Back to Journals » International Journal of General Medicine » Volume 16
Age and BRAFV600E Mutation Stratified Patients with Cytologically Benign Thyroid Nodules
Authors Huang G , Liu W, Han L, Zhang Y, Liu S, Zhang J, Niu B
Received 27 October 2023
Accepted for publication 12 December 2023
Published 22 December 2023 Volume 2023:16 Pages 6025—6039
DOI https://doi.org/10.2147/IJGM.S443711
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Guocong Huang,1 Wei Liu,2 Li Han,2 Yue Zhang,2 Siyao Liu,2 Jiali Zhang,2 Beifang Niu3,4
1Department of Thyroid and Breast Surgery, The First Hospital of Putian City, Fujian, People’s Republic of China; 2Beijing ChosenMed Clinical Laboratory Co. Ltd., Beijing, People’s Republic of China; 3Computer Network Information Center, Chinese Academy of Sciences, Beijing, People’s Republic of China; 4University of the Chinese Academy of Sciences, Beijing, People’s Republic of China
Correspondence: Guocong Huang, Department of Thyroid and Breast surgery, The First Hospital of Putian City, No. 449 South Gate West Road, Chengxiang District, Putian, Fujian Province, 351100, People’s Republic of China, Email [email protected] Beifang Niu, Computer Network Information Center, Chinese Academy of Sciences, CAS Informatization Plaza No. 2 Dong Sheng Nan Lu, Haidian District, Beijing, 100083, People’s Republic of China, Email [email protected]
Purpose: Our objective was to evaluate the diagnostic performance of BRAFV600E mutation for malignant, and to identify clinical characteristics associated with positive BRAFV600E mutation in low-risk cytological and ultrasound diagnostic thyroid nodules. This aims to identify patients who may benefit from BRAFV600E mutation testing and subsequent surgical intervention.
Patients and Methods: We analysis the clinical characteristics correlated with BRAFV600E mutation in our detection cohort, including 204 patients with 217 thyroid nodules, and separate analyses were performed in 103 thyroid nodules with benign cytological result. Signaling pathway and immune response associated with age and BRAFV600E mutation status were also evaluated in Asian patients with thyroid cancer from the Cancer Genome Atlas (TCGA) dataset.
Results: The positive BRAFV600E mutation was significantly associated with higher Ultrasound (US) classification (p< 0.001) and fine-needle aspiration (FNA) categories (p< 0.001). BRAFV600E mutation as a risk factor for malignancy, showing the optimal diagnostic efficacy for malignancy combined with FNA categories, with the AUC was 0.923. Otherwise, BRAFV600E mutation is a risk factor in screening malignancy in low-risk FNA and US classification, which is significant correlation with patients age. Patients over 50 years old exhibiting a higher percentage of positive BRAFV600E mutation when both ultrasound and FNA results indicate benign conditions, with higher risk of malignancy.
Conclusion: BRAFV600E mutation is an accurate adjunctive diagnostic marker on FNA to screen malignancy. In low risk of both ultrasound and FNA results, the positive BRAFV600E was significant increased in patients over 50 years old, which have higher risk of malignancy. Thus, the BRAFV600E mutation detection and further surgery should be strengthened in older patients with benign cytological and US results thyroid nodules.
Keywords: thyroid nodule, BRAFV600E mutation, benign cytology, older, diagnosis
Introduction
The thyroid gland is one of the largest endocrine glands in the human body. Thyroid lesions are often found in the gland, with a prevalence of 4% to 7%. Most of them are asymptomatic, and thyroid hormone secretion remains normal.1 Bernet et al have reported that thyroid nodules are associated with a 7–15% risk of malignancy.2 Ultrasound (US) is the most sensitive model to assess thyroid nodule morphology, aiding in identifying high-risk cases for malignancy.3 Certain sonographic features like hypoechogenicity, microcalcifications, irregular margins, absent halo sign, and increased intramodular blood flow are associated with a higher risk of malignancy.1 One of the newest radiology systems, the Chinese Thyroid Imaging Reporting and Data System (C-TIRADS), established by Chinese experts using a counting method, is suitable for Chinese clinical practices, and easy for clinical application and promotion.4 Nodules with higher C-TIRADS score are required to perform cytological analysis through fine-needle aspiration (FNA) to determine the risk of malignancy.5
Ultrasound-guided FNA (US-FNA) is a reliable and effective approach for evaluating thyroid nodules before surgery, providing cytopathological assessment with high sensitivity and specificity for malignancy.6 Otherwise, the false-negative rate of US-FNA diagnosis is approximately 12%. Based on the literature, approximately 70% of FNA are categorized as Bethesda II, with an anticipated 3% being false negatives.7 It is necessary to provide definitive diagnoses to distinguish malignant ones from benign cytological results for better management of patients.
