Back to Journals » OncoTargets and Therapy » Volume 14
AG-1024 Sensitizes Sorafenib-Resistant Hepatocellular Carcinoma Cells to Sorafenib via Enhancing G1/S Arrest
Authors Zhou W, Lou W, Chen J, Ding B, Chen B, Xie H, Zhou L, Zheng S , Jiang D
Received 29 October 2020
Accepted for publication 15 January 2021
Published 15 February 2021 Volume 2021:14 Pages 1049—1059
DOI https://doi.org/10.2147/OTT.S289324
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Alberto Bongiovanni
Wei Zhou,1– 3 Weiyang Lou,4 Junru Chen,1– 3 Bisha Ding,4 Binjie Chen,2,3,5 Haiyang Xie,1– 3 Lin Zhou,1– 3 Shusen Zheng,1– 3 Donghai Jiang2,3,5
1Department of Surgery, Division of Hepatobiliary and Pancreatic Surgery, First Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou, People’s Republic of China; 2NHFPC Key Laboratory of Combined Multi-Organ Transplantation, Hangzhou, People’s Republic of China; 3Key Laboratory of the Diagnosis and Treatment of Organ Transplantation, Hangzhou, People’s Republic of China; 4Department of Breast Surgery, First Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou, People’s Republic of China; 5Key Laboratory of Organ Transplantation, Hangzhou, People’s Republic of China
Correspondence: Shusen Zheng Tel +86-571-87236567
Fax +86-571-87236466
Email [email protected]
Donghai Jiang Tel +86-571-87236570
Fax +86-571-87236466
Email [email protected]
Purpose: The frequency in resistance to sorafenib accounts for the grim prognosis of advanced hepatocellular carcinoma (HCC). In the present study, we explore the anti-cancer efficacy of co-administration of sub-toxic AG-1024 with sorafenib in HCC cells to enhance the sensitivity of these cells to sorafenib.
Materials and Methods: Two acquired sorafenib-resistant HCC cells, SNU-sora-5 and SK-sora-5, were established and verified. The MTT assay, colony formation assay, cell morphology detection and flow cytometric analysis were then used to determine the anti-tumor effects of the co-administration of sub-toxic AG-1024 and sorafenib. Finally, the potential molecular mechanism was preliminarily examined.
Results: Compared to parental cell lines, the acquired sorafenib-resistant cell lines, SNU-sora-5 and SK-sora-5, were more resistant to sorafenib. Sub-toxic AG-1024 markedly enhanced sorafenib-mediated cell inhibition in acquired sorafenib-resistant HCC strains, with a reversal index (RI) of 4.64 in SNU-sora-5 and 4.58 in SK-sora-5 cell lines. Moreover, co-administration of sub-toxic AG-1024 and sorafenib exerted dramatic cytotoxicity compared with sorafenib alone in the intrinsic sorafenib-resistant HCC-LM3 cells. In contrast to high-dose sorafenib, sub-toxic AG-1024 combined with sorafenib had less impact on apoptosis while significantly enhancing G1/S arrest via activation of the mTOR/p21 signaling pathway. The more, pharmacological inhibition of mTOR activity by inhibitor Palomid 529 significantly antagonized the synergistic anti-cancer effects of AG-1024 and sorafenib in HCC cells.
Conclusion: The current findings indicate that sub-toxic AG-1024 may be a promising therapeutic agent in enhancing the sensitivity in HCC cells to sorafenib, bringing hope to HCC patients refractory to sorafenib treatment.
Keywords: hepatocellular carcinoma, sorafenib resistance, AG-1024, mTOR, G1/S arrest
Introduction
Hepatocellular carcinoma (HCC), the most common subtype of liver cancer often seen in people with underlying chronic liver disease, accounts for more than 90% of primary HCC.1,2 Currently, the associated morbidity and mortality of HCC are still on the rise in most countries, including China and the United States, with more than half of the cases occurring in China.1 Hepatocellular carcinoma is still considered a major threat to the health and lives of people in China, and across the world. Unfortunately, a large number of cases are diagnosed at the intermediate and late stages due to its insidious onset, rapid progression and difficulty in early detection.3 Till date, limited therapeutic approaches such as percutaneous hepatic arterial chemoembolization and combined medical treatment are applicable in patients with advanced HCC. Nonetheless, the overall efficacy of these procedures has not produced satisfactory results in most patients.
