Back to Journals » Journal of Pain Research » Volume 17
Acupuncture-Related Therapy for Knee Osteoarthritis: A Narrative Review of Neuroimaging Studies
Authors Qu Y , Peng Y, Xiong Y, Dong X, Ma P, Cheng S
Received 18 November 2023
Accepted for publication 21 February 2024
Published 27 February 2024 Volume 2024:17 Pages 773—784
DOI https://doi.org/10.2147/JPR.S450515
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Houman Danesh
Yuzhu Qu,1– 3,* Ying Peng,4,* Yan Xiong,5 Xiaohui Dong,3 Peihong Ma,6 Shirui Cheng2,3
1Post-Doctoral Scientific Research Workstation of Affiliated Sport Hospital, Chengdu Sport University, Chengdu, Sichuan, People’s Republic of China; 2Acupuncture and Tuina School, Chengdu University of Traditional Chinese Medicine, Chengdu, Sichuan, People’s Republic of China; 3Acupuncture and Brain Science Research Center, Chengdu University of Traditional Chinese Medicine, Chengdu, Sichuan, People’s Republic of China; 4Medical Aesthetics Department, Affiliated Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu, Sichuan, People’s Republic of China; 5Department of Osteoporosis, West China Fourth Hospital Sichuan University, Chengdu, Sichuan, People’s Republic of China; 6Medical Technology School, Tianjin University of Traditional Chinese Medicine, Tianjin, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Shirui Cheng, Chengdu University of Traditional Chinese Medicine, 1166 # Liutai Avenue, Chengdu, Sichuan, 611137, People’s Republic of China, Email [email protected] Peihong Ma, Tianjin University of Traditional Chinese Medicine, 10 # Poyanghu Road, Tuanpo Xincheng West District, Jinghai District, Tianjin, 301617, People’s Republic of China, Email [email protected]
Abstract: Acupuncture has been widely applied for treating knee osteoarthritis (KOA). Numerous studies have found that acupuncture can effectively alleviate KOA symptoms. With the advancement of neuroimaging techniques, integrating neuroimaging with in-depth investigations of acupuncture mechanisms has emerged as a hot topic in traditional Chinese medical neuroscience research. This review aimed to analyze the study design and main findings from neuroimaging studies of acupuncture-related therapy for KOA to provide a reference for future research. Original studies were sourced from English databases (PubMed, Embase, and Cochrane Library) and Chinese databases (Chinese National Knowledge Infrastructure, Chinese Biomedical Literature Database, the Chongqing VIP database, and Wanfang database). As a result, thirteen articles were ultimately included in this review. Functional magnetic resonance imaging was the most frequently used neuroimaging technique to explore cerebral responses to acupuncture-related therapy for KOA. Findings suggested that acupuncture-related therapy could regulate some brain regions in patients with KOA. Specifically, for acupuncture, it showed that the medial pain pathway and the limbic system were involved in the regulation of KOA. Meanwhile, moxibustion induced a wide range of functional activity throughout the entire brain.
Keywords: acupuncture-related therapy, knee osteoarthritis, cerebral response, neuroimaging study
Introduction
Osteoarthritis of the knee (KOA), the most prevalent joint disorder, is characterized by the loss of articular cartilage, progressive degeneration, pain, and physical dysfunction.1,2 Approximately 30% of individuals over 45 years old have radio-graphic evidence of KOA, and nearly half of them exhibit knee symptoms.3 Besides its significant impact on physical disability,4 KOA is also associated with an increased risk of all-cause mortality.5,6 Patients with KOA spend an average of over $10,000 in direct medical costs for this osteoarthritis throughout their lifetimes.3 The management of KOA spans non- or pharmacologic interventions, orthopedic aids, and surgery. Based on the treatment guidelines,7–9 acupuncture is recommended for the management of KOA symptoms. As a traditional complementary therapy originating in China, acupuncture-related therapy has been utilized for thousands of years to address knee-related disorders. According to traditional Chinese medicine theory, acupuncture and moxibustion are the most important components of acupuncture-related therapy. Specifically, acupuncture is characterized by inserting a metal needle into a subcutaneous acupoint, mainly including manual acupuncture, electro-acupuncture, auriculoacupuncture, and more.10 Moxibustion is identified as igniting moxa on the skin of an acupoint to produce warmth and cause redness around the peripheral.11 Clinically, acupuncture and moxibustion can be used alone or in combination to treat certain chronic diseases.12 Substantial studies have found that acupuncture-related therapy is beneficial to KOA.13–15
Advances in neuroimaging technologies for pain research have provided insights into cerebral mechanisms of acupuncture for KOA. For instance, KOA patients have exhibited abnormalities in brain functional activity and connectivity, such as the periaqueductal gray, raphe nuclei, medial frontal cortex, and bilateral hippocampus, while acupuncture could regulate these abnormalities to control KOA.16,17 Since the publication of the first neuroimaging study on acupuncture-related treatment for KOA in 2013,18 13 neuroimaging studies have been conducted in the past decade, supporting the effect of acupuncture on central regulation. These neuroimaging studies have described specific research methods, such as verum acupuncture (also known as real acupuncture, involving the insertion of a metal needle into an acupoint) and sham acupuncture (involving the use of a placebo needle device to prevent penetration of the skin at an acupoint or sham acupoint), heat-sensitive moxibustion and sham moxibustion, different neuroimaging techniques and analysis methods. Furthermore, they have reported different brain responses elicited by acupuncture. For instance, research has revealed that acupuncture could enhance functional connectivity (FC) between dorsal raphe and striatum,17 in the right frontoparietal network and the executive control network with the rostral anterior cingulate cortex/medial prefrontal cortex.19
Therefore, we summarized these available neuroimaging studies of acupuncture-related therapy for KOA, reviewed the methodological issues and the core regulated brain regions involved in acupuncture analgesia for KOA. Furthermore, this review provided prospects for future neuroimaging research on acupuncture-related therapy for KOA.