Molecular analysis can be used to enhance the diagnostic accuracy of cytological diagnosis and as a marker for targeted therapy.8 The main known genetic causes of thyroid cancer include point mutations in the BRAF, RAS, TERT, RET, and TP53 genes and the fusion genes RET/PTC, PAX8/PPAR-γ, and NTRK.9 Fusion gene positive PTCs were associated with more aggressive behavior disease than fusion gene negative PTCs.10 A comprehensive study reported by Pekova et al recently identified that RET fusion was associated with a 100% probability of malignancy.11 BRAFV600E is the most common genetic mutation in thyroid cancer, with a prevalence ranging from 37% to 83% in papillary thyroid cancer (PTC),12 and has shown a strong association with lymph node metastasis, extra thyroidal extension, disease recurrence, and lymph node metastasis-associated mortality.13,14 Several studies have proved BRAFV600E mutation is a valuable and accurate adjunctive diagnostic marker on US-FNA to screen malignancy in non-diagnostic and indeterminate thyroid nodules.15,16 Few studies have focused on the influence of BRAFV600E mutation in benign cytological analysis on malignancy, and the clinical characteristics associated with BRAFV600E mutation in the Bethesda System for Reporting Thyroid Cytopathology (TBSRTC) II remained unclear.17–19
In this study, we evaluated the diagnostic efficacy of BRAFV600E mutation in thyroid nodules for screening out malignancy, and the association with progression of thyroid cancer. On the other hand, in order to avoid overdiagnosis and overtreatment, we further focused on clinical characteristics and US features associated with positive BRAFV600E mutation in patients in low-risk cytology and US diagnosis to accurately locate the risk of BRAFV600E mutation and malignant risk.
Methods
Patients and Data
A total of 223 thyroid nodules in 210 patients underwent US and US-FNA with an additional BRAFV600E mutation detection in the Department of Thyroid and Breast Surgery of the First Hospital of Putian City (FHPT patients cohort) in Fujian Province from January 2021 to May 2022. Six patients with 6 nodules were excluded because of an incomplete cytopathological diagnosis or insufficient sonography diagnosis. As a result, 204 patients with 217 nodules were included in the analysis. The patients included 152 females and 52 males, aged from 17 to 91 years, mean age was 47.25±12.16 years. All the patients experienced clinical follow-up after six months, and no progression occurred during this period. Among all the samples, 44 thyroid nodules were treated with thyroidectomy, and 43 thyroid nodules had been diagnosed pathologically by postoperative paraffin sections, of which 38 were PTC and 1 was medullary thyroid cancer (MTC), three cases with nodular goiter, and 1 with thyroiditis. Patients characteristics including BRAFV600E mutation, the classification of US and FNA, sonography diagnosis information, gender, age, drinking, smoking, location, multiplicity, size, and pathological diagnosis were also recorded, the detail information was shown in Supplementary Tables 1 and 2. Both clinical data and BRAFV600E mutation detection results of 507 patients with thyroid cancer from the Cancer Genome Atlas (TCGA) were obtained from UCSC Xena (https://xenabrowser.net/datapages/), 501 patients among them with the RNA-seq expression data and 491 patients with mutation data. The diagram of the study group is shown in Figure 1.
 |
Figure 1 The diagram of the study. The sample used for analysis and the analysis diagram of the study. |
Ultrasound Examination
US examinations were performed by an experienced US radiologist (GH) used Siemens S2000 ultrasound diagnostic instrument equipped with a 5–12–MHz linear-array transducer. The thyroid glands were scanned in the supine position to determine the location, size (at their longest diameter), number, internal echo, morphology, margin, shape, and calcification. Suspicious US features of malignancy include microcalcification, irregular margins, taller-than-wide shape, hypoechogenicity and marked hypoechogenicity, irregular thick hole, and evidence of extrathyroidal growth.20 Two radiologists with more than ten years’ experience in thyroid ultrasonography classified all nodules into four category according to C-TIRADS: 3 (probably benign), 4A (low suspicion malignant), 4B (moderate suspicion malignant) and 4C (high suspicion malignant).4
FNA of Thyroid Nodules
FNA was performed under the guideline of US by experienced radiologists with a 0.7 mm syringe. Each lesion was aspired three or four passes in different directions to complete the sampling. Two or three of them were smeared on glass slides and fixed for cytopathological analysis. Based on TBSRTC, the results of FNA were categorized into six classifications, I–VI were non-diagnostic, benign, atypia undetermined significance/follicular lesion of undetermined significance (AUS/FLUS), suspicious for follicular neoplasm or suspicious for a Hurthle cell neoplasm, suspicious for malignancy and malignancy,21 respectively.
BRAFV600E Mutation Test
The specimens for BRAFV600E mutation analysis were from Formalin-Fixed Paraffin-Embedded (FFPE) tissues, thyroid FNA samples or serum samples. Genomic DNA extraction from FFPE tissues and thyroid FNA samples were used TargetingOne® FFPE TNA-P1, TargetingOne® FFPE TNA-P2 (TargetingOne® Biotech. Beijing, China), respectively. CfDNA from serum samples were used TargetingOne® cfDNA-B1 (TargetingOne® Biotech. Co. Ltd. Beijing, China). The quantity of isolated DNA was assessed by Nanodrop, all samples were adequate for BRAFV600E detection.
The BRAFV600E mutation was detected by human BRAFV600E mutation detection kit (TargetingOne® Biotech. Beijing, China) according to the manufacturer's instructions. The reaction components were prepared as follows: 7.5 μL of PCR reagent A (BRAFV600E), 7.5 μL of PCR reagent B (BRAFV600E), and 20–50 ng of DNA, sterilized water was used to make a volume of up to 30 μL. The optimal annealing temperature is determined to be 60°C. Each experiment included a negative and positive (BRAFV600E) control. The ddPCR platform system (TargetingOne® Biotech. Beijing, China) was utilized for evaluation. Droplets were generated and analyzed using the Drop Maker M1 and the Chip Reader R1. The quantitative results were estimated by modeling as a Poisson distribution using chip Analyzer-V1 (TargetingOne® Biotech. Beijing, China).