Sorafenib, a multi-tyrosine kinase inhibitor (TKI), was approved by the United States Food and Drug Administration (FDA) for advanced HCC more than a decade ago.4,5 Two clinical trials, the SHAPP and Oriental studies, have, respectively, confirmed that sorafenib can extend the median survival time and the median disease progression in patients with advanced HCC. Over the past decade, it has proven to be the most widely used molecular-targeted drug that effectively decrease mortality in HCC patients.5,6 Although sorafenib has shown survival benefit in patients with advanced HCC, drug resistance and the side effects of sorafenib remain a serious clinical concern. Some patients develop resistance due to long-term use, and some patients stop using the drug due to diarrhea, hand-foot syndrome and other side effects.4 While lenvatinib, cabozantinib and regorafenib have proven effective in recent clinical trials, their overall efficacy remains poor.7,8 Consequently, a search for drug combinations with sorafenib to increase the sensitivity of liver cancer cells to sorafenib while reducing sorafenib dosage and adverse effects warrants exploring.
AG-1024 (Tyrphostin), a reversible, competitive and selective insulin-like growth factor-1 receptor (IGF-1R) inhibitor, which can regulate the signaling activity of receptor protein tyrosine kinases (PTKs) to influence key cellular processes, such as apoptosis, differentiation and proliferation. Clinically, AG-1024 has been shown to inhibit MAA-related angiogenesis,9 enhance tumor radio-sensitivity,10 and inhibit NSCLC cell proliferation by blocking IGF-1R signal.11 It also has anti-proliferative effects and induces apoptosis in a variety of breast cancer and leukemia cell lines.12 Combined with paclitaxel in a separate study, AG-1024 sensitized K562 cells to paclitaxel-induced apoptosis and significantly inhibited cell proliferation.13 As IGF-1R is strongly expressed in HCC cells,14 AG-1024 can also inhibit the proliferation and induce apoptosis of HCC cells, and significantly inhibit the invasion of HCC cells in a dose-dependent manner.10
From a large-scale screening of small molecular compounds (>1800) with potential anti-tumor activity, we observed that sub-toxic AG-1024 could achieve synergistic cytotoxicity in combination with low dose sorafenib in HCC cells. In this study, we further investigated the antitumor effects of sub-toxic AG-1024 in combination with sorafenib and its underlying signaling pathways in vitro. In addition, we showed that the co-administration of sub-toxic AG-1024 and sorafenib can dramatically inhibit the proliferation of both acquired and intrinsic sorafenib-resistant HCC cells by inducing G1/S arrest. Moreover, the detailed molecular mechanism involved in this co-treatment-mediated growth inhibition was explored.
Materials and Methods
Cell Culture
The human HCC cell lines SNU-449, SK-hep-1 and HCC-LM3 were obtained from China Center for Type Culture Collection (CCTCC, Wuhan, China). The acquired sorafenib-resistant cell lines SNU-sora-5 and SK-sora-5 were established from SNU-449 and SK-hep-1, respectively. SNU-449 and SK-hep-1 were maintained in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS). SNU-sora-5 and SK-sora-5 were maintained in RPMI 1640 medium supplemented with 10% FBS and 5μM sorafenib. HCC-LM3 was maintained in MEM supplemented with 10% FBS.
Drugs and Treatments
Sorafenib (S1040) was purchased from Selleckchem (Selleckchem, TX, USA) and AG-1024 (MB4633) was purchased from Dalian Meilun Biotechnology (Meilunbio, China). Cells were evenly seeded in various plates or dishes in a drug-free medium for 24h. They were then treated with various doses of chemotherapeutic agents with or without co-administration of AG-1024. Palomid 529, the inhibitor of mTOR, was purchased from TargetMol (TargetMol, China).
MTT Assay
Cells were harvested and resuspended to a final concentration of 104 cells/mL. Aliquots of the cell suspension were evenly distributed into 96-wells tissue culture plates. After an overnight incubation, the designated columns were treated with various drug regimens. After 72h incubation, MTT was added to detect cell viability. The medium containing MTT was replaced with 150μL DMSO in each well to dissolve the formazan crystals after 4h incubation. The absorbance in individual wells was determined at 570nm using a microplate reader (Bio-Rad, USA).