Materials and Methods
Literature Search
The literature search was performed in the following databases from inception up to March 31, 2023 and updated on October 10, 2023: PubMed, Embase, Cochrane Library (CENTRAL), Chongqing VIP Chinese Science and Technology Periodical Database, Wanfang database, Chinese Biomedical Literature Database, and Chinese National Knowledge Infrastructure, without language restrictions. In addition, reference lists of related publications were checked for potentially applicable studies. A combination of MeSH terms, free-text terms, synonyms, and subject headings related to “knee osteoarthritis”, “acupuncture”, “moxibustion”, and “neuroimaging” were included in this strategy. The search strategy for PubMed is shown in Supplementary Table 1. Equivalent search terms were used in other databases.
Study Selection and Data Extraction
Studies were selected and included based on population, intervention, comparison, outcome and study design if they met the following criteria: 1) conducted among patients with KOA, 2) applied acupuncture and/or moxibustion as interventions, and 3) utilized neuroimaging techniques to explore cerebral responses. Conversely, animal studies, reviews, conference abstracts, commentaries, editorials, protocols, and clinical studies using experimental pain models were excluded.
Two reviewers (Ying Peng and Yan Xiong) independently screened all titles and abstracts, followed by full texts to identify eligible articles. The information of eligible articles was extracted by the two reviewers and then cross-checked by a third reviewer (Xiaohui Dong), including first author’s name, publication year, controls, sample size, interventions, neuroimaging modality and conditions, analytical methods, and outcomes of neuroimaging data. Any disagreements were resolved through discussion, with Yuzhu Qu serving as a judge if no agreement was reached.
Data Analysis
A descriptive analysis was conducted to summarize the methodological issues of the published A&M neuroimaging studies on KOA and cerebral responses elicited by A&M.
Results
Study Selection and Description
A total of 312 potential articles were initially identified using the primary search strategy. After removing duplicates and screening titles, abstracts, and full texts, 13 eligible clinical neuroimaging trials were ultimately included in this review (Figure 1). These articles were published between 2013 and 2023. Among them, six studies were conducted in the United States (Studies 1–6),19–24 and the remaining seven studies were carried out in China (Studies 7–13).25–31 Ten studies used acupuncture (eight used manual acupuncture, and two used electro-acupuncture), while three studies used moxibustion. Additionally, 12 studies applied blood-oxygen-level-dependent functional magnetic resonance imaging (BOLD-fMRI) as the neuroimaging tool, and one study used EEG (Study 13). Characteristics of included studies are shown in Table 1.
 |
Table 1 Characteristics of the 13 Included Studies |
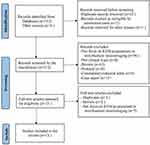 |
Figure 1 Flow diagram of this review. |
Participants
This review included a total of 561 KOA participants and 131 healthy subjects. The age of patients with KOA ranged between 40 and 69.4 years. The duration of KOA varied from 0.33 to 19 years (not stated in half of these studies).
Acupuncture-Related Interventions
Ten studies used acupuncture for KOA. Among them, three studies (Studies 1, 5, and 8) focused on the immediate effect of acupuncture, with a single treatment duration ranging from 6 to 25 minutes. Seven studies (Studies 2, 3, 4, 6, 7, 9, and 10) examined the long-term effect of acupuncture, employing 6 to 30 treatment sessions over 2 to 8 weeks, with each session lasting 25 to 30 minutes. The other three studies (Studies 11–13) focused on the immediate effect of moxibustion for KOA, with a single treatment duration of 30 minutes. The most frequently used acupoints for KOA were Dubi (ST35), Xiyan (EX-LE4 and EX-LE5), Yanglingquan (GB34), Yinlingquan (SP9), Xuanzhong (GB39), and Sanyinjiao (SP6). Almost all of included studies (except Study 1) stated the acquisition of needle sensation (deqi) or moxibustion sensation during each treatment session.