Different Expression Genes (DEGs) Identified and Immune Response Analysis
The R package “limma” was applied to calculate differentially expressed genes (DEGs) between older and younger and positive and negative BRAFV600E mutation, the threshold was set to p < 0.05 and | logFC | > 1.22 Then, Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment pathway analysis were performed on the DEGs within older and younger groups, and the intersection DEGs of positive and negative BRAFV600E mutation groups and younger and older age groups, respectively. The R package “clusterProfiler” (v.4.7.1.2) was used for functional annotation of the DEGs. An immune-related gene set (immune inhibitors and immunostimulators) was collected from the online tool TISIDB.23 The results were exhibited by bubble diagram and box plot drawn by “ggplot2” (v.3.4.1) package.
Somatic Mutation Analysis
We performed somatic mutation analysis based on thyroid carcinoma (THCA) samples. To reduce the false-positive rates, we deleted synonymous mutations. Mutational spectrum and mutational signature were depicted via “maftools” (v.2.12.0) R packages which provides a multiple of analysis modules to perform the visualization process. The mutation portrait matrix was factorized into two nonnegative matrices, “signatures” and “contributions” by the ExtractSignatures function of “maftools”, where “signatures” represented mutational processes and “contributions” reflected the associated mutational activities. This approach can automatically estimate the ideal number of retrieved mutational signatures because it is based on Bayesian variant nonnegative matrix factorization.
Statistical Analysis
The statistical analysis was performed with SPSS version 26.0 and R version 4.2.1. Fisher’s exact test or Pearson’s chi-squared test was used for categorical variables analysis. Normally distributed data were expressed as mean ± standard deviation and analyzed with a two-sample t-test, and non-normally distributed variables were presented as medians and interquartile range (IQR) using the Wilcoxon-Mann-Whitney test. The “ggalluvial” (v.0.12.3) R package was used to produce the alluvial diagrams. To measure the specificity and sensitivity of the prognostic capability of C-TIRADS, BRAFV600E, TBSRTC category, and the combined methods, we calculated the area under the curve (AUC) using the R “pROC” (v.1.18.0) package. A p-value of <0.05 was considered statistical difference and p-value of <0.01 was considered statistically significant difference.
Results
BRAFV600E Mutation Was Associated with Malignancy of Thyroid Nodules and Progression of Thyroid Cancer
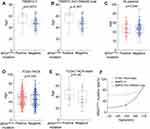
To evaluate the effect of BRAFV600E mutation on malignant progression of thyroid nodules, the BRAFV600E mutation detection was performed in all 204 patients with 217 thyroid nodules, the positive BRAFV600E mutation was detected in 89 (41.0%) thyroid nodules, and the other 128 nodules (59.0%) were negative. In TBSRTC II, the incidence of negative BRAFV600E mutation significantly higher than that of positive BRAFV600E mutation. Conversely, the positive BRAFV600E mutation was statistically significantly associated with TBSRTC III–IV (p=0.007) and TBSRTC V–VI (p<0.001), which poses a higher risk of malignancy. Patients in C-TIRADS 4b-4c had more frequent positive BRAFV600E mutation than patients with low-risk US classification (p<0.001) (Table 1). Notably, a substantial majority of thyroid nodules exhibiting positive BRAFV600E mutation were found to be situated in high-risk FNA and US categories (Figure 2A). We further investigated the correlation of BRAFV600E mutation with different pathological stages of thyroid cancers based on TCGA database. The BRAFV600E mutation was increased in advanced stages (stage III–IV) compared with early stages (stage I–II) (p<0.001) (Figure 2B), similarly, the proportion of positive BRAFV600E mutation was higher in advanced pathological T stage (T3-T4) (p<0.001) and N1 stage (p<0.001) (Figure 2C and D). With the development of M stage, there was no statistically significant difference between positive and negative BRAFV600E mutation (Figure 2E). When separated from Asian patients, the BRAFV600E mutation increased as the pathological stage and T stage increased (Supplementary Figure 1A and B), but the results were not statistically significant. No differences were found in M stage and N stage (Supplementary Figure 1C and D). The expression of BRAF has no statistically significant difference between positive and negative BRAFV600E mutation (Figure 2F and Supplementary Figure 1E), indicated that the BRAFV600E mutation has no influence in expression of BRAF, associated with malignant in thyroid nodules directly. We further analyzed the molecular features of BRAFV600E mutation in thyroid cancer using TCGA-THCA cohort, we found that BRAFV600E was the most commonly mutated gene. Interestingly, most patients with positive BRAFV600E mutation did not have any other mutations in the top 30 most frequently mutated genes (Supplementary Figure 2A). And the coincident and exclusive associations across mutated genes showed that the top 30 genes with the highest mutation frequency were mutual exclusivity with positive BRAFV600E mutation (Supplementary Figure 2B). The results indicate that positive BRAFV600E mutation is associated with higher risk of malignancy in thyroid nodules.
 |
Table 1 Correlation Between Clinical Characteristics and BRAF600E Mutation in All Thyroid Nodules |
BRAFV600E Was an Adjunctive Diagnostic Marker on FNA to Screen Malignancy in Benign Cytology
Among all the thyroid nodules, 43 of them have postoperative pathological diagnosis, of which 4 cases with nodular goiter or thyroiditis were benign, and the other 39 were malignant, including 38 PTC and 1 MTC. As shown in Table 2, the percentage of positive BRAFV600E mutation is 74.4% (29/39) in PTC cases in our study, which was significantly associated malignant of thyroid nodules (p=0.008). All the thyroid nodules belonged to TBSRTC V–VI were confirmed malignant, however, the results were not statistically significant (p=0.072). Seven (17.9%) samples classified as TBSRTC II and 18 (47.2%) samples classified as C-TIRADS 3–4a confirmed to be malignant by pathological diagnosis, indicating that the sensitivity for malignancy diagnosis of the TBSRTC and C-TIRADS classification was low in our study. No statistically significant differences were observed in gender, age, drinking, and smoking between the benign and malignant thyroid nodules. Noteworthily, the median age in malignancy is 45.92±12.55, which was younger than those in benign, but there was no statistical significance between them (p=0.664).