Cell Morphology Detection
Cells were inoculated in a 6cm petri dish with a density of 105 cells, and maintained for 72h with different drug regiments. The setup was observed and photographed under phase contrast microscope. As well, the cell contour and morphology were observed and inspect under microscope.
Colony Formation Assay
Cells were seeded into 6cm petri dish at a density of 2000 cells and were maintained with different drug regimens for about 2 weeks. The cell clones were stained with Crystal Violet (CV). The attached cells were fixed by methanol for 10 minutes and then stained with CV for an additional 10 minutes after washing the plates with PBS twice.
Analysis of Cell Cycle
Cell cycle distribution was determined by flow cytometry. After drug exposure for 72h, both detached and attached cells were harvested and washed twice with PBS, followed by fixation in 75% ethanol diluted in PBS. Cells were then incubated in PBS containing 100μg/mL RNase-A and 40μg/mL propidium iodide (PI) at room temperature for 0.5h prior to flow cytometric analysis. Cell cycle distribution and DNA content were analyzed using a Coulter Epics V instrument (Beckman Coulter, USA).
Analysis of Apoptosis
Annexin V/PI apoptosis detection kit (Beyotime, China) was used to detect cell apoptosis according to the manufacturer’s instructions. Cells were harvested and washed twice with PBS after a 72h treatment. They were then suspended in 400μL of binding buffer, 5μL of Annexin V antibody conjugated with fluorescein isothiocyanate (FITC) and 5μL of PI solution. After incubation in the mixture for 15 minutes at 4°C in the dark, the percentage of apoptotic cells was determined via flow cytometry.
Western Blot Analysis and Antibodies
The antibodies used were: poly-ADP-ribose polymerase (PARP) (db1841), Cle-PARP (db3552), CDK2 (db299), CDK4 (db1682) and CDK6 (db807) from Diagbio Technology, LTD. (Diagbio, China); Bax (#5023), Bcl-2 (#4223), Bcl-xl (#2764), β-actin (#8457), Phospho-Rb (#8516), p21 (#2947), Cyclin E (#20,808), Cyclin D (#2978), IGF-I Receptor β (#9750), Phospho- IGF-I Receptor β (#3021), mTOR (#2983), Phospho-mTOR (#5536), AKT (#4691) and Phospho-AKT (#4060) were obtained from Cell Signaling Technology, Inc. (CST, USA); p53 (sc-126) was obtained from Santa Cruz Biotechnology, Inc.; goat anti-rabbit and goat anti-mouse IgG peroxidase-conjugated secondary antibodies (31,460 and 31,430) were purchased from Thermo-Pierce (Rockford, USA). The cultured cells were collected and lysed by RIPA lysate to extract total protein after different treatments for Western blot analysis. Equal amounts (10μg/lane) of proteins were separated on 10% SurePAGE gels (GenScript, China) and transferred to polyvinylidene difluoride membranes. After blocking with 5% bovine serum albumin for 1h at room temperature, the membranes were incubated with the respective antibodies mentioned above at 4°C overnight. After washing with 0.1% (v/v) Tween 20 in TBS, the membranes were incubated with peroxidase-conjugated goat anti-rabbit secondary antibodies for 1h at room temperature and the proteins were detected by enhanced chemiluminescence detection system (ECL).
Statistical Analysis
Results are presented as means ± standard errors. The difference was considered statistically significant at a level of p < 0.05, and statistical power was set at 0.80. Student’s t-test was used for two-group comparisons, and multiple-treatment groups were analyzed by one-way analysis of variance (ANOVA). Statistical analysis of the data presented was performed using SPSS version 22.0 (SPSS, USA).
