Back to Journals » Infection and Drug Resistance » Volume 13
Abscess Drainage with or Without Antibiotics in Lactational Breast Abscess: Study Protocol for a Randomized Controlled Trial
Authors Luo J, Long T, Cai Y, Teng Y, Fan Z, Liang Z, Zhu C, Ma H, Li G
Received 28 June 2019
Accepted for publication 24 December 2019
Published 21 January 2020 Volume 2020:13 Pages 183—190
DOI https://doi.org/10.2147/IDR.S221037
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Eric Nulens
Jiayue Luo, Tianzhu Long, Yuanxuan Cai, Yuan Teng, Zhe Fan, Zhen Liang, Cairong Zhu, Hongmin Ma, Guanhua Li
Department of Breast Surgery, Guangzhou Women and Children`s Medical Center, Guangzhou Medical University, Guangzhou, Guangdong 510623, People’s Republic of China
Correspondence: Guanhua Li; Hongmin Ma
Department of Breast Surgery, Guangzhou Women and Children`s Medical Center, Guangzhou Medical University, Guangzhou, Guangdong 510623, People’s Republic of China
Tel/Fax +86 20 8188 6332
Email [email protected]; [email protected]
Background: Lactational breast abscess, a complication from lactational mastitis, is a common cause of breastfeeding discontinuation. No consensus has been reached regarding the necessity of antibiotics in this disease. The purpose of this trial is to determine if surgical drainage is non-inferior to drainage together with a standard course of antibiotics, in the treatment of lactational breast abscess.
Methods: Breastfeeding females with breast abscess from 18 to 50 years old are eligible for study inclusion. An expected number of 306 patients will be randomly allocated in parallel to the intervention arm (simple drainage without antibiotics) or the control arm (abscess drainage with standard 5-day-course of antibiotics). The primary outcomes include the time to resolution of breast abscess and disease recurrence rate. Secondary outcomes of interests are 3-day-improvement proportion, rate of continuing breastfeeding, treatment failure rate, procedural-related complications, and length of hospital stay. An expected non-inferiority margin for the difference in the primary outcome of interest is set at 1 day, on the basis of a one-sided 97.5% confidence interval.
Discussion: This trial will provide first-hand evidence on whether simple abscess drainage is non-inferior to drainage together with a standard course of antibiotics, in lactational mothers with breast abscess. The indication of antibiotic prophylaxis could be revised if non-inferiority is set up, and guidelines for lactational breast abscess require amendments correspondingly.
Trial Registration: This study has been registered in the Chinese Clinical Trial Registry, and the trial registration number is ChiCTR1900024008.
Keywords: lactational breast abscess, antibiotics, drainage, randomized controlled trial, study protocol
Introduction
Breast abscess is defined as localized collection of inflammatory exudate in breast tissue.1 Lactational breast abscess is a complication of lactational mastitis if not managed in an expeditious fashion.2,3 For breastfeeding mothers, the incidence of mastitis ranges from 3% to 20%,4,5 and 4.6% to 11%6,7 of lactational abscesses develop from mastitis. Importantly, lactational breast abscess is a common cause of breastfeeding early cessation.8,9
Nipple fissures and milk stasis are primary reasons for the formation of lactational breast abscess.9–11 Other risk factors contributing to breast abscess as the deterioration of lactational mastitis include age, primiparity, gestational age over 41 weeks, obesity, and tobacco consumption.8,12 The most frequent causative pathogen for lactational breast abscess is Staphylococcus aureus,13,14 however, the methicillin-resistant Staphylococcus aureus (MRSA) infection as an etiological factor is on the increase.15–18
Current approaches in managing lactational breast abscess include milk drainage either by continuing breastfeeding or pumping, removal of pus by needle aspiration, catheter drainage, open surgical drainage, and the use of antibiotics.19,20 Needle aspiration is an effective treatment for draining abscesses less than 5 cm with a small volume of infected pus.21,22 For patients who require repeated aspiration, an alternative option is to temporarily place a catheter into the abscess cavity with regular irrigation.23,24 For those with ischemia or necrosis of the overlying skin or those with abscesses over 5 cm in diameter, open surgical drainage is the preferred option.24,25