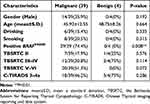 |
Table 2 Clinical Characteristics Associated with Malignant in Thyroid Nodules |
We analyzed the sensitivity and specificity of TBSRTC, BRAFV600E mutation, and C-TIRADS along with the combination of both or three methods. The results revealed that the specificity of TBSRTC was as high as 100%; however, the sensitivity was lower compared with BRAFV600E mutation and C-TIRADS classification. The combination of TBSRTC and BRAFV600E mutation greatly increase the sensitivity of TBSRTC, and showed the highest AUC, which was 0.923 (Figure 3 and Table 3). All samples with positive BRAFV600E mutation were malignant by pathological diagnosis, including benign cytology diagnosis TBSRTC II (Table 4). Several studies had assessed the risk of malignancy in patients with benign cytology,17–19 the results revealed that the majority thyroid nodules with positive BRAFV600E mutation confirmed malignant (Table 5). The results above indicated that there was a risk of malignant in benign cytological results, which may be associated with positive BRAFV600E mutation.
 |
Table 3 Diagnostic Efficacy of Different Methods for Discrimination Malignant in Thyroid Nodules |
 |
Table 4 Correlations of BSRTC Categories with BRAFV600E Mutation and Pathological Results |
 |
Table 5 The Prediction of BRAFV600E Mutation on Malignancy in Thyroid Nodules with Benign Cytology |
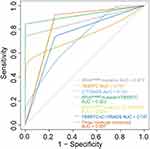 |
Figure 3 ROC analysis of diagnosis methods for malignancy. The ROC analysis of TBSRTC, BRAFV600E mutation status, and C-TIRADS for malignancy in thyroid nodules. |
The Prediction of Clinicopathologic and US Characteristics to BRAFV600E Mutation in Patients with Low-Risk Cytological and US Classification
Based on the results described above, the positive BRAFV600E mutation was a risk factor of malignancy in patients with benign cytology. However, according to the 2015 American Thyroid Association (ATA) guideline, the BRAFV600E mutation detection was un-practical and not required immediately in benign cytology,24 and the clinicopathologic and US characteristics associated with BRAFV600E mutation in patients with benign cytology were unclear. In this study, all the cases classified TBSRTC II had BRAFV600E mutation detection, 18 of those were positive. The mean age of patients with positive BRAFV600E mutation was 56.35±11.89 years, significantly higher than that of negative BRAFV600E mutation, which was 47.67±9.38 years (p=0.0012), other clinicopathologic and US features had no statistically significant differences between positive and negative BRAFV600E mutation. Meanwhile, higher US classification C-TIRADS 4b-4c was not significantly correlated with positive BRAFV600E mutation (Table 6). Considering the majority samples with benign cytology belonged to C-TIRADS 3–4a, we further analyzed the clinical and US features associated with positive BRAFV600E mutation in patients with C-TIRADS 3–4a in TBSRTC II results. Consisted with the result of Table 6, positive BRAFV600E mutation was upregulated in older patients (p=0.001), multiple nodules and right location also showed difference between positive and negative BRAFV600E mutation, with p value were 0.031 and 0.036, respectively (Table 7). Other suspicious malignant US features, such as hypoechogenicity, irregular morphology, unclear boundary and larger thyroid nodules size have no significant association with BRAFV600E mutation.
 |
Table 6 Correlation Between BRAFV600E Mutation and Clinical Characteristics in Thyroid Nodules with Benign Cytology |
 |
Table 7 The Relationship Between BRAFV600E Mutation and Clinical Characteristics in C-TIRADS 3–4a Patients with Benign Cytology |
BRAFV600E Mutation Was Upregulated as Age Increased in Patients with Low Risk FNA and US Diagnosis
Patients of older age exhibit a higher proportion of BRAFV600E mutations in benign cytology TBSRTC II (p=0.0012) and in C-TIRADS 3–4a patients of benign cytology (p=0.001) (Figure 4A and B), but no significant difference was found in the entire population in our cohort (Figure 4C). We then conducted a statistical analysis using the TCGA database on thyroid cancer, there was no correlation between age and BRAFV600E mutation in all the patients (p=0.343) and separated Asian patients (p=0.08) (Figure 4D and E). Graphing the positive probability of the three thyroid groups, including patients in benign cytology, patients in C-TIRADS 3–4a of benign cytology, and thyroid cancer in TCGA Asian patients, we found that the probability of BRAFV600E mutations was significantly higher in thyroid cancer patients than in other two groups, regardless of age. In all three groups, the probability of positive BRAFV600E mutations in the thyroid significantly increased with age over 50 years (Figure 4F). Therefore, greater attention should be paid to patients older than 50 years in benign cytological results, and BRAFV600E testing should be conducted under appropriate conditions for screening high risk of malignancy, followed by surgical treatment.