Results
Establishment and Verification of Acquired Sorafenib-Resistant HCC Cells
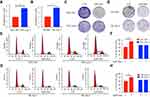
As we described previously,15 acquired sorafenib-resistant cell line SK-sora-5 was established from SK-hep-1. SNU-449 was initially exposed to sorafenib. Subsequently, a stepwise increase of sorafenib concentrations from 1µM to 5µM was performed to establish the novel acquired sorafenib-resistant cell line SNU-sora-5, which proliferated normally in 5µM sorafenib concentration. To investigate the effects of sorafenib on these cells, the MTT assay was used to detect the proliferation inhibition at different concentrations of sorafenib (0.05~20µM). As shown in Figure 1A and B, the half-maximal inhibitory concentration (IC50) values of sorafenib for SNU-449 and SK-hep-1 were 3.68±0.11µM and 4.31±0.06µM, which were statistically lower than their corresponding sorafenib-resistant cell lines (SNU-sora-5: 6.93±0.26µM; SK-sora-5: 8.09±0.35µM). The colony-forming assay’s result shown in Figure 1C and D, suggested that the size and number of colonies were markedly reduced after treatment with 5μM sorafenib in the SNU-449 and SK-hep-1 cells compared with SNU-sora-5 and SK-sora-5 cells. Furthermore, we explored the role of sorafenib in manipulating cell cycle. As shown in Figure 1E–H, treatment with 5µM sorafenib significantly increased the percentage of cells in the G1 phase from 49.54% to 73.81% in SNU-449 and 59.28% to 79.77% in SK-hep-1. On the contrary, no statistical differences were observed in SNU-sora-5 and SK-sora-5 in regards to G1 phase.
Sub-Toxic AG-1024 and Sorafenib Exerts Synergistic Anti-Cancer Effects in Acquired Sorafenib-Resistant HCC Cells
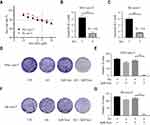
To investigate the effect of sub-toxic AG-1024 combined with sorafenib in acquired sorafenib-resistant HCC cells, we first determined the appropriate concentration of AG-1024 in SNU-sora-5 and SK-sora-5. As shown in Figure 2A, AG-1024 exhibited little cytotoxicity to SNU-sora-5 and SK-sora-5 in the range of 0.2 to 5µM. With exposure to 5µM AG-1024 for 72h, the survival rate of SNU-sora-5 and SK-sora-5 was approximately 80%. Hence, 5µM AG-1024 was chosen as sub-toxic dosage for the subsequent combination regimen. Intriguingly, 5µM AG-1024 dramatically improve the cytotoxicity of sorafenib in acquired sorafenib-resistant HCC cells as presented in Figure 2B and C. The IC50 values of combined sorafenib/sub-toxic AG-1024 in SNU-sora-5 and SK-sora-5 were significantly decreased from 6.93±0.27µM to 1.37±0.35µM and from 8.09±0.35µM to 1.59±0.10µM, respectively. Compared to sorafenib alone, the reversal index (RI) of sub-toxic AG-1024 combined with sorafenib was 4.64 in SNU-sora-5 and 4.58 in SK-sora-5. Furthermore, in the colony-forming assays, SNU-sora-5 and SK-sora-5 co-treated with sub-toxic AG-1024 and sorafenib exhibited significantly decreased colony formation ability compared to control and monotherapy groups (Figure 2D–G). These results demonstrate that sub-toxic AG-1024 has remarkable potential in reversing acquired sorafenib resistance in HCC cells.
Sub-Toxic AG-1024 and Sorafenib Exerts Synergistic Anti-Cancer Effects in Intrinsic Sorafenib-Resistant HCC Cells
The IC50 value of sorafenib for the HCC-LM3 was determined. The result revealed that HCC-LM3 is more resistant to sorafenib than SNU-449 and SK-hep-1 (Figure 3A). To further demonstrate the wide applicability of AG-1024 in sensitizing sorafenib to HCC cells, intrinsic sorafenib-resistant HCC cell HCC-LM3 was included. Sub-toxic AG-1024 was introduced to combine with sorafenib to assess whether there is a similar synergetic therapeutic effect in HCC-LM3. As shown in Figure 3B and C, the survival rate of HCC-LM3 was markedly decreased in the co-treated group. The IC50 value of sorafenib in combination with 5µM AG-1024 significantly decreased from 6.12±0.16µM to 0.57±0.14µM. Compared to sorafenib alone, the RI of sub-toxic AG-1024 combined with sorafenib was 10.82. Furthermore, the size and number of colonies were also significantly reduced after co-treatment with sub-toxic AG-1024 and sorafenib (Figure 3D and E). These findings demonstrate that co-administration of sub-toxic AG-1024 and sorafenib can significantly enhance the sensitivity of sorafenib in intrinsic-resistant HCC cells.