As for antibiotics, no consensus has ever been reached with respect to its necessity. To the best of our knowledge, very few well-powered studies have so far focused explicitly on the impact of routine antibiotic usage for lactational breast abscess.20 Previous studies have showed that Staphylococcus aureus, a frequent causative pathogen, is diversely resistant to antibiotics,26,27 while in clinical practice, antibiotics are empirically administrated in many cases without definite etiology of infection, or even without the report of antibiotic sensitivity test.26,27 This might worsen the condition of drug resistance and repeated courses might even increase the risk for opportunistic infection, such as Candida.28–30
On account of inherent limitations of the studies available in the literature, well-designed studies on a larger scale are thus warranted to properly assess the role of antibiotics for the treatment of breast abscess in breastfeeding women. Therefore, we launched a randomized controlled study to determine if abscess drainage either with percutaneous catheter implantation or surgical drainage is non-inferior to abscess drainage plus a standard course of antibiotics, in breastfeeding women with breast abscess. Our study is prepared in line with the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) guidelines.31
Trial Objective & Hypothesis
The main purpose of the current study is to determine the efficacy and safety of abscess drainage without antibiotic treatment, compared to the standard course of antibiotics together with drainage for lactational breast abscess. It is hypothesized that abscess drainage without antibiotic treatment is non-inferior to abscess drainage plus antibiotic treatment and will not lead to exacerbation of infection. The rate of breastfeeding continuation, the incidence of recurrence, treatment failure rate, and length of hospital stay are also analyzed.
Methods
Trial Design
This trial is designed as a prospective, single-center, non-blinded, open-label, non-inferior randomized controlled trial. Two parallel groups of patients are randomly allocated to standard course of antibiotics therapy (5 days, intravenous) plus abscess drainage (surgical or catheter-based) (control arm) or abscess drainage without antibiotic therapy (intervention arm). The primary endpoints of interest are time to resolution of breast abscess and disease recurrence rate. Patient enrollment, randomization, and intervention in the current trial are outlined in Figure 1. Figure 2 depicts the trial schedule according to Standard Protocol Items Recommendations for Interventional Trials (SPIRIT) guidelines.
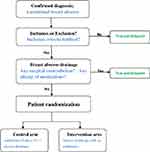 |
Figure 1 Flowchart of inclusion and randomization. Abbreviation: IV, intravenous. |
Trial Setting
This trial will run in the Department of Breast Surgery, Guangzhou Women and Children`s Medical Center, which is the largest breastfeeding care research center in south China. This study will be launched in line with the Declaration of Helsinki and in accordance with Good Clinical Practice. Approval of ethics for this study was obtained from the Ethics Committee of Guangzhou Women and Children`s Medical Center on 6 June 2019 (approval no. 2019KL27001). This trial: the Guangzhou Lactational-Breast-Abscess Antibiotics and Drainage Randomized Trial (the GLAD trial) has been registered in the Chinese Clinical Trial Registry (registration no. ChiCTR1900024008). Eligible patients will be informed with respect to trial details. Written informed consents will be obtained from all eligible patients prior to study inclusion by surgeons in our department. Research data will be kept and analyzed anonymously and confidentially. The results of this study will be released through a publication in a medical journal.
Eligibility Criteria
Confirmation of the diagnosis for lactational breast abscess is based on both clinical manifestation and ultrasonography. Features of clinical manifestation include a localized mass with fever, pain, tenderness, skin redness, and swelling. Sonographic characteristics of lactational breast abscess include a multi-formed, localized swelling, with irregular fluid sonolucent area, non-homogenous echoes, and inflammatory axillary lymph nodes.
Inclusion criteria are:
- Breastfeeding females, age ranging from 18 to 45
- Confirmed diagnosis of lactational breast abscess (as mentioned above)
- Measurable swelling by ultrasonography
- Obtainment of written informed consent before inclusion
- Awareness of trial details as well as agreement with the study process, intervention, and follow-up.