Neuroimaging Scan and Data Analysis
Twelve studies (Studies 1–12) applied BOLD-fMRI with resting scans to investigate brain responses, followed by functional segregation analysis (regional homogeneity, ReHo; amplitude of low-frequency fluctuations/fractional amplitude of low-frequency fluctuations, ALFF/fALFF), functional integration analysis (functional connectivity, FC; independent component analysis, ICA; brain network), and structural analysis (cortical thickness; sub-cortical volume). Study 13 utilized EEG with a task condition to analyze the power spectral density of the central region in KOA patients.
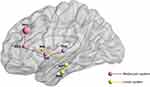
Cerebral responses of acupuncture-related therapy for KOA are shown in Table 2. Frequently reported brain regions included the prefrontal cortex (PFC, including the medial, lateral, dorsolateral, and orbital PFC), cingulate gyrus (anterior cingulate cortex, ACC; rostral ACC, rACC;), temporal gyrus, periaqueductal grey (PAG), insula, thalamus, hippocampus, parahippocampal gyrus, and nucleus accumbens (NAc) (Figure 2).
 |
Table 2 Brain Imaging Data of Acupuncture-Related Therapy Neuroimaging Studies in KOA |
Results of Risk-of-Bias Assessments
The risk-of-bias assessment in clinical neuroimaging research is not well established. In this review, based on previous acupuncture neuroimaging reviews,32 the risk-of-bias was assessed using the RoB 2 and ROBINS-I for RCTs and non-RCTS, respectively, as shown in Table 1. Among the eight RCTs, two studies were rated as having a “low” overall risk of bias (Studies 9 and 10). The remaining six were rated as having a “some concerns” due to acupuncturists always being aware of group assignments; it is concerning that there was no reporting on whether outcome evaluators were blinded to the group assignment (Study 1–6). The five included non-RTCs were rated as having a “moderate” overall risk of bias, also because it was not clear whether outcome evaluators were aware of participants’ interventions (Study 7–8 and 11–13).
Discussion
Over the past decade, there has been increased attention given to explore the cerebral mechanism of acupuncture for KOA by neuroimaging techniques. To date, 13 articles measuring brain activities have been considered, providing visualized evidence for a deeper understanding of the central mechanism of acupuncture in treating KOA.
Neuroimaging Techniques and Analysis Methods Applied in Acupuncture-Related Therapy for KOA
Since the 1990s, neuroimaging techniques, particularly fMRI (12 out of the 13 included studies), have been widely applied to explore the central mechanisms of acupuncture-related therapy. fMRI offers advantages such as good spatiotemporal resolution,29 timely and visualized imaging, non-invasion,30 and lack of radiation31 to investigate cerebral response. Within the review, ReHo, ALFF/fALFF, and FC were the most frequently used to analyze the fMRI data. ReHo reflects the consistency of functional activities of adjacent brain regions,33 with a higher ReHo value indicating a consistent local functional activity in a brain region and a lower value implying local abnormal neural activities. Due to its good repeatability, one quarter of the included fMRI studies used ReHo to explore the central mechanism of acupuncture for KOA. In this review, one-third of the included fMRI studies used ALFF/fALFF as an indicator of neuronal activity, reflecting the spontaneous neuronal activity through the magnitude of spontaneous BOLD signal. Meanwhile, fALFF is an improvement on ALFF to avoid interference from physiological signals.34 The abnormality of ALFF/fALFF is thought to be a candidate biomarker for the prognosis of various pain conditions.35 More than half of the included fMRI studies in this review selected FC as an indicator of the brain network, measuring neuronal activities based on the specific region of interest of the brain, representing neural communication among brain regions.36 These analytical methods are sensitive and reliable to be used in investigating the cerebral mechanism of acupuncture-related therapy.
Cerebral Response to Acupuncture for KOA Patients
Based on the neuroimaging results, acupuncture treatment induced brain regulation mainly in several brain regions, including PFC, ACC, insula, thalamus, nucleus NAc, hippocampus, and parahippocampal gyrus, with an increased FC in PFC-rACC/PAG. A more extensive and notable brain response was exhibited in verum acupuncture compared to sham acupuncture, including PFC, PAG, ACC, thalamus, insula, etc., with an enhanced FC in PFC-ACC/insula/PAG.
