Back to Journals » International Journal of General Medicine » Volume 14
Accuracy of Antibiotic Allergy Documentation and the Validity of Physicians’ Decision in a Pediatric Tertiary Care Setting
Authors Al Jeraisy M , Al Osaimi S , Al Hawas A, Muamar A, Aleidi L, Khonain N, Abolfotouh MA
Received 30 September 2021
Accepted for publication 27 October 2021
Published 8 November 2021 Volume 2021:14 Pages 7819—7823
DOI https://doi.org/10.2147/IJGM.S341629
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Majed Al Jeraisy,1,2 Shaden Al Osaimi,1 Abdullah Al Hawas,1 Alanoud Muamar,1 Lamia Aleidi,3 Njoud Khonain,4 Mostafa A Abolfotouh2
1College of Pharmacy, King Saud Bin Abdulaziz University for Health Sciences, Ministry of National Guard-Health Affairs, Riyadh, Saudi Arabia; 2King Abdullah International Medical Research Center, King Saud Bin Abdulaziz University for Health Sciences, Ministry of National Guard-Health Affairs, Riyadh, Saudi Arabia; 3Unaizah College of Pharmacy, Qassim University, Riyadh, Saudi Arabia; 4College of Pharmacy, Princess Noura Bint Abdulrahman University, Riyadh, Saudi Arabia
Correspondence: Mostafa A Abolfotouh
King Abdullah International Medical Research Center (KAIMRC)/King Saud bin-Abdulaziz University for Health Sciences (KSAU-HS), Ministry of National Guard- Health Affairs, P.O. Box 3660, Riyadh, 11426, Saudi Arabia
Tel +966 11 429-4460
; +966 503659204
Fax +966 11 429-4440
Email [email protected]
Background: Patients allergic to antibiotics are at higher risk of receiving treatment with a broader spectrum, more harmful, and expensive agents. The aims of this study were (1) to assess the quality of documentation of antibiotics allergies in the electronic medical records (EMR) in a Pediatric tertiary care setting, and (2) to determine the validity of physicians’ decision to hold antibiotics prescriptions.
Methods: This is a retrospective cohort study at King Abdullah Specialized Children Hospital, Riyadh, Saudi Arabia. A review of the EMR and all Adverse Drug Reaction (ADR) reports of pediatric patients 1– 14 years old, with a documented allergy to antibiotics from June 2016 until June 2019. The quality of documentation of antibiotics allergy was assessed based on the presence of four parameters: 1) allergy alert notification, 2) allergy severity classification, 3) setting notes, and 4) symptoms’ description. In addition, all physicians’ reports of allergy to antibiotics were cross-classified according to their corresponding ADR reports, and the validity of physicians’ documentation of allergy was assessed.
Results: Of a total of 105 Pediatric patients’ EMR, documentation of antibiotics allergy was available in 98 (93.3%), with the presence of symptoms description (83%), allergy notes (87%), severity (67%), and signs of alert (50.8%). Overall documentation quality was good for only 23.5% of patients, while it was poor for 35.7%. Physicians’ documentation of antibiotics allergy was 0.82 sensitive [with 0.18 risk of allergy] and 0.60 specific [with 0.40 unnecessary restrictions of prescriptions]. Of all children with possible/actual allergies, only 38.9% were referred to the immunology clinic.
Conclusion: The quality of documentation of antibiotic allergy in children and the validity of physicians’ decisions are less than satisfactory. Therefore, improving communications between all healthcare providers regarding patients’ allergy status and follow-up for further assessment of the reaction is recommended to improve patient care.
Keywords: flagging, drug allergy, allergy documentation, quality, Naranjo scale
Background
Drug allergy is an atypical drug reaction that over-stimulates the immunological response to any medication.1,2 Drug allergy appears most commonly with antimicrobial medicines. Patients allergic to antibiotics are at higher risk of receiving treatment with a broader spectrum or more harmful and expensive agents.3 Generally, true allergies are uncommon; most reported drug allergies are infusion-related adverse drug reactions.4
Entering incomplete or inaccurate information in the patient’s record about drug allergy results in poor allergy documentation, which could lead to misinterpretations causing several unnecessary medical errors,5 in addition, it will deprive the patient of the benefits of some of these medications. Thus, improving the documentation of drug allergies in patients’ medical records by assuring complete and comprehensive information would be a reasonable approach to avoid such errors.6,7 To achieve full documentation, it is advised that all healthcare staff contribute to identifying the nature and severity of any occurring reactions associated with drug allergies or adverse drug reactions. Allowing better communications between patients and the medical staff is vital in reaching a more comprehensive conclusion of the causality relationship between drugs and their allergies.8
Although new options for allergy and adverse drug reactions documentation like digital and electronic forms have been introduced, they are still not devoid of documentation errors. Therefore, it is important to spread knowledge and encourage understanding of the limitations and benefits of such options.9 For example, 8% and 13% of inpatient and outpatient medication errors, respectively, were caused by patients receiving a medication to which they had a known allergy.5,10 However, with computerized physician order entry (CPOE), the rate of known allergy errors decreased by 56%.11
In a previous study to assess the quality of allergy documentation, 250 patients were interviewed, and only 95 of them had an actual allergic reaction, 20 (21%) of documents were complete, 55 (58%) were partially finished, and 20 (21%) were incomplete. Of the 20 papers categorized as incomplete, 18 had no information recorded about their allergy status, neither in drug charts nor in medical records.12 To the best of our knowledge, there are no studies to assess the quality of documentation of drug allergies in pediatric patients in a Saudi medical care setting. Thus, our aim in this study is to evaluate the practice of antibiotic allergies documentation in the electronic medical records (EMR) and determine the validity of physicians’ decisions to hold antibiotics prescriptions.