Exclusion criteria are:
- Incapable of giving informed consent (eg, linguistic reasons)
- Concomitant vital organ dysfunction, hematological diseases, mental disorders
- History of breast carcinoma or history of breast surgeries
- Patients with surgical contradictions (eg, severe hyperglycemia, severe coagulation disorders, and unstable hemodynamics)
- Suspicion of sepsis, or refractory infections
- Immunocompromised conditions (eg, patients with histories of chemotherapy, organ transplant, and any immuno-deficient diseases)
- Concomitant antibiotic usage for other indications
- Known allergy to study medications
- Repeated high fever during the course of antibiotics
- Patients with other concomitant infections
Interventions
Abscess Drainage
All study participants will be treated with a drainage procedure, either by percutaneous catheter insertion or by surgical placement. Two drainage approaches are routinely used in our department: the 14G catheter for percutaneous insertion, and the 18Fr tube for surgical placement. The decision of abscess draining method depends on the diameter of breast abscess as measured by ultrasonography. Typically, a 14G catheter is indicated in abscesses less than 5 cm in diameter; otherwise, the 18Fr tube is applied. Each drainage implantation is connected to a continuous vacuum draining device, of which the recommended duration is 3 to 6 days.
Antibiotic Treatment
Study participants will be randomized into two groups to receive either: a 5-day-course of postprocedural antibiotic management (control group); or no antibiotic treatment (intervention group). Antibiotics are administered intravenously, with clindamycin (three times a day, 900 mg ×5 days) plus cefuroxime (three times a day, 1500 mg ×5 days) or azithromycin (once a day, 500 mg ×5 days) as an alternative option for those with cephalosporin contraindications. A switch to oral antibiotic administration is not permitted. As a safety concern for breastfeeding, no other antibiotics are allowed. Cessation of breastfeeding is not recommended for all participants.
Treatment Modifications
Antibiotic administration plans may be restarted or altered only if a confirmed source of infection is proven. A revision of the antibiotic treatment plan to guarantee effective anti-infection management is permitted only if an adverse effect is observed or if drug resistance is confirmed by the culture result.
Discharge & Follow-Up
Discharge criteria include normal body temperature for at least 1 day, blood leukocyte counting within normal range, absence of breast abscess, and no evidence of other infections. After discharge, study participants are asked to fulfill standard outpatient visits once a week from the day of discharge to 4 weeks after discharge. Readmission is suggested in case of disease recurrence. At 6 months after discharge, a standard follow-up via telephone will be performed.
Outcomes
Primary Outcomes
The primary outcomes of interest of this study include the time to resolution of breast abscess and the recurrence rate. Resolution is defined as relief of symptoms, absence of infection, absence of lesion confirmed by ultrasonography. The duration from the time of hospitalization to disease resolution is recorded. Recurrence is defined as re-development of breast abscess at the same location after discharge, and the recurrence rate is documented.
Secondary Outcomes
Secondary outcomes of interest are 3-day improvement proportion, the rate of continuing breastfeeding, treatment failure rate, procedural-related complications, and length of hospital stay.
Three-day improvement proportion is defined as the reduction percentage of abscess volume compared with that of the baseline level. The criteria for treatment failure are repeated high fever, abscess volume increase to at least 25% of the baseline level, worsening of infectious symptoms and increase of purulent drainage. Procedure-related complications include milk fistula, residual abscess, surgical site infection, and unfavorable cosmetic outcome.
Sample Size Calculation
This trial is a prospective, non-inferior, parallel, randomized controlled trial, with a primary outcome of time (measured in days) to abscess resolution. A power analysis is conducted on the basis of a one-sided 97.5% confidence interval for the effect of intervention, with an expected non-inferiority margin of 1 (day). To achieve a power of 90%, 138 participants are required for each group. Considering the possibility of missing data and loss of follow-up (10% in total), we will recruit 153 participants for each group.
Recruitment
Recruitment of patients will commence on 1 Aug 2019. Additional participants may be recruited to maintain trial feasibility. The expected 306 participants are anticipated to be completed in 2021.
Allocation
Web-based randomization (stratified by the diameter of abscess) will be done via the “Research Randomizer Program” (http://www.randomizer.org) within 24 hrs after admission. The randomization sequence number is individually unique and will be performed by an independent staff for study data management who is not involved in the recruitment of participants and clinical practice. Sequence numbers and the randomization results will be disclosed to study investigators until enrolled participants have completed all baseline assessments.
Trial Execution
All study personnel are Good Clinical Practice (GCP)-qualified. Prior to trial initiation, all department faculties are gathered by the research personnel to instruct the study protocol and relevant procedures. Surgeons and research investigators are trained to evaluate the type of lactational breast abscess, to decide the eligibility of study recruitment, and to implement treatments according to the study protocol. A research-coordinating committee is established so that all surgeons can send patients directly to study investigators. This way, patients will be screened for eligibility and have the interventions performed after randomization only by study investigators.