KOA patients with chronic pain experience abnormal brain functional activity in somatosensory, affective, and cognitive, involving multiple brain regions and networks associated with the pain processing systems.37–39 Öztürk et al found that KOA patients with chronic pain exhibited abnormal functional activity of the dorsolateral PFC (dlPFC).40 Liu et al demonstrated that KOA patients showed dysregulated FC between the hippocampus and thalamus/superior frontal gyrus.41 Furthermore, Barroso et al revealed that KOA pain was linked to disruption of whole-brain and local FC, with major nodal connectivity changes identified in the primary somatosensory cortex/primary motor cortex, parahippocampal gyrus, and insula.36 In this review, studies showed that acupuncture activated lateral and orbital PFC and insula/putamen and regulate FC among PFC, ACC, thalamus, insula, PGA, etc. These brain regions, including the thalamus, ACC, and PFC, are thought to participate in processing motivation-affective, cognitive-evaluative, and memory aspects of pain,42–45 which are associated with the medial pain system. The medial pain system originates from pain-specific neurons in the superficial dorsal horn of the spinal cord, projects via thalamus to ACC and insula, and transmits the cognitive-emotional component of pain to the PFC.42 The thalamus is a relay station for transmitting pain information to the PFC, which is the key to pain awareness.46 Previous research demonstrated that KOA with chronic pain caused altered FC of the thalamus to several brain regions, which also correlated with negative affect in these brain regions.39 ACC is crucial for responding to pain-related anxiety and fear,47–49 and KOA pain is connected to the abnormal FC of ACC to other brain areas.50 While the PFC receives ascending, nociceptive input, it also exerts a substantial top-down control role in pain sensation, which relies on its connections to hippocampus, PAG, thalamus, amygdala, etc.51–53 Although the altered functional activity of these brain regions was represented in KOA, the abnormality could be reversed once pain is controlled by acupuncture. Thus, the findings suggested that the medial pain system is involved in the central response to acupuncture for KOA pain relief in regulating cognitive, memory, and emotion.
According to the included neuroimaging studies, acupuncture was found to regulate abnormal functional activity in the cortical structure (the cingulate gyrus) and subcortical structures (hippocampus, parahippocampal gyrus, and NAc) of patients with KOA. This regulation was associated with the limbic system, which plays a role in the affective aspects of pain, regulating emotional and motivational responses.54,55 Acupuncture with expectancy was found to alleviate KOA pain better than regular acupuncture, and this central mechanism was correlated with an increased FC between NAc and the medial PFC/rACC/dlPFC.23 Since NAc and ACC are involved in emotion, motivation and reward-related behaviors,56,57 functional changes in these regions can lead to emotional and cognitive problems in patients with KOA pain. Acupuncture could improve KOA pain by regulating these brain areas in the limbic system. Notably, these brain regions were functionally interconnected and participated in pain processing together. Acupuncture not only enhanced FC among hippocampus, parahippocampal gyrus, PFC and ACC21 but also increased FC between hippocampus and PAG.16 The hippocampus is involved in forming emotional memory of pain through the Papez circuit and transmitting information to the limbic system.58,59 Meanwhile, the parahippocampal gyrus is understood to play a role in pain sensitivity.60 The pain sensitivity is correlated with the altered function of parahippocampal gyrus in KOA patients.37 Studies showed that acupuncture could regulate altered functional activity of these brain regions. Acupuncture reduces the intensity of KOA pain may be correlated to regulating these regions in the limbic system to improve negative emotions. Therefore, the effect of acupuncture in treating KOA may be partly attributed to the regulation of the medial pain and limbic systems, which presents potential targets for pain modulation in KOA.
Intriguingly, it is known that psychological factors influence chronic pain to some extent.61 Some studies have suggested that baseline psychological conditions, such as expectancy, partly determined KOA pain responses to acupuncture.62 Hashmi et al found that the psychologically conditioned analgesia was invariant to sham versus real treatment.26 It plays a direct role in modulating pain, independent of any direct analgesic effect of acupuncture, suggesting acupuncture may or may not be more effective than a placebo intervention in treating KOA. Although the placebo of psychological factors showed an effect on relieving pain, some studies found that psychological condition controls KOA pain through different pathways based on different treatment modalities. For instance, Gollub et al reported that expectancy similarly modulated KOA pain in both verum and sham acupuncture, while they showed different regulation patterns in brain response.20 Therefore, these studies provide new insights into combining positive psychological factors with treatment modalities to enhance clinical efficacy.
Cerebral Response to Moxibustion for KOA Patients
Moxibustion, an important component of acupuncture therapy, is also commonly used for KOA. Similar to acupuncture, moxibustion regulated brain functional activity of KOA patients in frontal lobe, thalamus, hippocampus, cingulate cortex, and occipital lobe. It is inferred that acupuncture and moxibustion had some similarities in activating brain regions through exogenous stimulation on acupoints and meridians.63–65 However, moxibustion differs from acupuncture in that it also showed a more extensive response in the cerebrum, midbrain, cerebellum, central hemisphere, white matter, etc. Specifically, moxibustion was found to increase the fALFF values in the cerebrum, left cerebellum, central hemisphere, extra-nucleus, and white matter, and decrease the fALFF values in precentral gyrus, frontal lobe, and occipital lobe. Additionally, moxibustion could increase the ReHo values in thalamus, extra-nucleus and parietal lobe while decrease it in the cerebrum and frontal lobe in KOA patients. The effect of moxibustion on KOA patients involves complex coordinated regulation among multiple brain regions. For example, Zheng et al reported that moxibustion for KOA increased the fALFF values in parietal precuneus, posterior cingulate cortex, anterior cerebellum, central hemisphere, and white matter while decreased the fALFF values in superior temporal gyrus and occipital cuneiform.27 It is speculated that the central mechanism between acupuncture and moxibustion for KOA may differ to some extent. For acupuncture, it involves the process of stimulating specific acupoint by inserting metal needles and manipulates the needle, so its effect is produced by the physical stimulation that penetrates the skin.66 While moxibustion is used to treat diseases by burning moxa, it possesses features of heat, light, moxa smoke, and drug properties.67 The thermal product of moxibustion is an essential factor in achieving clinical effects. Moxibustion’s unique thermal effect can activate local specific receptors and transmit the signal to the thermal cortical center, thereby eliciting a broad response in cerebral areas.68 Despite moxibustion could elicit a wide cerebral response, the central mechanism of moxibustion for KOA is not yet clear. Therefore, further research is necessary to elucidate the cerebral mechanism of moxibustion in the future.