Methods
Study Design
A retrospective cohort study of pediatric patients from 1 to 14 years with a reported allergy to antibiotics was conducted to collect data on the documentation of allergy to antibiotics.
Study Setting
This study was conducted at King Abdullah Specialized Children Hospital (KASCH), Riyadh, Saudi Arabia. KASCH is a tertiary pediatric hospital in Riyadh, Saudi Arabia, with a total capacity of 552 beds. The hospital policy states that the physician must report the drug allergy in the Safety Reporting System (SRS). Therefore, in many cases, the physician will flag the patient as allergic to this medication. Then, the Medication Safety Officer (MSO) must send it to the clinical pharmacist for evaluation. The clinical pharmacist will evaluate the drug allergy based on the reported incident in the EMR and, if necessary, will interview the bedside nurse for more details, then will apply the criteria of the Naranjo scale to recommend the appropriate action and whether to agree or disagree to flag the patient as allergic to the medication. Based on the pharmacist’s assessment, MSO will take the appropriate action. The hospital policy mandates the clinical pharmacist to evaluate any ADRs generated by the Safety Reporting System (SRS). However, a lack of communication and feedback between physicians, pharmacists, and medication safety officers leads to discrepancies between the recommendation of the clinical pharmacist and the physician’s action of the allergy status.
Study Subjects
This study included pediatric patients aged 1 to 14 years, with a reported antibiotic allergy, from June 2016 until June 2019.
Data Collection
A review of the EMR and all Adverse Drug Reaction (ADR) reports, from June 2016 until June 2019, was conducted for pediatric patients from 1 to 14 years with a documented allergy to antibiotics to collect data on the documentation of allergy to antibiotics.
Assessment of the documentation was done by comparing its quality in the EMR with the ADR reports. Documentations were assessed based on the presence of the following four parameters: (1) allergy alert notification, (2) allergy severity classification, (3) setting notes, and (4) symptoms description. Symptoms’ description was considered to be present only if it was complete. It was considered complete when it was indicative of an informative illustration about the duration and severity of the symptoms and the management that was performed to resolve the symptoms. Based on these parameters, for the flagged patients, documentation was considered good if it had all four parameters recorded, fair if it had three parameters and poor if it had ≤2 parameters. For the non-flagged patients, the evaluation of ADRs was based on the last two parameters: setting notes and symptoms description, so it was considered good if both were present and immediately poor if one was missing. The research team developed such criteria for quality assessment internally as we are not aware of any published tools for quality assessment.
Results
A total of 105 pediatric patients were included in this study; sixty-five (61.90%) were males. The mean age of the patients was 6.93±3.89 years. Documentation of allergy to antibiotics was available in 98 (93.3%) of patients. Specifically, documentation existed in the records of the majority of patients for symptoms (83%) and allergy notes (87%). In comparison, it existed for severity and alert signs in only 67% and 41% of patients’ records, respectively. Overall, the documentation quality was good for only 23.5% of patients, while it was poor for 35.7%. Naranjo score was available for 82 patients. The majority (56, 60.8%) had the score of 5–8 (doubtful/probable), while 36 (38%) had a score of 1–4 (possible/definite). Flagging was reported for 63 patients, while pharmacist decision was available for 56 (57.1%) patients, Table 1.
