00497-2022
11. Zamorano Cuervo N, Grandvaux N. ACE2: evidence of role as entry receptor for SARS-CoV-2 and implications in comorbidities. Elife. 2020;9. doi:10.7554/eLife.61390
12. Folegatti PM, Ewer KJ, Aley PK, et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet. 2020;396(10249):467–478. doi:10.1016/S0140-6736(20)31604-4
13. Walsh EE, Frenck RW, Falsey AR, et al. Safety and immunogenicity of two RNA-based covid-19 vaccine candidates. N Engl J Med. 2020;383(25):2439–2450. doi:10.1056/NEJMoa2027906
14. Jackson LA, Anderson EJ, Rouphael NG, et al. An mRNA vaccine against SARS-CoV-2 — preliminary report. N Engl J Med. 2020;383(20):1920–1931. doi:10.1056/NEJMoa2022483
15. Farahat RA, Abdelaal A, Umar TP, et al. The emergence of SARS-CoV-2 Omicron subvariants: current situation and future trends. Infez Med. 2022;30(4):480–494. doi:10.53854/liim-3004-2
16. D’Arminio N, Giordano D, Scafuri B, et al. In silico analysis of the effects of omicron spike amino acid changes on the interactions with human proteins. Molecules. 2022;27(15):4827. doi:10.3390/molecules27154827
17. Nyberg T, Ferguson NM, Nash SG, et al. Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B.1.1.529) and delta (B.1.617.2) variants in England: a cohort study. Lancet. 2022;399(10332):1303–1312. doi:10.1016/S0140-6736(22)00462-7
18. Agusti A, Celli BR, Criner GJ, et al. Global initiative for chronic obstructive lung disease 2023 report: GOLD executive summary. Eur Respir J. 2023;61(4):2300239. doi:10.1183/13993003.00239-2023
19. Chaiwong W, Takheaw N, Laopajon W, et al. Neutralizing antibody and T-cell responses against SARS-CoV-2 wild-type and variants of concern in chronic obstructive pulmonary disease subjects after ChAdOx-1/ChAdOx-1 homologous vaccination: a preliminary study. Vaccines. 2022;10(12):2176. doi:10.3390/vaccines10122176
20. Havervall S, Marking U, Svensson J, et al. Anti-spike mucosal IgA protection against SARS-CoV-2 omicron infection. N Engl J Med. 2022;387(14):1333–1336. doi:10.1056/NEJMc2209651
21. Focosi D, Maggi F, Casadevall A. Mucosal vaccines, sterilizing immunity, and the future of SARS-CoV-2 virulence. Viruses. 2022;14(2):187. doi:10.3390/v14020187
22. Marking U, Bladh O, Havervall S, et al. 7-month duration of SARS-CoV-2 mucosal immunoglobulin-a responses and protection. Lancet Infect Dis. 2023;23(2):150–152. doi:10.1016/S1473-3099(22)00834-9
23. Bladh O, Marking U, Havervall S, et al. Mucosal immune responses following a fourth SARS-CoV-2 vaccine dose. Lancet Microbe. 2023;4(7):e488. doi:10.1016/S2666-5247(23)00102-7
24. Tut G, Lancaster T, Krutikov M, et al. Strong peak immunogenicity but rapid antibody waning following third vaccine dose in older residents of care homes. Nat Aging. 2023;3(1):93–104. doi:10.1038/s43587-022-00328-3
25. Ozbay Kurt FG, Lepper A, Gerhards C, et al. Booster dose of mRNA vaccine augments waning T cell and antibody responses against SARS-CoV-2. Front Immunol. 2022;13:1012526. doi:10.3389/fimmu.2022.1012526
26. Forgacs D, Silva-Moraes V, Sautto GA, et al. The effect of waning on antibody levels and memory B cell recall following SARS-CoV-2 infection or vaccination. Vaccines. 2022;10(5):696. doi:10.3390/vaccines10050696
27. Ferdinands JM, Rao S, Dixon BE, et al. Waning of vaccine effectiveness against moderate and severe covid-19 among adults in the US from the VISION network: test negative, case-control study. BMJ. 2022;379:e072141. doi:10.1136/bmj-2022-072141
28. Terreri S, Piano Mortari E, Vinci MR, et al. Persistent B cell memory after SARS-CoV-2 vaccination is functional during breakthrough infections. Cell Host Microbe. 2022;30(3):400–408 e4. doi:10.1016/j.chom.2022.01.003
29. Muecksch F, Wang Z, Cho A, et al. Increased memory B cell potency and breadth after a SARS-CoV-2 mRNA boost. Nature. 2022;607(7917):128–134. doi:10.1038/s41586-022-04778-y
30. Antonelli M, Pujol JC, Spector TD, et al. Risk of long COVID associated with delta versus omicron variants of SARS-CoV-2. Lancet. 2022;399(10343):2263–2264. doi:10.1016/S0140-6736(22)00941-2
31. Zhao X, Zhang R, Qiao S, et al. Omicron SARS-CoV-2 neutralization from inactivated and ZF2001 vaccines. N Engl J Med. 2022;387(3):277–280. doi:10.1056/NEJMc2206900
32. Nadesalingam A, Cantoni D, Aguinam ET, et al. Vaccination and protective immunity to SARS-CoV-2 omicron variants in people with immunodeficiencies. Lancet Microbe. 2023;4(2):e58–e59. doi:10.1016/S2666-5247(22)00297-X
33. Rosenblum HG, Wallace M, Godfrey M, et al. Interim recommendations from the advisory committee on immunization practices for the use of bivalent booster doses of COVID-19 vaccines — United States, October 2022. MMWR Morb Mortal Wkly Rep. 2022;71(45):1436–1441. doi:10.15585/mmwr.mm7145a2
34. Lee IT, Cosgrove CA, Moore P, et al. Omicron BA.1-containing mRNA-1273 boosters compared with the original COVID-19 vaccine in the UK: a randomised, observer-blind, active-controlled trial. Lancet Infect Dis. 2023;23(9):1007–1019. doi:10.1016/S1473-3099(23)00295-5
35. Aksyuk AA, Bansal H, Wilkins D, et al. AZD1222-induced nasal antibody responses are shaped by prior SARS-CoV-2 infection and correlate with virologic outcomes in breakthrough infection. Cell Rep Med. 2023;4(1):100882. doi:10.1016/j.xcrm.2022.100882
36. Liew F, Talwar S, Cross A, et al. SARS-CoV-2-specific nasal IgA wanes 9 months after hospitalisation with COVID-19 and is not induced by subsequent vaccination. EBioMedicine. 2023;87:104402. doi:10.1016/j.ebiom.2022.104402
37. Curlin ME, Bates TA, Guzman G, et al. Omicron neutralizing antibody response following booster vaccination compared with breakthrough infection. Med. 2022;3(12):827–837 e3. doi:10.1016/j.medj.2022.09.001
38. Naaber P, Tserel L, Kangro K, et al. Protective antibodies and T cell responses to omicron variant after the booster dose of BNT162b2 vaccine. Cell Rep Med. 2022;3(8):100716. doi:10.1016/j.xcrm.2022.100716
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.