Future Perspectives
First, acupuncture-related therapy is known to have both immediate69 and cumulative effects.70 Some studies have explored the cerebral response caused by the immediate effect of acupuncture, and others have focused on the response elicited by its cumulative effect. However, few studies have investigated the differences in changes of cerebral response in KOA patients with different doses of acupuncture-related stimulation. It remains unclear how many intervention sessions could produce the maximum improvement in KOA symptoms and to what extent brain functional activity/structure changed. Hence, future studies could investigate the correlation between the amount of acupuncture-related stimulation and changes in cerebral activity or structure in KOA patients. Second, there may exist differences in efficacy among acupuncture, moxibustion, and other therapies, as well as their underlying mechanisms. Therefore, in the future, the effects of different therapies on the cerebral response could be compared to identify specific therapeutic targets of acupuncture-related therapy. Third, previous studies have reported mild degeneration of white matter in patients with KOA.18,27 However, few studies of acupuncture or moxibustion for KOA have focused on brain structure. This may be related to the short treatment course of clinical effect-acting, which is insufficient to observe significant changes in brain structure. Future research could use relatively long-term courses to investigate whether and how A&M regulates brain structure in KOA patients. Finally, it has been found that individuals’ brain functional activities are continuously changing, even in the resting state.71 Meanwhile, current studies primarily explore KOA patients’ cerebral responses in a resting state after acupuncture-related stimulation. Future research could consider a task-state study design to investigate the regulation of dynamic functional brain activities by acupuncture-related therapy in KOA patients.
Conclusion
Acupuncture is effective in treating KOA, while its underlying cerebral mechanism remains unclear. This review synthesized the study design and main neuroimaging research findings on acupuncture-related therapy for KOA. The results suggested that acupuncture could regulate pain-related cerebral regions in KOA patients associated with the medial pain and limbic systems, while moxibustion could regulate a wide range of brain regions. Future neuroimaging research is needed to reveal the central mechanisms of acupuncture for KOA.
Acknowledgments
This study was supported by the Sichuan Science and Technology Program (No. 2022NSFSC0856), China Postdoctoral Science Foundation (No. 2021MD703796), National Natural Science Foundation of China (No. 82205288), and Medical Technology Project of Health Commission of Sichuan Province (No. 21PJ110).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Bijlsma JW, Berenbaum F, Lafeber FP. Osteoarthritis: an update with relevance for clinical practice. Lancet. 2011;377(9783):2115–2126. doi:10.1016/S0140-6736(11)60243-2
2. Shi X, Yu W, Wang T, et al. Electroacupuncture alleviates cartilage degradation: improvement in cartilage biomechanics via pain relief and potentiation of muscle function in a rabbit model of knee osteoarthritis. Biomed Pharmacother. 2020;123:109724. doi:10.1016/j.biopha.2019.109724
3. Katz JN, Arant KR, Loeser RF. Diagnosis and treatment of hip and knee osteoarthritis: a review. JAMA. 2021;325(6):568–578. doi:10.1001/jama.2020.22171
4. Jamshidi A, Pelletier JP, Martel-Pelletier J. Machine-learning-based patient-specific prediction models for knee osteoarthritis. Nat Rev Rheumatol. 2019;15(1):49–60. doi:10.1038/s41584-018-0130-5
5. Cooper C, Arden NK. Excess mortality in osteoarthritis. BMJ. 2011;342:d1407.
6. Wang Y, Nguyen UDT, Lane NE, et al. Knee osteoarthritis, potential mediators, and risk of all-cause mortality: data from the osteoarthritis initiative. Arthritis Care Res. 2021;73(4):566–573. doi:10.1002/acr.24151
7. Kolasinski SL, Neogi T, Hochberg MC, et al. 2019 American College of Rheumatology/arthritis foundation guideline for the management of osteoarthritis of the hand, hip, and knee. Arthritis Care Res. 2020;72(2):149–162. doi:10.1002/acr.24131
8. Skou ST, Roos EM. Physical therapy for patients with knee and Hip osteoarthritis: supervised, active treatment is current best practice. Clin Exp Rheumatol. 2019;37(Suppl 120):112–117.