Blinding
Blinding in this study is difficult to achieve. Blinding is not expected since satisfactory clinical outcome requires the awareness whether antibiotics have been applied to the patient or not. Hence, this is an open-label, non-blinded trial.
Trial Data Management
Statistical managers from Guangzhou Women and Children`s Medical Center will be responsible for data management, data collection, monitoring, and collaboration with trial investigators. Baseline demographics, treatments, perioperative variables, and follow-up data will be obtained from hospital-based electronic medical records. Quality control will be conducted by statistical managers to ensure data correctness and completeness. Every missing file or inconsistency must be clarified and reported to the trial coordinators by the responsible investigator.
Statistics
An expected non-inferiority margin of 1 day in the primary outcome of interest is considered acceptable between two arms. The non-inferiority margin is deemed acceptable due to the fact that in-hospital mortality is ignorable and infection-related complications can be well managed.
Primary Outcome Analyses
The time to resolution (relief of symptoms, absence of infection, and absence of lesion confirmed by ultrasonography) of breast abscess and the recurrence rate will be compared between both trial arms. The Student`s t-test and chi-square test will be used to compare time to resolution and the recurrence rate, respectively. Regression analysis will be performed to figureout possible predictors and risk factors of the composite primary outcomes. Independent variables include treatments, age, the approach of drainage, and volume of the abscess. A two-sided level of 0.05 is deemed statistically significant.
Secondary Outcome Analyses
Baseline demographics and secondary outcome parameters will be compared with independent samples between both trial arms. The student`s t-test or Mann–Whitney U-test will be performed in continuous variables, while chi-square or Fisher`s exact test will be conducted in categorical variables. With adjustment for independent variables mentioned above, linear regression is used for continuous outcome measures, whereas logistic regression is applied to dichotomous outcome measures. A two-sided level of 0.05 is deemed statistically significant.
Safety
Every adverse event, regardless of treatment relevance, should be recorded in detail within 1 day by the investigator. If a severe adverse event occurs, whatever it is relevant to the study or not, must be reported within 6 hrs by the investigator to the research-coordinating committee through an “adverse event form”, on which type of adverse event, the date of occurrence, severity, association with the study, and outcome evaluation. The time-span during which adverse events should be collected starts from the day on which the written informed consent is obtained to the completion of follow-up (6 months).
Data Monitoring
A data safety monitoring board (DSMB) is in charge of data monitoring and security control throughout the study. The DSMB consists of a statistician, two gynecologists, two surgeons, a pharmacist, and a microbiologist, all of whom will not participate in the trial, without any conflict of interest with all trial investigators. The DSMB members will visit the trial-participating department, monitor protocol adherence, and evaluate study data on a regular basis. The DSMB members will be invited for the first time when the first five randomized participants have discharged. Subsequent DSMB monitoring visits will be scheduled every 3 months.
Discussion
The current study is to investigate a debatable question: whether antibiotics are necessary for the treatment of lactational breast abscess? It would decrease the administration of antibiotics if single drainage is not inferior to drainage plus antibiotics. The findings of this randomized clinical trial will provide us evidence on the role of antibiotics for the management of lactational breast abscess.
It is the global public health issue regarding the overuse of antibiotics, which leads to microbial multiple-drug resistance. More studies are warranted to update the evidence and guidelines for using antibiotics for many diseases. For many infectious diseases, like pneumonia, osteomyelitis, and endocarditis, shorter course or a shift from intravenous therapy to oral administration have proven to be as effective as standard courses of antibiotics.32,33 For mastitis or lactational breast abscess, however, proper studies that rule out the suitability and optimization of antibiotics are not available. To date, no randomized clinical trial with sufficient power has ever assessed the efficacy of drainage without postoperative antibiotics in lactational breast abscess.