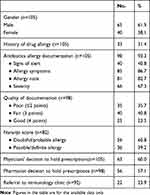 |
Table 1 Antibiotics Allergy Documentation Among Children at King Abdullah Specialized Children Hospital |
Table 2 showed high sensitivity (0.82), with ten patients wrongly documented as not allergic (0.18 false negatives). Those are at risk of being given a drug that might provoke an anaphylactic reaction. Although the allergy information was available in their medical records, those patients were not flagged, emphasizing the importance of supporting physicians with pertinent information. Meanwhile, the table showed moderate specificity (0.60), with 17 patients wrongly documented (flagged) as being allergic to a drug (0.40 false positives). Those patients could be denied potentially effective or lifesaving medicine.
 |
Table 2 Validity of Physicians’ Decision to Hold Antibiotics Prescriptions |
Concerning the referral to the immunology clinic, most patients (70, 76.1%) had no referral notes or recommendations to follow-up, and less than one-fourth (22, 23.9%) had immunology referral notes. Those constituted 38.9% of patients whose Naranjo scale was possible/definite, compared to only 14.3% of patients whose Naranjo scale was doubtful/probable (x2=7.29, p=0.007), Table 3.
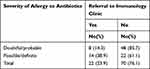 |
Table 3 Referral to Immunology Clinic and Severity of Allergy Based on Naranjo Scale |
Discussion
In this study, we aimed to assess the practice of documentation of drug allergies in the EMR and the accuracy of the physicians’ decisions. Our review of the medical records demonstrated that the majority have poor allergy documentation, with good documentation for only 23.5% of patients. These findings were similar to the findings of a pilot study12 that reported missing information in EMR. In addition, several patients with several drug allergies had only one of their allergies documented.2 In a pilot study of 55 patients who were introduced to drug allergy clarification (DAC) Standardized Questionnaire,13 54.5% (n=30) of those patients were classified as either having drug allergies or drug intolerance, and at least one inconsistency between EMR documentation and interviews was found in 29 patients; with no illustration on the details of allergy. In our study, documentation of allergy to antibiotics was available in 98 (93.3%). Documentation existed in the records of the majority of patients for symptoms’ description (83%) and allergy notes (87%). In comparison, it existed for severity and allergy notes in only 67% and 41% of patients’ records, respectively. Such poor documentation or lack of information would result in unnecessary medical errors and avoidance of beneficial therapies.
Most clinicians and nurses are mixing up between drug allergies and side effects. Documenting adverse antibiotic reactions as true allergy is associated with negative consequences and deprives patients of the best selection of antibiotics. This was shown in a previous study,3 manifesting that Penicillin-allergic children have increased comorbidities, more extended hospital stays, and greater hospitalization expenses.3
Although hospital policy mandates the clinical pharmacist to evaluate any ADRs generated by the Safety Reporting System (SRS), there was a discrepancy between the recommendation of clinical pharmacist and physician’s action of the allergy status. This inadequate documentation could lead to inappropriate prescribing and unnecessary avoidance of beneficial therapies. Our study revealed that 17 patients were wrongly documented (flagged) as allergic to a drug (0.40 false positives). Those patients could be denied potentially effective or lifesaving medicine, with a modest specificity of 0.60. Meanwhile, ten patients were wrongly documented as not allergic (0.18 false negatives), with a high sensitivity of 0.82. Those are at risk of being given a drug that might provoke an anaphylactic reaction. This finding was in agreement with the findings of two previous studies, where 8% and 13% of inpatient and outpatient medication errors, respectively, were caused by patients receiving a medication to which they had a known allergy.5,10 Although the allergy information was available in their medical records, those patients were not flagged, emphasizing the importance of supporting physicians with pertinent information. The sensitivity of flagging allergic patients to antibiotics was relatively high (0.82), while the specificity was low (0.60), resulting in unnecessary restrictions of prescriptions. Our explanation for this finding is the lack of communication between physicians, pharmacists, and medication safety officers.
According to the Allergy Status Identification and Documentation policy in the Ministry of National Guard-Health Affairs (MNG-HA), it is suggested that patients with actual allergic reactions are to be seen by immunologists. In the present study, the Naranjo score was available for 82 patients. The majority (56, 60.8%) had a score of 5–8 (doubtful/probable), while 36 (38%) had a score of 1–4 (possible/definite). However, only 22% of the patients were referred to follow up with an immunologist. Of the 82 (78%) cases that had no referral, 21 cases had the Naranjo score of 1–4 (Possible), 44 cases had the Naranjo score of 5–8 (Probable), and 1 case had a Naranjo score of >9 (Definite). Improving communications between all healthcare providers regarding patients’ allergy status and follow-up for further assessment of the reaction is necessary to improve patient care.
Limitations
This study has some limitations that should be highlighted. First, this is a retrospective chart review of EMRs, which might be a limitation due to the likely incomplete and inaccurate documentation. Second, this is a single-site study with a small sample size that would not generalize the study’s conclusion. Third, we came up with our parameters to evaluate documentation of allergy to antibiotics based on our best judgment choice, as no previous parameters were used or validated for such measurement.