9. Schnitzer TJ. Update of ACR guidelines for osteoarthritis: role of the coxibs. J Pain Symptom Manage. 2002;23(4):S24–S30; discussion S31–4. doi:10.1016/S0885-3924(02)00372-X
10. Wan R, Fan Y, Zhao A, et al. Comparison of efficacy of acupuncture-related therapy in the treatment of rheumatoid arthritis: a network meta-analysis of randomized controlled trials. Front Immunol. 2022;13:829409. doi:10.3389/fimmu.2022.829409
11. Wang QM, Gao M, Li SX, et al. Effect of mild moxibustion with moxa stick and infrared mild moxibustion on skin blood perfusion at Waiguan (TE 5). Zhongguo Zhen Jiu. 2023;43(11):1269–1274. doi:10.13703/j.0255-2930.20221115-k0001
12. Wang X, Wang X, Hou M, et al. Warm-needling moxibustion for knee osteoarthritis: a randomized controlled trial. Zhongguo Zhen Jiu. 2017;37(5):457–462. doi:10.13703/j.0255-2930.2017.05.001
13. Lv ZT, Shen LL, Zhu B, et al. Effects of intensity of electroacupuncture on chronic pain in patients with knee osteoarthritis: a randomized controlled trial. Arthritis Res Ther. 2019;21(1):120. doi:10.1186/s13075-019-1899-6
14. Zhao L, Cheng K, Wang L, et al. Effectiveness of moxibustion treatment as adjunctive therapy in osteoarthritis of the knee: a randomized, double-blinded, placebo-controlled clinical trial. Arthritis Res Ther. 2014;16:R133.
15. Chen N, Wang J, Mucelli A, et al. Electro-acupuncture is beneficial for knee osteoarthritis: the evidence from meta-analysis of randomized controlled trials. Am J Chin Med. 2017;45(05):965–985. doi:10.1142/S0192415X17500513
16. Egorova N, Gollub RL, Kong J. Repeated verum but not placebo acupuncture normalizes connectivity in brain regions dysregulated in chronic pain. Neuroimage Clin. 2015;9:430–435. doi:10.1016/j.nicl.2015.09.012
17. Gao N, Shi H, Hu S, et al. Acupuncture enhances dorsal raphe functional connectivity in knee osteoarthritis with chronic pain. Front Neurol. 2021;12:813723. doi:10.3389/fneur.2021.813723
18. Xie H, Xu F, Chen R, et al. Image formation of brain function in patients suffering from knee osteoarthritis treated with moxibustion. J Tradit Chin Med. 2013;33(2):181–186. doi:10.1016/S0254-6272(13)60122-3
19. Chen X, Spaeth RB, Freeman SG, et al. The modulation effect of longitudinal acupuncture on resting state functional connectivity in knee osteoarthritis patients. Mol Pain. 2015;11:67. doi:10.1186/s12990-015-0071-9
20. Gollub RL, Kirsch I, Maleki N, et al. A functional neuroimaging study of expectancy effects on pain response in patients with knee osteoarthritis. J Pain. 2018;19(5):515–527. doi:10.1016/j.jpain.2017.12.260
21. Kong J, Wang Z, Leiser J, et al. Enhancing treatment of osteoarthritis knee pain by boosting expectancy: a functional neuroimaging study. Neuroimage Clin. 2018;18:325–334. doi:10.1016/j.nicl.2018.01.021
22. Qu B, Wang H, Zhao C, et al. Clinical efficacy evaluation and central mechanism study of acupuncture in treating chronic knee osteoarthritis. J Xinjiang Med Univ. 2021;44:600–604.
23. Wang X, Li JL, Wei XY, et al. Psychological and neurological predictors of acupuncture effect in patients with chronic pain: a randomized controlled neuroimaging trial. Pain. 2023;164(7):1578–1592. doi:10.1097/j.pain.0000000000002859
24. Zhou J, Zeng F, Cheng S, et al. Modulation effects of different treatments on periaqueductal gray resting state functional connectivity in knee osteoarthritis knee pain patients. CNS Neurosci Ther. 2023;29(7):1965–1980. doi:10.1111/cns.14153
25. Chen X, Spaeth RB, Retzepi K, et al. Acupuncture modulates cortical thickness and functional connectivity in knee osteoarthritis patients. Sci Rep. 2014;4(1):6482. doi:10.1038/srep06482
26. Hashmi JA, Kong J, Spaeth R, et al. Functional network architecture predicts psychologically mediated analgesia related to treatment in chronic knee pain patients. J Neurosci. 2014;34(11):3924–3936. doi:10.1523/JNEUROSCI.3155-13.2014
27. Zheng J, Chen B, Di X, et al. A resting state fMRI study of heat-sensitive moxibustion at Dubi (ST 35) in patients with knee osteoarthritis. J New Chin Med. 2015;47:243–245.
28. Huang X, Li Q, Xie D, et al. Study on the characteristics of EEG power spectral density in suspension moxibustion at Dubi (ST 35) of different states. World Chin Med. 2019;14:1936–1941.