Breast abscess is deemed as a progression of mastitis with infection involvement. Although Staphylococcus aureus is the major pathogen involved in breast abscesses, the microbiology of this disease is complicated. The breastfeeding-related skin injury can be a potential risk for microbial entrance, and coagulase-negative staphylococci are part of the breast skin flora, including S. hominis, S. epidermidis, S. hemolyticus, and S. lugdunensis, as identified in the BREAST-MF study by Jianu et al.34,35 Flora from the breast skin can temporarily colonize the nipple ducts, and if pathogenic microorganisms are involved, this may also cause infectious mastitis, and subsequently results in the formation of breast abscess.36
Antibiotics are empirically recommended as an anti-infection approach. However, for lactating mothers, antibiotics should be cautious. The drug should be active, with good concentration in the abscess; moreover, it should not be harmful to the baby.5 Based on our institutional practice, a multidisciplinary team should be included in the management of lactational breast abscess. Firstly, ultrasound features predict outcomes and guide means of treatment. Secondly, abscess drainage is performed, either with percutaneous catheter implantation or with surgical placement. Thirdly, adequate breast support and manual breast emptying help relaxing the breast and reducing tissue edema, which are essential for treatment. In our experience, antibiotics are not routinely used in the comprehensive treatment of lactational breast abscess, and the treatment success rate is similar as compared with appropriate management plus antibiotics.
Besides bacteriology, multiple factors contribute to the outcome of breast abscess. Size (small or large), location (central or peripheral), shape (round, septal, or irregular), and thickness are all influential factors. Although it is controversial that using the size of the abscess to decide means of drainage,37 a diameter of 5 cm is frequently applied in our institution as a cutoff in which percutaneous implantation enables adequate drainage of small abscesses, whereas surgical tube insertion capacitates a definite cure for large abscesses over 5 cm. Similarly, the recurrence rate may be influenced by factors such as surgical treatment,20,38 age, pathogen, and some lifestyle behaviors, like smoking.24,39,40 Also, literature has implicated that nipple piercing41 and obesity3 are relevant to increased risk of occurrence. Based on our institutional practice, which constitutes the largest population of lactational diseases in south China, effective abscess drainage is vitally essential and is empirically sufficient for a definite cure without antibiotics at all. Treatment with antibiotics is not effective without the removal of infectious fluid.20
If non-antibiotic therapy is not inferior to the standard treatment, the length of hospital stay will be shortened, accompanying with lower hospital cost. For long-term interest, the optimization strategy for antibiotics retards the urgency of worldwide bacteriological drug resistance. Some limitations of this study merit further considerations. Firstly, this is a non-blinded trial. Blinding in the present study is difficult to achieve, since satisfactory clinical outcome requires the awareness of whether antibiotics have been used or not. Secondly, this study is based on a single center. Agreements and consensus on therapeutic options for this disease are difficult to reach among institutions. It is also a matter of fact that only a limited number of institutions provide full-scale medical service to lactational mothers in south China. Finally, another limitation of this study is that heterogeneities pertaining to surgeons` expertise may exist. For quality control, ultrasonographic images and a short video of the draining procedure are taken for each patient included in this study. In this way, we may improve the reliability and reproducibility of our findings.
Trial Status
The current trial: the Guangzhou Lactational-Breast-Abscess Antibiotics and Drainage Randomized Trial (the GLAD trial) has been registered in the Chinese Clinical Trial Registry (registration no. ChiCTR1900024008). The first recruitment of patients will commence on 1 Aug 2019. The expected 306 participants are expected to be completed in 2021.
Acknowledgment
The current study is supported by the initial research funding of Guangzhou Women and Children`s Medical Center (Grant numbers: 5001-1600012 and 5001-1600027).
Disclosure
The authors report no conflicts of interest in this work.