The Molecular Mechanisms Underlying the Increased Frequency of BRAFV600E Mutations in Older Patients
Differential gene expression analysis was performed between patient age groups (≤50 years vs >50 years) in TCGA-THCA-Asian cohort, 277 differentially expressed genes were identified, which were significantly enriched in Ras signaling pathway (Figure 5A and B), closing interconnected with BRAF mutation. 1174 genes between the BRAFV600E mutation groups were also identified (Figure 5C). The intersection differential genes of BRAFV600E mutations and age were significantly enriched in immune-related signaling pathways (Figure 5D). Therefore, we hypothesized that the association between age and BRAFV600E mutations may be immune-related. Furthermore, we investigated the correlation of immune response with patients age and BRAFV600E mutation, we demonstrated the majority of immunostimulator were down regulated in older patients and patients with positive BRAFV600E mutation, BTNL2, CXCR4, TNFRSF14 and TNFRSF25 were significant higher in younger patients and CXCR4 was significant higher in patients with negative BRAFV600E mutation (Figure 5E). No statistic differences in immunoinhibitors were obtained between patients age groups (≤50 years vs >50 years) (Supplementary Figure 3).
Discussion
Accurately distinguishing between benign and malignant thyroid nodules is crucial to prevent patient overtreatment and tailor personalized treatment strategies. High-risk malignant thyroid nodules typically require surgical excision. Regarding surgical approaches, studies suggest no significant difference in early postoperative complications between total thyroidectomy (TT) and subtotal thyroidectomy (STT).25 Similarly, LigaSure vessel (LS) or harmonic scalpel (HS) exhibit comparable safety in thyroidectomy.26 For low-risk malignant thyroid nodules, repetitive ultrasound examinations and follow-ups can mitigate the discomfort associated with surgery. Ultrasound examination, widely utilized for assessing thyroid nodules, remains the most prevalent method. A study by Grimmichova et al indicates an 18% malignancy rate in thyroid nodules classified as low-risk by ultrasound.27 In China, the C-TIRADS is extensively employed for thyroid ultrasound classification, utilizing a counting method based on suspicious malignant features. Nodules classified as 4b-6 are recommended for FNA testing.4,28 FNA has traditionally been considered the gold standard diagnostic method for thyroid nodules before surgery, demonstrating high sensitivity and specificity in diagnosing thyroid malignancy. However, in cases of indeterminate FNA results, both sensitivity and specificity for malignancy significantly decrease.27 In Mulita et al studies, the malignant of thyroid nodules that fall within Bethesda categories II and III–IV was 1.58% and 15–40%, respectively.7,29 The BRAFV600E mutation detection has proved can be used as a marker in FNA evaluation to diagnosis malignant in thyroid nodules.30,31
In our study, 46.2% of thyroid nodules classified as C-TIRADS 3–4a and 17.9% categorized as BSRTC II were diagnosed as PTC, a proportion significantly higher than previously reported data. This discrepancy may be attributed to our comprehensive integration of ultrasound, FNA, and BRAFV600E mutation detection results and selected high-risk thyroid nodules for surgery and pathological diagnosis, contributing to a notable increase in the overall malignancy rate. Our finding also revealed the sensitivity and specificity of C-TIRADS and TBSRTC has significant improved when combined with BRAFV600E mutation detection, and the combination of BRAFV600E mutation and TBSRTC categories had optimal diagnostic performance of thyroid nodules, with the AUC, sensitivity, and specificity being 0.923, 84.6%, and 100%, respectively. A large number of studies have reported that BRAFV600E mutation was significantly associated with PTC or papillary thyroid microcarcinoma (PTMC),13,32,33 and the specificity of BRAFV600E mutation for PTC was nearly 100%.34 Consisted with the report, in pathological diagnosed group, all the patients with positive BRAFV600E mutation were confirmed malignant, including those belonged to low-risk cytological categories TBSRTC II and C-TIRADS 3–4a.
Positive BRAFV600E mutation could provide prognostic information for thyroid cancer, showed significantly higher risk of mortality and recurrence.35,36 In addition, the BRAFV600E mutation has an additive effect with various clinical features, such as older age, male gender, and lymph node metastasis, significantly increasing the risk of mortality in PTC.14,37,38 Actually, positive BRAFV600E mutation was significantly associated with high-risk FNA and US classification, along with advanced pathological T stage and lymph node metastases in thyroid cancer in our study. Thus, the thyroid nodules with positive BRAFV600E mutation should take more attention for repeating US examination to make the final decision on whether to proceed with surgical intervention and remove thyroid nodules.
Few studies have focused on BRAFV600E mutation analysis in thyroid nodules with benign cytology, and all three literature reports we retrieved have confirmed that the majority thyroid nodules in benign cytology with positive BRAFV600E mutation confirmed malignant.17–19 Consisted with the reports, three patients in TBSRTC II with positive BRAFV600E mutation confirmed malignant by pathological diagnosis, confirming that there is a malignant risk even in the low-risk category of BSRTC II, and the BRAFV600E mutation can identify it. However, for thyroid nodules with benign cytologic evaluation, in order to avoid overdiagnosis and overtreatment, the BRAFV600E mutation detection was un-practical and not required immediately according to the 2015 ATA guideline.24 Using clinical and ultrasound features to identify positive BRAFV600E mutation, and including the BRAFV600E mutation status in the prognostic risk stratification can lead to more precise management of patients with low-risk cytologic category.