29. Clark VP. A history of randomized task designs in fMRI. Neuroimage. 2012;62(2):1190–1194. doi:10.1016/j.neuroimage.2012.01.010
30. Cai RL, Shen GM, Wang H, et al. Brain functional connectivity network studies of acupuncture: a systematic review on resting-state fMRI. J Integr Med. 2018;16(1):26–33. doi:10.1016/j.joim.2017.12.002
31. Panych LP, Madore B. The physics of MRI safety. J Magn Reson Imaging. 2018;47(1):28–43. doi:10.1002/jmri.25761
32. Wen Q, Ma P, Dong X, et al. Neuroimaging studies of acupuncture on low back pain: a systematic review. Front Neurosci. 2021;15:730322. doi:10.3389/fnins.2021.730322
33. Zang Y, Jiang T, Lu Y, et al. Regional homogeneity approach to fMRI data analysis. Neuroimage. 2004;22(1):394–400. doi:10.1016/j.neuroimage.2003.12.030
34. Liu Z, Dong L, Tang W, et al. Within- and across-network alterations in the default network and in visual network patients with somatic symptom disorder. Psychiatry Res Neuroimaging. 2022;327:111563. doi:10.1016/j.pscychresns.2022.111563
35. Yu Z, Wang RR, Wei W, et al. A coordinate-based meta-analysis of acupuncture for chronic pain: evidence from fMRI studies. Front Neurosci. 2022;16:1049887. doi:10.3389/fnins.2022.1049887
36. Tomasi D, Volkow ND. Association between brain activation and functional connectivity. Cereb Cortex. 2019;29(5):1984–1996. doi:10.1093/cercor/bhy077
37. Barroso J, Wakaizumi K, Reis AM, et al. Reorganization of functional brain network architecture in chronic osteoarthritis pain. Hum Brain Mapp. 2021;42(4):1206–1222. doi:10.1002/hbm.25287
38. Parks EL, Geha PY, Baliki MN, et al. Brain activity for chronic knee osteoarthritis: dissociating evoked pain from spontaneous pain. Eur J Pain. 2011;15(8):843.e1–14. doi:10.1016/j.ejpain.2010.12.007
39. Iwabuchi SJ, Drabek MM, Cottam WJ, et al. Medio-dorsal thalamic dysconnectivity in chronic knee pain: a possible mechanism for negative affect and pain comorbidity. Eur J Neurosci. 2023;57(2):373–387. doi:10.1111/ejn.15880
40. Öztürk Ö, Algun ZC, Bombacı H, et al. Changes in prefrontal cortex activation with exercise in knee osteoarthritis patients with chronic pain: an fNIRS study. J Clin Neurosci. 2021;90:144–151. doi:10.1016/j.jocn.2021.05.055
41. Liu J, Tao J, Cai G, et al. The altered hippocampal functional connectivity and serum brain-derived neurotrophic factor level predict cognitive decline in patients with knee osteoarthritis. Cereb Cortex. 2023;33(20):10584–10594. doi:10.1093/cercor/bhad305
42. Sewards TV, Sewards MA. The medial pain system: neural representations of the motivational aspect of pain. Brain Res Bull. 2002;59(3):163–180. doi:10.1016/s0361-9230(02)00864-x
43. Shen Z, Zhang H, Wu Z, et al. Electroacupuncture alleviates chronic pain-induced anxiety disorders by regulating the rACC-thalamus circuitry. Front Neurosci. 2020;14:615395. doi:10.3389/fnins.2020.615395
44. Yoshino A, Okamoto Y, Onoda K, et al. Sadness enhances the experience of pain via neural activation in the anterior cingulate cortex and amygdala: an fMRI study. Neuroimage. 2010;50(3):1194–1201. doi:10.1016/j.neuroimage.2009.11.079
45. Mutschler I, Ball T, Wankerl J, et al. Pain and emotion in the insular cortex: evidence for functional reorganization in major depression. Neurosci Lett. 2012;520(2):204–209. doi:10.1016/j.neulet.2012.03.095
46. Yang Y, Xu H, Deng Z, et al. Functional connectivity and structural changes of thalamic subregions in episodic migraine. J Headache Pain. 2022;23(1):119. doi:10.1186/s10194-022-01491-z
47. Bliss TV, Collingridge GL, Kaang BK, et al. Synaptic plasticity in the anterior cingulate cortex in acute and chronic pain. Nat Rev Neurosci. 2016;17(8):485–496. doi:10.1038/nrn.2016.68
48. Chen QY, Li XH, Zhuo M. NMDA receptors and synaptic plasticity in the anterior cingulate cortex. Neuropharmacology. 2021;197:108749. doi:10.1016/j.neuropharm.2021.108749
49. Zhou YS, Meng FC, Cui Y, et al. Regular aerobic exercise attenuates pain and anxiety in mice by restoring serotonin-modulated synaptic plasticity in the anterior cingulate cortex. Med Sci Sports Exerc. 2022;54(4):566–581. doi:10.1249/MSS.0000000000002841