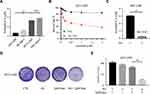
Sub-Toxic AG-1024 Combined with Sorafenib Has Less Impact on Apoptosis
Next, we probed the effects of co-administration of sub-toxic AG-1024 and sorafenib in the modulation of HCC cell apoptosis. As depicted in Figure 4A, which shows that the morphology of SNU-sora-5 did not significantly change after 72h treatment with 5µM AG-1024 and 2µM sorafenib. Albeit, after co-administration of sub-toxic AG-1024 and sorafenib, the growth of SNU-sora-5 was obviously inhibited and the abnormal morphology was observed, evidenced by fusiform adherent cells. Distinctly, a variety of bright suspended cells were observed after treatment with high-dose sorafenib (10µM). Furthermore, Annexin V/propidium iodide apoptosis detection assay was used to investigate whether AG-1024 enhances sorafenib-induced tumor cell apoptosis. As depicted in Figure 4B and C, co-administration of sub-toxic AG-1024 and sorafenib did not significantly increase the apoptotic ratio in SNU-sora-5. However, high-dose sorafenib significantly enhances apoptosis of SNU-sora-5 compared with the combination group (26.30% vs 7.24% P<0.01). Hence, co-administration of sub-toxic AG-1024 and sorafenib and high-dose sorafenib seemed to have different effects on cell morphology and apoptosis. Subsequently, the expression levels of several apoptosis-associated proteins were analyzed by Western blot. As shown in Figure 4D, the protein expression levels of PARP and cleaved PARP were not markedly altered by the co-administration of sub-toxic AG-1024 and sorafenib, but higher expression levels of both were observed in the high-dose sorafenib group. In addition, there was no significant changes in Bax and Bcl-2 in the combination group compared to the high-dose sorafenib group (Figure 4E).
Sub-Toxic AG-1024 Combed with Sorafenib Significantly Enhance G1/S Arrest
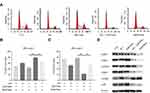
The effect of the co-administration of sub-toxic AG-1024 and sorafenib on the cell cycle was determined. As demonstrated in Figure 5A–C, SNU-sora-5 showed obvious arrest of G1 phase (79.39% vs 43.86% P<0.01) compared with 2µM sorafenib monotherapy. Consequently, a significant decline in percent S phase was seen in the combination group (11.25% vs 38.36% P<0.01), suggesting that co-treatment with sub-toxic AG-1024 and sorafenib led to G1/S arrest of SNU-sora-5 cells. Additionally, the effect of G1/S arrest was evident in the combination group compared to the high-dose sorafenib group. To better understand the mechanism by which this co-administration inhibits G1/S transition, we detected the expression of multiple critical modulators involved in this process, in particular CDK4, CDK6, Cyclin D, CDK2, Cyclin E and p-Rb. As shown in Figure 5D, among all these protein markers, the lower expression levels of CDK6, CDK2 and p-Rb and higher expression levels of Cyclin E and Cyclin D were observed in the combination group, which partially indicate G1/S phase retardation. In conclusion, our results clearly show that co-treatment with sub-toxic AG-1024 enhances sorafenib-induced G1/S arrest.
Sub-Toxic AG-1024 Combined with Sorafenib Enhance G1/S Arrest via Activating the mTOR/P21 Signaling Pathway
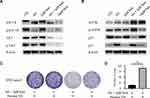
Subsequently, we investigated the potential molecular action mechanism of co-administration of sub-toxic AG-1024 and sorafenib in SNU-sora-5. We first studied the effect of this co-treatment on IGF-1R signaling. As an inhibitor of IGF-1R, AG-1024 monotherapy or combined with sorafenib downregulated expression of IGF-1R. In addition, AG-1024 could change the state of IGF-1R. As shown in Figure 6A, p-IGF-1R was significantly down-regulated. Further, lower AKT and p-AKT expression levels were observed in the combination group and high-dose sorafenib group (Figure 6A). As shown in Figure 6B, the increased expression level of mTOR was also noted in both the combination group and high-dose sorafenib groups. Even so, an apparent increase in p-mTOR expression was observed in the combination group while the high-dose sorafenib group showed much greater decrease in p-mTOR expression. Concurrently, an increase in p21 expression was noted in the combination group. It was reported that the expression of p21 was induced by p53.16 However, p53 was down-regulated in the combination group. Furthermore, Palomid 529, the inhibitor of mTOR, was used to inhibit the activity of mTOR17 in SNU-sora-5. As shown in Figure 6C, cellular growth inhibition mediated by the co-administration of sub-toxic AG-1024 and sorafenib could be significantly antagonized by Palomid 529. Additionally, the number of colonies was significantly increased after treatment with Palomid 529 (Figure 6D). Thus, the upregulation of mTOR phosphorylation might play a key role in the sub-toxic AG-1024 and sorafenib-induced G1/S arrest by up-regulating the p21 expression. These results suggest that mTOR/p21 signaling pathway is involved in the sub-toxic AG-1024 and sorafenib-induced synergistic anti-cancer effects.