References
1. LH A. ABM clinical protocol #4: mastitis, revised March 2014. Breastfeeding Med. 2014;9(5):239–243. doi:10.1089/bfm.2014.9984
2. Amir LH. ABM clinical protocol #4: mastitis, revised March 2014. Breastfeeding Med. 2014;9(5):239–243. doi:10.1089/bfm.2014.9984
3. Bharat A, Gao F, Aft RL, Gillanders WE, Eberlein TJ, Margenthaler JA. Predictors of primary breast abscesses and recurrence. World J Surg. 2009;33(12):2582–2586. doi:10.1007/s00268-009-0170-8
4. Foxman B, D’Arcy H, Gillespie B, Bobo JK, Schwartz K. Lactation mastitis: occurrence and medical management among 946 breastfeeding women in the United States. Am J Epidemiol. 2002;155(2):103–114. doi:10.1093/aje/155.2.103
5. Kataria K, Srivastava A, Dhar A. Management of lactational mastitis and breast abscesses: review of current knowledge and practice. Indian J Surg. 2013;75(6):430–435. doi:10.1007/s12262-012-0776-1
6. Amir LH, Forster D, McLachlan H, Lumley J. Incidence of breast abscess in lactating women: report from an Australian cohort. BJOG. 2004;111(12):1378–1381. doi:10.1111/bjo.2004.111.issue-12
7. Merz L, De Courten C, Orasch C. [Breast infections]. Rev Med Suisse. 2014;10(427):
8. Dener CIA. Breast abscesses in lactating women. World J Surg. 2003;27(2):130–133. doi:10.1007/s00268-002-6563-6
9. Ahluwalia IB, Morrow B, Hsia J. Why do women stop breastfeeding? Findings from the pregnancy risk assessment and monitoring system. Pediatrics. 2005;116(6):1408–1412. doi:10.1542/peds.2005-0013
10. Dennis CL, Schottle N, Hodnett E, McQueen K. An all-purpose nipple ointment versus lanolin in treating painful damaged nipples in breastfeeding women: a randomized controlled trial. Breastfeeding Med. 2012;7(6):473–479. doi:10.1089/bfm.2011.0121
11. Leung SS. Breast pain in lactating mothers. Hong Kong Med j. 2016;22(4):341–346. doi:10.12809/hkmj154762
12. Kvist LJ, Rydhstroem H. Factors related to breast abscess after delivery: a population-based study. BJOG. 2005;112(8):1070–1074. doi:10.1111/bjo.2005.112.issue-8
13. Branch-Elliman W, Lee GM, Golen TH, Gold HS, Baldini LM, Wright SB. Health and economic burden of post-partum Staphylococcus aureus breast abscess. PLoS One. 2013;8(9):e73155. doi:10.1371/journal.pone.0073155
14. Chen CY, Anderson BO, Lo SS, Lin CH, Chen HM. Methicillin-resistant Staphylococcus aureus infections may not impede the success of ultrasound-guided drainage of puerperal breast abscesses. J Am Coll Surg. 2010;210(2):148–154. doi:10.1016/j.jamcollsurg.2009.11.003
15. Gould IM. Antibiotics, skin and soft tissue infection and meticillin-resistant Staphylococcus aureus: cause and effect. Int J Antimicrob Agents. 2009;34(Suppl 1):S8–11. doi:10.1016/S0924-8579(09)70542-4
16. Moazzez A, Kelso RL, Towfigh S, Sohn H, Berne TV, Mason RJ. Breast abscess bacteriologic features in the era of community-acquired methicillin-resistant Staphylococcus aureus epidemics. Arch Surg. 2007;142(9):881–884. doi:10.1001/archsurg.142.9.881
17. Schoenfeld EM, McKay MP. Mastitis and methicillin-resistant Staphylococcus aureus (MRSA): the calm before the storm? J Emerg Med. 2010;38(4):e31–e34. doi:10.1016/j.jemermed.2008.11.021
18. Chen KT, Huard RC, Della-Latta P, Saiman L. Prevalence of methicillin-sensitive and methicillin-resistant Staphylococcus aureus in pregnant women. Obstet Gynecol. 2006;108(3 Pt 1):482–487. doi:10.1097/01.AOG.0000227964.22439.e3
19. Dixon JM, Khan LR. Treatment of breast infection. BMJ. 2011;342:d396. doi:10.1136/bmj.d396
20. Irusen H, Rohwer AC, Steyn DW, Young T. Treatments for breast abscesses in breastfeeding women. Cochrane Database Syst Rev. 2015;8:Cd010490.