50. Terry EL, Tanner JJ, Cardoso JS, et al. Associations between pain catastrophizing and resting-state functional brain connectivity: ethnic/race group differences in persons with chronic knee pain. J Neurosci Res. 2022;100(4):1047–1062. doi:10.1002/jnr.25018
51. Kummer KK, Mitrić M, Kalpachidou T, et al. The medial prefrontal cortex as a central hub for mental comorbidities associated with chronic pain. Int J Mol Sci. 2020;22(1):21. doi:10.3390/ijms22010021
52. Zhou H, Zhang Q, Martinez E, et al. A novel neuromodulation strategy to enhance the prefrontal control to treat pain. Mol Pain. 2019;15:1744806919845739. doi:10.1177/1744806919845739
53. Ong WY, Stohler CS, Herr DR. Role of the prefrontal cortex in pain processing. Mol Neurobiol. 2019;56(2):1137–1166. doi:10.1007/s12035-018-1130-9
54. Bushnell MC, Ceko M, Low LA. Cognitive and emotional control of pain and its disruption in chronic pain. Nat Rev Neurosci. 2013;14(7):502–511. doi:10.1038/nrn3516
55. Leknes S, Tracey I. A common neurobiology for pain and pleasure. Nat Rev Neurosci. 2008;9(4):314–320. doi:10.1038/nrn2333
56. Porreca F, Navratilova E. Reward, motivation, and emotion of pain and its relief. Pain. 2017;158(Suppl 1):S43–s49. doi:10.1097/j.pain.0000000000000798
57. Ottenheimer D, Richard JM, Janak PH. Ventral pallidum encodes relative reward value earlier and more robustly than nucleus accumbens. Nat Commun. 2018;9(1):4350. doi:10.1038/s41467-018-06849-z
58. Roxo MR, Franceschini PR, Zubaran C, Kleber FD, Sander JW. The limbic system conception and its historical evolution. ScientificWorldJournal. 2011;11:2428–2441. doi:10.1100/2011/157150
59. Eggers AE. Redrawing Papez’ circuit: a theory about how acute stress becomes chronic and causes disease. Med Hypotheses. 2007;69(4):852–857. doi:10.1016/j.mehy.2007.01.074
60. Grant JA, Courtemanche J, Duerden EG, et al. Cortical thickness and pain sensitivity in zen meditators. Emotion. 2010;10(1):43–53. doi:10.1037/a0018334
61. Vachon-Presseau E, Berger SE, Abdullah TB, et al. Brain and psychological determinants of placebo pill response in chronic pain patients. Nat Commun. 2018;9(1):3397. doi:10.1038/s41467-018-05859-1
62. Linde K, Witt CM, Streng A, et al. The impact of patient expectations on outcomes in four randomized controlled trials of acupuncture in patients with chronic pain. Pain. 2007;128(3):264–271. doi:10.1016/j.pain.2006.12.006
63. Takamoto K, Urakawa S, Sakai K, et al. Effects of acupuncture needling with specific sensation on cerebral hemodynamics and autonomic nervous activity in humans. Int Rev Neurobiol. 2013;111:25–48.
64. Chae Y, Chang DS, Lee SH, et al. Inserting needles into the body: a meta-analysis of brain activity associated with acupuncture needle stimulation. J Pain. 2013;14(3):215–222. doi:10.1016/j.jpain.2012.11.011
65. Campbell A. Point specificity of acupuncture in the light of recent clinical and imaging studies. Acupunct Med. 2006;24(3):118–122. doi:10.1136/aim.24.3.118
66. Lin JG, Kotha P, Chen YH. Understandings of acupuncture application and mechanisms. Am J Transl Res. 2022;14(3):1469–1481.
67. Pan S, Wang S, Li J, et al. Moxibustion for primary dysmenorrhea: an adjuvant therapy for pain relief. Evid Based Complement Alternat Med. 2022;2022:6864195. doi:10.1155/2022/6864195
68. Huang K, Liang S, Sun Z, et al. Startup mechanism of moxibustion warming and dredging function. Zhongguo Zhen Jiu. 2017;37(9):1023–1026. doi:10.13703/j.0255-2930.2017.09.031
69. Wei XY, Luo SL, Chen H, et al. Functional connectivity changes during migraine treatment with electroacupuncture at Shuaigu (GB8). J Integr Med. 2022;20(3):237–243. doi:10.1016/j.joim.2022.01.009
70. Li C, Yang J, Park K, et al. Prolonged repeated acupuncture stimulation induces habituation effects in pain-related brain areas: an FMRI study. PLoS One. 2014;9(5):e97502. doi:10.1371/journal.pone.0097502
71. Ganzetti M, Mantini D. Functional connectivity and oscillatory neuronal activity in the resting human brain. Neuroscience. 2013;240:297–309. doi:10.1016/j.neuroscience.2013.02.032
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.