Recently, a study revealed that more than two suspicious US features in benign cytologic analysis should consider further treatment of BRAFV600E mutation detection.17 Several US features, such as microcalcification, marked hypoechogenicity and irregular margins were more frequently observed in thyroid nodules harbouring positive BRAFV600E mutation than negative BRAFV600E mutation.30,39 In our study, there were no significant differences in C-TIRADS classification, along with US features between positive and negative BRAFV600E mutation in benign cytologic results. The reason may be that the majority of cases were classified as 3–4a, so the result of C-TIRADS classification was not referential. Surprisingly, the positive BRAFV600E mutation was increased in older patients in benign cytology and in the nodules classified both C-TIRADS 3–4a and TBSRTC II.
For thyroid cancer, The American Joint Committee on Cancer (AJCC) staging system has emphasized that the age as the part of progression for malignant, treated as a dichotomous variable into risk stratification and treatment,40,41 and the mortality risk of PTC was increased as increasing of patients age.42 A study has reported that in patients older ≥60 years, the papillary microcarcinomas (PMCs) were most likely to enlarge or show clinical node metastases.43 Shen et al has reported that the patient age exhibits a linear correlation with mortality rates in individuals with the positive BRAFV600E mutation, conversely, among patients with the negative BRAFV600E mutation, mortality rates remain relatively low and stable with increasing age.37 In our study, we observed a significant upregulation of the BRAFV600E mutation in patients aged over 50, particularly in the context of low-risk FNA and US diagnoses. Specifically, when dealing with thyroid nodules exhibiting lower suspicious US features and negative FNA findings, older patients warrant heightened attention for additional BRAFV600E testing and further surgery.
We identified the different expression genes between two age groups (≤50 years vs >50 years) were significantly enriched in Ras signaling pathway, which are closely interconnected with BRAF mutation.44,45 The signaling pathway enriched in the different genes intersection of age groups and BRAFV600E mutation played a crucial role in modulating immune response. Previous study has shown that in thyroid cancer, BRAFV600E mutation exhibits a largely immunosuppressive profile and demonstrates disruptions in the immune surveillance.46 Consisted with the study, our results showed a decrease expression of most immunostimulators in the patients with positive BRAFV600E mutation and older age. However, immunoinhibitors had no significant difference between patients age groups. More samples are necessary for further analysis.
There are several limitations of this study. Firstly, the malignancy rate in our study was higher than other studies before, the reason may be that the nodules with the pathological diagnosis in our study were at high risk of malignant, such as higher TBSRTC category, more suspicious US features, and positive BRAFV600E mutation, which made the proportion of malignant was high. Additionally, all the thyroid nodules were followed up by regular clinic visits for half of the year, which is shorter and inadequate for detecting the progression. On the other hand, for the correlation of BRAFV600E mutation and patient age, the results were obtained by Asian patients in TCGA-THCA, the number of samples was limited and there was no clinical data for analysis and validation. Subsequently, more prospective data were required for a deeper analysis. Otherwise, this is a retrospective study of single-institution analysis, we need more cases and prospective studies to verify our results in future studies.
Conclusions
The BRAFV600E mutation serves as a risk factor in screening malignancy in thyroid nodules, and its combination with TBSRTC classification can enhance diagnostic sensitivity. In cases of TBSRTC II and C-TIRADS 3–4a in benign cytology, thyroid nodules with the positive BRAFV600E mutation exhibit an increased malignant risk. Additionally, the proportion of patients aged 50 and above with the BRAFV600E mutation is significantly higher than those below 50 years old. The new results may help to make a better decision to screen thyroid nodules in low-risk FNA and US diagnostic results to have further BRAFV600E mutation detection, which have higher risk of malignancy, and further surgery should be considered.
Abbreviations
PTC, Papillary thyroid cancer; C-TIRADS, Chinese Thyroid imaging reporting and data system; TCGA, The Cancer Genome Atlas; FNA, Fine needle aspiration; US, Ultrasound; US-FNA, Ultrasound-guided fine needle aspiration; TBSRTC, The Bethesda System for Reporting Thyroid Cytopathology; MTC, Medullary thyroid cancer; DEGs, Different expression genes; KEGG, Kyoto Encyclopedia of Genes and Genomes; THCA, Thyroid carcinoma; AUC, Area under the curve; ATA, American Thyroid Association; PTMC, Papillary thyroid microcarcinoma; DOR, Diagnostic odds ratio; AJCC, American Joint Committee on Cancer.
Data Sharing Statement
The datasets supporting the conclusions of this article are included within the Supplementary Tables 1 and 2. The data generated in the present study may be requested from the corresponding author.
Ethics Approval and Consent to Participate
This study was conducted according to the guidelines of the Declaration of Helsinki. The study protocol was approved by the Ethics Institutional Review Board of The First Hospital of Putian City (approval number: 2023-066). The patients/participants provided their written informed consent to participate in this study.
Consent to Publication
The details of any images and tables can be published, and that the persons providing consent have been shown the article contents to be published, all authors agree with the publication.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research was funded by National Natural Science Foundation of China (grant number 92259101, 31771466) and Strategic Priority Research Program of the Chinese Academy of Sciences, China (grant number XDB38040100).
Disclosure
The Authors declare that there is no conflict of interests.