21. Schwarz RJ, Shrestha R. Needle aspiration of breast abscesses. Am J Surg. 2001;182(2):117–119. doi:10.1016/S0002-9610(01)00683-3
22. Ozseker B, Ozcan UA, Rasa K, Cizmeli OM. Treatment of breast abscesses with ultrasound-guided aspiration and irrigation in the emergency setting. Emerg Radiol. 2008;15(2):105–108. doi:10.1007/s10140-007-0683-0
23. Falco G, Foroni M, Castagnetti F, et al. Ultrasound-guided percutaneous catheter drainage of large breast abscesses in lactating women: how to preserve breastfeeding safely. Breastfeeding Med. 2016;11:555–556. doi:10.1089/bfm.2016.0121
24. Christensen AF, Al-Suliman N, Nielsen KR, et al. Ultrasound-guided drainage of breast abscesses: results in 151 patients. Br J Radiol. 2005;78(927):186–188. doi:10.1259/bjr/26372381
25. Berna-Serna JD, Madrigal M, Berna-Serna JD. Percutaneous management of breast abscesses. An experience of 39 cases. Ultrasound Med Biol. 2004;30(1):1–6. doi:10.1016/j.ultrasmedbio.2003.10.003
26. Marin M, Arroyo R, Espinosa-Martos I, Fernandez L, Rodriguez JM. Identification of emerging human mastitis pathogens by MALDI-TOF and assessment of their antibiotic resistance patterns. Front Microbiol. 2017;8:1258. doi:10.3389/fmicb.2017.01258
27. Delgado S, Arroyo R, Jimenez E, et al. Staphylococcus epidermidis strains isolated from breast milk of women suffering infectious mastitis: potential virulence traits and resistance to antibiotics. BMC Microbiol. 2009;9:82. doi:10.1186/1471-2180-9-82
28. Dinsmoor MJ, Viloria R, Lief L, Elder S. Use of intrapartum antibiotics and the incidence of postnatal maternal and neonatal yeast infections. Obstet Gynecol. 2005;106(1):19–22. doi:10.1097/01.AOG.0000164049.12159.bd
29. Dabbas N, Chand M, Pallett A, Royle GT, Sainsbury R. Have the organisms that cause breast abscess changed with time?–implications for appropriate antibiotic usage in primary and secondary care. Breast J. 2010;16(4):412–415. doi:10.1111/j.1524-4741.2010.00923.x
30. Lam E, Chan T, Wiseman SM. Breast abscess: evidence based management recommendations. Expert Rev Anti Infect Ther. 2014;12(7):753–762. doi:10.1586/14787210.2014.913982
31. Chan AW, Tetzlaff JM, Altman DG, Dickersin K, Moher D. SPIRIT 2013: new guidance for content of clinical trial protocols. Lancet. 2013;381(9861):91–92. doi:10.1016/S0140-6736(12)62160-6
32. Spellberg B. The new antibiotic mantra-”shorter is better”. JAMA Intern Med. 2016;176(9):1254–1255. doi:10.1001/jamainternmed.2016.3646
33. Boucher HW. Partial oral therapy for osteomyelitis and endocarditis - is it time? N Engl J Med. 2019;380(5):487–489. doi:10.1056/NEJMe1817264
34. Jianu DM, Sandulescu O, Streinu-Cercel A, et al. Microbiologic safety of the transareolar approach in breast augmentation. Aesthetic Surg j. 2016;36(1):51–57. doi:10.1093/asj/sjv106
35. Jianu DM, Streinu-Cercel A, Blidaru A, et al. Breast ecology assessment in the study of local microflora - study protocol. Germs. 2013;3(1):14–17. doi:10.11599/issn.2248-2997
36. Streinu-Cercel A, Jianu DM, Sandulescu O, Streinu-Cercel A. Response to “commentary on: microbiologic safety of the transareolar approach in breast augmentation. Aesthetic Surg j. 2016;36(4):Np177. doi:10.1093/asj/sjv264
37. David M, Handa P, Castaldi M. Predictors of outcomes in managing breast abscesses-A large retrospective single-center analysis. Breast J. 2018;24(5):755–763. doi:10.1111/tbj.2018.24.issue-5
38. Trop I, Dugas A, David J, et al. Breast abscesses: evidence-based algorithms for diagnosis, management, and follow-up. Radiographics. 2011;31(6):1683–1699. doi:10.1148/rg.316115521
39. Gollapalli V, Liao J, Dudakovic A, Sugg SL, Scott-Conner CE, Weigel RJ. Risk factors for development and recurrence of primary breast abscesses. J Am Coll Surg. 2010;211(1):41–48. doi:10.1016/j.jamcollsurg.2010.04.007
40. Giess CS, Golshan M, Flaherty K, Birdwell RL. Clinical experience with aspiration of breast abscesses based on size and etiology at an academic medical center. J Clin Ultrasound. 2014;42(9):513–521. doi:10.1002/jcu.v42.9
41. Versluijs-Ossewaarde FN, Roumen RM, Goris RJ. Subareolar breast abscesses: characteristics and results of surgical treatment. Breast J. 2005;11(3):179–182. doi:10.1111/j.1075-122X.2005.21524.x
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.