Discussion
HCC, a common malignant tumor, ranks the second leading cause of cancer-related death worldwide. As an inhibitor targeting multiple tyrosine protein kinases (VEGFR, PDGFR and Raf family kinases), sorafenib acts on numerous targets to inhibit cancer proliferation and tumor angiogenesis, which can effectively lead to a decrease in mortality in HCC patients.18,19 Advanced stage HCC patients can benefit from 400mg twice daily sorafenib therapy.5,6,20 To date, however, clinical trials have revealed that only about 30% patients show sensitivity to sorafenib, and HCC usually progresses within 6 months resulting from resistance to sorafenib.2,21 Furthermore, various side effects have been documented in nearly 90% of sorafenib-treated HCC patients, including diarrhea, rash, hand-foot reaction (HFS) and fatigue.3,22 Eventually, some patients have to stop taking the medication due to these adverse effects. As a result, high-dose sorafenib was not often recommended. In a nutshell, there is an urgent need to search and develop a therapeutic agent(s) which can enhance the sensitivity of cells to sorafenib, overcome drug resistance and reduce the overall toxicity as wells as the side effects.
AG-1024, an IGF-IR inhibitor, can prohibit proliferation, induce apoptosis and decrease invasion in HCC via the blockage of the IGF-IR signaling pathway.10 In this study, we discovered that sub-toxic AG-1024 significantly improves the sensitivity of various types of liver cancer cells to sorafenib in both acquired and intrinsic-resistant HCC cells. Compared with AG-1024 monotherapy or sorafenib, the lethal effect of their combination treatment group was pronounced, indicating that AG-1024 might be a promising drug that can enhance sorafenib-mediated cell death in HCC.
Numerous studies have documented the close correlation between apoptosis and cell cycle progression.23 Cellular apoptosis is often mediated by extrinsic and intrinsic pathways. The extrinsic pathway is mediated by the death receptors in the cyto-membrane, which further activates downstream caspase followed by the cleavage of PARP. In contrast, the intrinsic pathway is tightly correlated with the Bcl-2 family of proteins, including pro-apoptotic protein Bax, the anti-apoptotic protein Bcl-2 and Bcl-xl, which regulate apoptosis by controlling the mitochondrial outer membrane permeability.24,25 Our results suggest that co-administration of sub-toxic AG-1024 and sorafenib had a lesser impact on apoptosis. However, high-dose sorafenib significantly promoted apoptosis. Hence, apoptosis might not play a key role in the growth inhibition-mediated by co-administration of sub-toxic AG-1024 and sorafenib. The molecular mechanisms of sub-toxic AG-1024 and sorafenib-induced anti-cancer effect differ from high-dose sorafenib.