Conclusion
Doctors’ decisions about allergies to antibiotics and their documentation are not up to par. Proper documentation of drug allergies in pediatric patients results in better care and an appropriate selection of antibiotics. Improved communication between all healthcare providers regarding patients’ allergy status and follow-up for further evaluation will enhance patient care. Other prospective studies with interventional educational programs are needed.
Abbreviations
KAMC, King Abdulaziz Medical city; MNG-HA, Ministry of National Guard-Health Affairs; KASCH, King Abdullah Specialized Children’s Hospital; IRB, Institutional Review Board; EMR, Electronic medical records; ADR, Adverse Drug Reaction; CPOE, computerized physician order entry; DAC, drug allergy clarification; SRS, Safety Reporting System.
Data Sharing Statement
Most of the data supporting our findings is contained within the manuscript, and all others, excluding identifying/confidential respondent data, will be shared upon request by contacting the corresponding author [email protected].
Ethics Approval and Consent to Participate
This research was approved by the Institutional Review Board (IRB) of The Ministry of National Guard-Health Affairs. Riyadh, Saudi Arabia. Informed consent to participate in the study was waived as per the policy of the ethics committee, since this is a retrospective chart review study, with no risk to participants. This study was conducted in accordance with the Declaration of Helsinki.
Acknowledgments
This study was fully supported by King Abdullah International Research Center (KAIMRC), Riyadh, Saudi Arabia. It was approved by the IRB of Ministry of National Guard-Health Affairs, Riyadh, Saudi Arabia. It was presented in a poster during the 11th Research Summer School program at KAIMRC, August, 2019.
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Dias de Castro E, Carolino F, Ribeiro L, Cernadas J. Overview of drug allergy: from immunogenetic basis to practice. Acta Med Port. 2018;31(10):581–588. doi:10.20344/amp.10092
2. Khan DA, Solensky R. Drug allergy. J Allergy Clin Immunol. 2010;125(2):S126–S137. doi:10.1016/j.jaci.2009.10.028
3. Borch JE, Andersen KE, Bindslev‐Jensen C. The prevalence of suspected and challenge‐verified penicillin allergy in a university hospital population. Basic Clin Pharmacol Toxicol. 2006;98(4):357–362. doi:10.1111/j.1742-7843.2006.pto_230.x
4. Kuperman GJ, Gandhi TK, Bates DW. Effective drug-allergy checking: methodological and operational issues. J Biomed Inform. 2003;36(1–2):70–79. doi:10.1016/S1532-0464(03)00063-7
5. Leape LL, Bates DW, Cullen DJ, et al. Systems analysis of adverse drug events. JAMA. 1995;274(1):35–43. doi:10.1001/jama.1995.03530010049034
6. Benkhaial A, Kaltschmidt J, Weisshaar E, Diepgen TL, Haefeli WE. Prescribing errors in patients with documented drug allergies: comparison of ICD-10 coding and written patient notes. Pharm World Sci. 2009;31(4):464–472. doi:10.1007/s11096-009-9300-5
7. Jones TA, Como JA. Assessment of medication errors that involved drug allergies at a university hospital. Pharmacotherapy. 2003;23(7):855–860. doi:10.1592/phco.23.7.855.32729
8. Barton L, Futtermenger J, Gaddi Y, et al. Simple prescribing errors and allergy documentation in medical hospital admissions in Australia and New Zealand. Clin Med (Northfield Il). 2012;12(2):119. doi:10.7861/clinmedicine.12-2-119
9. Khalil H, Leversha A, Khalil V. Drug allergy documentation-time for a change? Int J Clin Pharm. 2011;33(4):610–613. doi:10.1007/s11096-011-9525-y
10. Gandhi TK, Burstin HR, Cook EF, et al. Drug complications in outpatients. J Gen Intern Med. 2000;15(3):149–154. doi:10.1046/j.1525-1497.2000.04199.x
11. Nuckols TK, Smith-Spangler C, Morton SC, et al. The effectiveness of computerized order entry at reducing preventable adverse drug events and medication errors in hospital settings: a systematic review and meta-analysis. Syst Rev. 2014;3(1):1–2. doi:10.1186/2046-4053-3-56
12. Sim L, Barras M, Cottrell N. Patients’ understanding of drug allergy and documentation—is there a link? J Pharm Pract Res. 2005;35(4):276–278. doi:10.1002/j.2055-2335.2005.tb00362.x
13. Deas CM, White CW. Pilot study to assess outcomes of a drug allergy clarification service on a general medicine floor at a local community hospital. Inov Pharm. 2018;9(3):1. doi:10.24926/iip.v9i3.1463
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