References
1. Mulita F, Anjum F. Thyroid adenoma. In: StatPearls [Internet]. StatPearls Publishing; 2023.
2. Bernet VJ, Chindris AM. Update on the Evaluation of thyroid nodules. J Nucl Med. 2021;62(Suppl 2):13S–19S. doi:10.2967/jnumed.120.246025
3. Brito JP, Gionfriddo MR, Al Nofal A, et al. The accuracy of thyroid nodule ultrasound to predict thyroid cancer: systematic review and meta-analysis. J Clin Endocrinol Metab. 2014;99(4):1253–1263.
4. Zhou J, Yin L, Wei X, et al. 2020 Chinese guidelines for ultrasound malignancy risk stratification of thyroid nodules: the C-TIRADS. Endocrine. 2020;70(2):256–279. doi:10.1007/s12020-020-02441-y
5. Detweiler K, Elfenbein DM, Mayers D. Evaluation of thyroid nodules. Surg Clin North Am. 2019;99(4):571–586. doi:10.1016/j.suc.2019.04.001
6. Wong R, Farrell SG, Grossmann M. Thyroid nodules: diagnosis and management. Med J Aust. 2018;209(2):92–98. doi:10.5694/mja17.01204
7. Mulita F, Iliopoulos F, Tsilivigkos C, et al. Cancer rate of Bethesda category II thyroid nodules. Med Glas. 2022;19(1):1413–1421.
8. Poller DN, Glaysher S. Molecular pathology and thyroid FNA. Cytopathology. 2017;28(6):475–481. doi:10.1111/cyt.12492
9. Hlozek J, Pekova B, Rotnágl J, Holý R, Astl J. Genetic changes in thyroid cancers and the importance of their preoperative detection in relation to the general treatment and determination of the extent of surgical intervention—a review. Biomedicines. 2022;10(7). doi:10.3390/biomedicines10071515
10. Pekova B, Sykorova V, Dvorakova S, et al. RET, NTRK, ALK, BRAF, and MET fusions in a large cohort of pediatric papillary thyroid carcinomas. Thyroid. 2020;30(12):1771–1780. doi:10.1089/thy.2019.0802
11. Bulanova Pekova B, Sykorova V, Mastnikova K, et al. RET fusion genes in pediatric and adult thyroid carcinomas: cohort characteristics and prognosis. Endocr Relat Cancer. 2023;30(12). doi:10.1530/ERC-23-0117
12. Kim Y, Kim YS, Bae JS, Kim JS, Kim K. Is gross extrathyroidal extension to strap muscles (T3b) only a risk factor for recurrence in papillary thyroid carcinoma? A Propensity Score Matching Study. Cancers. 2022;14(10). doi:10.3390/cancers14102370
13. Chen H, Song A, Wang Y, et al. BRAF V600E mutation test on fine-needle aspiration specimens of thyroid nodules: clinical correlations for 4600 patients. Cancer Med. 2022;11(1):40–49. doi:10.1002/cam4.4419
14. Tao Y, Wang F, Shen X, et al. BRAF V600E status sharply differentiates lymph node metastasis-associated mortality risk in papillary thyroid cancer. J Clin Endocrinol Metab. 2021;106(11):3228–3238. doi:10.1210/clinem/dgab286
15. Lin K, Xiang Y, Qiao L, Liu R, Dong S, Zhang X. A predictive model for selecting malignant thyroid nodules in patients with nondiagnostic or indeterminate fine-needle aspiration cytologic findings. J Ultrasound Med. 2015;34(7):1245–1251. doi:10.7863/ultra.34.7.1245
16. Jinih M, Foley N, Osho O, et al. BRAF(V600E) mutation as a predictor of thyroid malignancy in indeterminate nodules: a systematic review and meta-analysis. Eur J Surg Oncol. 2017;43(7):1219–1227. doi:10.1016/j.ejso.2016.11.003
17. Chen X, Zhou Q, Wang F, et al. Value of BRAF V600E in high-risk thyroid nodules with benign cytology results. AJNR Am J Neuroradiol. 2018;39(12):2360–2365. doi:10.3174/ajnr.A5898
18. Kim SY, Kim EK, Kwak JY, Moon HJ, Yoon JH. What to do with thyroid nodules showing benign cytology and BRAF(V600E) mutation? A study based on clinical and radiologic features using a highly sensitive analytic method. Surgery. 2015;157(2):354–361. doi:10.1016/j.surg.2014.09.003
19. Zhang Y, Lu F, Shi H, et al. Predicting malignancy in thyroid nodules with benign cytology results: the role of Conventional Ultrasound, Shear Wave Elastography and BRAF V600E. Clin Hemorheol Microcirc. 2022;81(1):33–45. doi:10.3233/CH-211337
20. Gharib H, Papini E, Garber JR, et al. American association of clinical endocrinologists, American college of endocrinology, and associazione medici endocrinologi medical guidelines for clinical practice for the diagnosis and management of thyroid nodules--2016 update. Endocr Pract. 2016;22(5):622–639. doi:10.4158/EP161208.GL
21. Cibas ES, Ali SZ. The 2017 Bethesda system for reporting thyroid cytopathology. Thyroid. 2017;27(11):1341–1346. doi:10.1089/thy.2017.0500
22. Ritchie ME, Phipson B, Wu D, et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43(7):e47. doi:10.1093/nar/gkv007
23. Ru B, Wong CN, Tong Y, et al. TISIDB: an integrated repository portal for tumor-immune system interactions. Bioinformatics. 2019;35(20):4200–4202. doi:10.1093/bioinformatics/btz210
24. Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26(1):1–133.