Compared with high-dose sorafenib, co-administration of sub-toxic AG-1024 and sorafenib induced greater G1/S arrest in HCC cells. This was confirmed by the analysis of expression of several key regulators involved in cyclin associated proteins. Co-administration of sub-toxic AG-1024 and sorafenib enhanced G1/S arrest through partial downregulation of CDK2, CDK6 and p-Rb expression levels, while upregulating cyclin E expression. Contrary to high-dose sorafenib, the expression of CDK4 and cyclin D were significantly upregulated. Hence, the detailed mechanisms associated with these observed difference between the co-administration and high-dose sorafenib groups need further exploration. In 40–50% of HCC cells, mTOR is activated to regulate the cell cycle cell, proliferation and resist apoptosis, which is closely linked to acquired drug resistance of HCC.9,11,12 Moreover, it has also been established that IGF-IR fuels mTOR.26,27 To validate whether AG-1024 enhances sorafenib sensitivity by regulating mTOR-related signaling pathway, a Western blot analysis together with the administrations of an mTOR inhibitor was performed. Although the mTOR expression level was significantly upregulated in both the combination and high-dose sorafenib groups, a considerable up-regulation of mTOR phosphorylation and p21 was only observed in the combination group. It was reported that CDKN1A (p21) acts as the downstream target gene of TP53 (p53). The expression of p21 is induced by wild-type p53 and is not associated with mutant p53.16 Notably, p21 plays a significant role in tumor development through p53-dependent and p53-independent pathways. In this study, low expression of p53 showed an opposite trend to the high expression of p21 in the combination group. Through the p53-independent pathways, p21 played an important role in synergistic injury mediated by AG-1024 in combination with sorafenib. The molecular mechanism needs further study. This high phosphorylation level suggests that co-administration of sub-toxic AG-1024 and sorafenib enhances G1/S arrest via activation of the mTOR/p21 signaling pathway. Besides, the co-administration of sub-toxic AG-1024 and sorafenib-mediated growth inhibition could be significantly antagonized by Palomid 529, which also demonstrates the important role of mTOR in sub-toxic AG-1024 and sorafenib-induced synergistic anti-cancer effects.
Although this work explicitly demonstrates that sub-toxic AG-1024 combined with sorafenib exerts a significant anti-tumor effect in hepatocellular carcinoma cells, there are some limitations that should be further investigated in the future. In particular, the lack of in vivo anti-tumor experiments; the important role AG-1024 plays in controlling other cancer types; and the combined efficacy of AG-1024 with other molecular targeted drugs in cancer.
Conclusion
Collectively, our study demonstrates that AG-1024 sensitizes HCC cells to sorafenib via enhancing G1/S arrest. We propose for the first time that AG-1024 holds potential as a promising drug to stimulate and enhance the antitumor effect of sorafenib in HCC. It may play an essential role in the quest to overcome sorafenib resistance in clinical practice in the future and offer hope to patients with sorafenib-resistant HCC.
Acknowledgments
This work was supported by Fundamental Research Funds for the Central Universities (2020FZZX003-01-08); National S&T Major Project (No. 2017ZX10203205); Innovative Research Groups of National Natural Science Foundation of China (No. 81721091); Zhejiang Provincial Natural Science Foundation (LY18H160017); Zhejiang International Science and Technology Cooperation Project (NO.2016C04003); Research Unit Project of Chinese Academy of Medical Sciences (2019-I2M-5-030).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. doi:10.3322/caac.21262
2. Tang WW, Chen ZY, Zhang WL, et al. The mechanisms of sorafenib resistance in hepatocellular carcinoma: theoretical basis and therapeutic aspects. Signal Transduct Target Ther. 2020;5(1):1–15. doi:10.1038/s41392-020-0187-x
3. Morse DC, Chockalingam R, Pye A, Huen A. Hidradenitis suppurativa associated with sorafenib initiation. J Am Acad Dermatol. 2019;81:AB219–AB219.
4. Ray E, Sanoff H. Optimal therapy for patients with hepatocellular carcinoma and resistance or intolerance to sorafenib: challenges and solutions. J Hepatocell Carcinoma. 2017;4:131–138. doi:10.2147/JHC.S124366
5. Grazie ML, Biagini MR, Tarocchi M, Polvani S, Galli A. Chemotherapy for hepatocellular carcinoma: the present and the future. World J Hepatol. 2017;9(21):907–920. doi:10.4254/wjh.v9.i21.907
6. Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–390. doi:10.1056/NEJMoa0708857
7. Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a Phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10(1):25–34. doi:10.1016/S1470-2045(08)70285-7
8. Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised Phase 3 non-inferiority trial. Lancet. 2018;391:1163–1173.
9. Totoki Y, Tatsuno K, Covington KR, et al. Trans-ancestry mutational landscape of hepatocellular carcinoma genomes. Nat Genet. 2014;46(12):1267–1273. doi:10.1038/ng.3126
10. Yao WF, Liu JW, Sheng GL, Huang DS. Blockade of IGF-IR exerts anticancer effects in hepatocellular carcinoma. Mol Med Rep. 2011;4(4):719–722. doi:10.3892/mmr.2011.486
11. Morgensztern D, McLeod HL. PI3K/Akt/mTOR pathway as a target for cancer therapy. Anticancer Drugs. 2005;16(8):797–803. doi:10.1097/01.cad.0000173476.67239.3b
12. Chen KF, Tai WT, Hsu CY, et al. Blockade of STAT3 activation by sorafenib derivatives through enhancing SHP-1 phosphatase activity. Eur J Med Chem. 2012;55:220–227. doi:10.1016/j.ejmech.2012.07.023
13. Cheng HY, Ko FH. Studying the enhancement of programmed cell death by combined AG-1024 and paclitaxel in a model of chronic myelogenous leukemia. Life Sci. 2014;102(2):118–126. doi:10.1016/j.lfs.2014.03.014
14. Zhang ZQ, Lei B, Chai W, Liu RH, Li TJ. Increased expression of insulin-like growth factor-1 receptor predicts poor prognosis in patients with hepatocellular carcinoma. Medicine. 2019;98(44):e17680. doi:10.1097/MD.0000000000017680
15. Chen RG, Cheng QY, Owusu-Ansah KG, et al. Cabazitaxel, a novel chemotherapeutic alternative for drug-resistant hepatocellular carcinoma. Am J Cancer Res. 2018;8(7):1297–1306.
16. Xiao B, Zhao Y, Jia X, Wu J, Wang Y, Huang F. Multifaceted p21 in carcinogenesis, stemness of tumor and tumor therapy. World J Stem Cells. 2020;12(6):481–487. doi:10.4252/wjsc.v12.i6.481
17. Mark A. RES-529: a PI3K/AKT/mTOR pathway inhibitor that dissociates the mTORC1 and mTORC2 complexes. Anticancer Drugs. 2016.
18. Cabral LKD, Tiribelli C, Sukowati CHC. Sorafenib resistance in hepatocellular carcinoma: the relevance of genetic heterogeneity. Cancers. 2020;12(6):1576. doi:10.3390/cancers12061576
19. Fan G, Wei X, Xu X. Is the era of sorafenib over? A review of the literature. Ther Adv Med Oncol. 2020;12:175883592092760. doi:10.1177/1758835920927602
20. Prejac J, Kekez D, Belev B, et al. Leukocytoclastic vasculitis associated with sorafenib treatment for hepatocellular carcinoma. Anticancer Drugs. 2020;31(1):76–79. doi:10.1097/CAD.0000000000000840
21. Chen W, Yang J, Zhang Y, Cai H, Chen X, Sun D. Regorafenib reverses HGF-induced sorafenib resistance by inhibiting epithelial-mesenchymal transition in hepatocellular carcinoma. FEBS Open Bio. 2019;9(2):335–347. doi:10.1002/2211-5463.12578
22. Jang HJ, Lee SA, Seong S, Kim S, Han G. Combined treatment for lung metastasis from hepatocellular carcinoma: a case report. Explore. 2018;14(5):385–388. doi:10.1016/j.explore.2017.11.004
23. Huang JL, Cao SW, Ou QS, et al. The long non-coding RNA PTTG3P promotes cell growth and metastasis via up-regulating PTTG1 and activating PI3K/AKT signaling in hepatocellular carcinoma. Mol Cancer. 2018;17(1):93. doi:10.1186/s12943-018-0841-x
24. Han W, Yu F, Wang R, Guan W, Zhi F. Valproic acid sensitizes glioma cells to luteolin through induction of apoptosis and autophagy via akt signaling. Cell Mol Neurobiol. 2020. doi:10.1007/s10571-020-00930-2
25. Wang CH, Yang JM, Guo YB, Shen J, Pei XH. Anticancer activity of tetrandrine by inducing apoptosis in human breast cancer cell line MDA-MB-231 in vivo. Evid Based Complement Alternat Med. 2020;2020:1–11.
26. Liu H, Moroi Y, Yasumoto S, et al. Expression of insulin-like growth factor-1 receptor, p-AKT and p-ERK1/2 protein in extramammary paget’s disease. Br J Dermatol. 2006;155(3):586–591. doi:10.1111/j.1365-2133.2006.07366.x
27. Hixon ML, Paccagnella L, Millham R, Perez-Olle R, Gualberto A. Development of inhibitors of the IGF-IR/PI3K/Akt/mTOR pathway. Rev Recent Clin Trials. 2010;5(3):189–208. doi:10.2174/157488710792007329
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.