25. Bouchagier K, Anesidis S, Maroulis I, et al. Thyroidectomy for the management of differentiated thyroid carcinoma and their outcome on early postoperative complications: a 6-year single-centre retrospective study. Chirurgia. 2022;117(5):556–562.
26. Mulita F, Theofanis G, Verras G-I, et al. Comparison of postoperative bleeding using harmonic scalpel and LigaSure in thyroid surgery: a 15-year single-centre retrospective study. Medicinski Glasnik. 2023;20(2):1–6.
27. Grimmichova T, Pacesova P, Hill M, et al. Thyroid cancer detection in a routine clinical setting: performance of ACR TI-RADS, FNAC, and molecular testing in prospective cohort study. Biomedicines. 2022;10(5):954.
28. Qi Q, Zhou A, Guo S, et al. Explore the diagnostic efficiency of Chinese thyroid imaging reporting and data systems by comparing with the other four systems (ACR TI-RADS, Kwak-TIRADS, KSThR-TIRADS, and EU-TIRADS): a Single-Center Study. Front Endocrinol. 2021;12. doi:10.3389/fendo.2021.763897
29. Mulita F, Plachouri M-K, Liolis E, Vailas M, Panagopoulos K, Maroulis I. Patient outcomes following surgical management of thyroid nodules classified as Bethesda category III (AUS/FLUS). Endokrynologia Polska. 2021;72(2):143–144. doi:10.5603/EP.a2021.0018
30. Du J, Han R, Chen C, et al. Diagnostic efficacy of ultrasound, cytology, and BRAFV600E mutation analysis and their combined use in thyroid nodule screening for papillary thyroid microcarcinoma. Front Oncol. 2022;11:4697.
31. Seo JY, Kim E-K, Kwak JY. Additional BRAF mutation analysis may have additional diagnostic value in thyroid nodules with “suspicious for malignant” cytology alone even when the nodules do not show suspicious US features. Endocrine. 2014;47(1):283–289.
32. Du J, Han R, Chen C, et al. Diagnostic efficacy of ultrasound, cytology, and BRAF(V600E) mutation analysis and their combined use in thyroid nodule screening for papillary thyroid microcarcinoma. Front Oncol. 2021;11:746776. doi:10.3389/fonc.2021.746776
33. Moon WJ, Choi N, Choi JW, Kim SK, Hwang TS. BRAF mutation analysis and sonography as adjuncts to fine-needle aspiration cytology of papillary thyroid carcinoma: their relationships and roles. AJR Am J Roentgenol. 2012;198(3):668–674. doi:10.2214/AJR.11.7185
34. Li J, Liu J, Yu X, Bao X, Qian L. BRAFv600e mutation combined with thyroglobulin and fine-needle aspiration in diagnosis of lymph node metastasis of papillary thyroid carcinoma. Pathol Res Pract. 2018;214(11):1892–1897.
35. Xing M, Alzahrani AS, Carson KA, et al. Association between BRAF V600E mutation and mortality in patients with papillary thyroid cancer. JAMA. 2013;309(14). doi:10.1001/jama.2013.3190
36. Xing M, Alzahrani AS, Carson KA, et al. Association between BRAF V600E mutation and recurrence of papillary thyroid cancer. J Clin Oncol. 2015;33(1):42–50. doi:10.1200/jco.2014.56.8253
37. Shen X, Zhu G, Liu R, et al. Patient age–associated mortality risk is differentiated by BRAF V600E status in papillary thyroid cancer. J Clin Oncol. 2018;36(5):438.
38. Wang F, Zhao S, Shen X, et al. BRAF V600E confers male sex disease-specific mortality risk in patients with papillary thyroid cancer. J Clin Oncol. 2018;36(27):2787.
39. Liang S, Huang K. Correlation between ultrasonographic appearance of papillary thyroid microcarcinoma and BRAF V600E mutation. J Oncol. 2022;2022. doi:10.1155/2022/5916379
40. Edge SB, Byrd DR, Carducci MA, Compton CC, Fritz A, Greene F. AJCC Cancer Staging Manual. Vol. 7. Springer; 2010.
41. Amin MB, Edge SB, Greene FL, et al. AJCC Cancer Staging Manual. Vol. 1024. Springer; 2017.
42. Adam MA, Thomas S, Hyslop T, Scheri RP, Roman SA, Sosa JA. Exploring the relationship between patient age and cancer-specific survival in papillary thyroid cancer: rethinking current staging systems. J Clin Oncol. 2016;34(36):4415–4420. doi:10.1200/JCO.2016.68.9372
43. Ito Y, Miyauchi A, Kihara M, Higashiyama T, Kobayashi K, Miya A. Patient age is significantly related to the progression of papillary microcarcinoma of the thyroid under observation. Thyroid. 2014;24(1):27–34. doi:10.1089/thy.2013.0367
44. Patton EE, Mitchell DL, Nairn RS. Genetic and environmental melanoma models in fish. Pigm Cell Melanoma Res. 2010;23(3):314–337. doi:10.1111/j.1755-148X.2010.00693.x
45. Maraka S, Janku F. BRAF alterations in primary brain tumors. Discov Med. 2018;26(141):51–60.
46. Angell TE, Lechner MG, Jang JK, Correa AJ, LoPresti JS, Epstein AL. BRAF V600E in papillary thyroid carcinoma is associated with increased programmed death ligand 1 expression and suppressive immune cell infiltration. Thyroid. 2014;24(9):1385–1393. doi:10.1089/thy.2014.0134
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.