Back to Journals » Neuropsychiatric Disease and Treatment » Volume 19
A Systematic Review Assessing Patient-Related Predictors of Response to Transcranial Magnetic Stimulation in Major Depressive Disorder
Authors Gonterman F
Received 6 October 2022
Accepted for publication 17 February 2023
Published 8 March 2023 Volume 2023:19 Pages 565—577
DOI https://doi.org/10.2147/NDT.S388164
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Roger Pinder
Fernando Gonterman1,2
1Department of Population Health Science & Policy, Icahn School of Medicine at Mount Sinai, New York, NY, USA; 2Department of Counseling & Clinical Psychology, Teachers College Columbia University, New York, NY, USA
Correspondence: Fernando Gonterman, Icahn School of Medicine at Mount Sinai, One Gustave L. Levy Place, Box 1077, New York, NY, 10029, USA, Email [email protected]
Objective: The safety and efficacy of transcranial magnetic stimulation (TMS) in the acute treatment of major depressive disorder (MDD) is well established. However, it is not well understood which patient-related factors are associated with a more robust antidepressant response. Identifying predictive factors for therapeutic response to TMS treatment in depression will guide clinicians in patient selection.
Methods: By systematic review of clinical trial data, the current study aims to identify and analyze reported patient-specific predictors of response to an acute course of TMS treatment for MDD. PubMed was searched for randomized controlled trials of TMS for patients with depression. Studies were appraised for risk of bias using components recommended by the Cochrane Collaboration and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Results: TMS data were available from 375 studies, 18 of which were included in this review. Treatment response is inversely associated with treatment refractoriness and age.
Conclusion: Inadequate sample size and large heterogeneity in study parameters among clinical trials limit any strong conclusions from being drawn; nonetheless, despite these limitations, there is mounting evidence, which points to age and treatment refractoriness as candidate variables for predicting clinical outcome. Implications of these findings for treatment of MDD are discussed.
Keywords: depression, neurostimulation, TMS
Introduction
The principle of electromagnetism demonstrated by Michael Faraday during the 1830s is the basis of transcranial magnetic stimulation (TMS). Dr. Anthony T. Barker introduced the first stable TMS device in 1985. Originally, it was developed as a neurodiagnostic tool to noninvasively examine the connection between the central nervous system and skeletal muscle.1 Using a pulsed magnetic field, responses are elicited in those muscles that receive corticomotor input from the stimulated motor area.2
Not long after TMS was introduced, reports of a therapeutic effect began to emerge.3 Antidepressant effects, according to Grunhaus et al,4 were observed as early as 1993, and after several landmark clinical trials repetitive transcranial magnetic stimulation (rTMS) was shown to have a clinically significant benefit for depression.5–7 In October of 2008, the United States Food and Drug Administration approved the rTMS protocol employed in a multisite trial8 for use for major depressive disorder (MDD), specifically to alleviate symptoms in patients with treatment-resistant depression who have not found relief from prior antidepressant medication for the current depressive episode.
Patients who exhibit depressive symptomatology that follows a medication resistant course may find clinical benefit from TMS. Evidence suggests that for every 10 patients undergoing TMS, three9,10 to five11–13 will respond but will not necessarily achieve remission. This certainly begs the question, what patient-related factors influence outcome? However, a proper analysis of patient-related predictors must necessarily include an analysis of the procedural aspects of TMS, for variations in outcome are highly dependent upon treatment parameters. As a corollary, it should be borne in mind that the optimal treatment parameters for the management of depression have yet to be established at the writing of this review.
TMS Procedure
TMS treatment involves placing an insulated coil over the scalp surface. The coil is connected to a stimulator, which generates an electric current, resulting in a magnetic field around the coil. Magnetic pulses pass through hair, scalp, and skull unimpeded, stimulating the underlying cortex.
Regarding treatment location, magnetic pulses are delivered to a region of the brain implicated in the pathophysiology of depression, namely the dorsolateral prefrontal cortex (DLPFC). Due to its proximity to the scalp surface and its connections with remote areas that are also involved in mood (eg, the subgenual anterior cingulate cortex), the DLPFC is an ideal treatment target. With functional neuroimaging showing a reduction in glucose metabolism of depressed patients in the left DLPFC, TMS has generally been directed to this region.14 Historically, the DLPFC was located using the “5-centimeter method” in accordance with which the coil is placed 5 centimeters (cm) anterior along a parasagittal line from the motor spot that elicited motor-evoked potentials in the abductor pollicis brevis. While practical and simple, this method fails to account for anatomical differences such as skull size and cortical morphology. Eventually, it was determined to be more effective to move the treatment location slightly more anterior and lateral. For this reason, many researchers and clinicians – if they do not have access to more reliable targeting methods – move 5.5 or 6 cm forward from the motor threshold location.15,16
Meta-analyses support superior efficacy of high-frequency TMS administered to the left DLPFC.17 There is also evidence from a small number of studies that suggests low-frequency TMS administered to the right DLPFC may produce a similar antidepressant effect.18,19 On the basis thereof, conventional clinical approaches generally follow one of these two treatment paradigms. However, as more research is conducted and more advanced technology is produced, more techniques arise. A combination of the two protocols just described has been investigated for example. Commonly referred to as bilateral rTMS, this protocol consists of high-frequency TMS administered to the left DLPFC followed by low-frequency TMS administered to the right DLPFC, applied sequentially during the same session. Questions surrounding its efficacy led researchers to test whether bilateral rTMS is superior or inferior to unilateral stimulation of the left or right DLPFC. The consensus is that bilateral rTMS is comparable to unilateral rTMS in treating major depression.15,20–23
Frequency, as it relates to TMS treatment, modulates cortical excitability. Protocols using 5 hertz (Hz) or higher are considered high-frequency TMS and low-frequency TMS corresponds to approximately 1 Hz.24 An increase or decrease in neuronal activity is achieved by using a higher or lower frequency, respectively. As aforementioned, imaging studies correlate left-right DLPFC imbalance with depression, specifically, left DLPFC hypoactivity and right DLPFC hyperactivity.14,25 Thus, it is hypothesized that to bring neuronal activity in the left DLPFC to a normalized state high-frequency TMS would be administered, given that it induces an excitatory effect, and whereas high-frequency TMS has an excitatory effect, low-frequency TMS has an inhibitory effect on the receiving neurons. Right DLPFC hyperactivity would thus be moderated by low-frequency TMS. In consideration of the foregoing, stimulation parameters alone do not determine cortical excitability. That the modulation of cortical excitability depends, in part, on the baseline neural activity prior to stimulation, is of paramount importance, given that the direction and amplitude of stimulation is partly determined by the activity of neural tissue preceding stimulation.26
Changes in cortical excitability, as studies have demonstrated, are sustained beyond the stimulation period.27 A meta-analysis reporting on the durability of the antidepressant effect of rTMS among patients who responded to an initial acute course of treatment stated that 67% of patients sustained response after 3 months and 46% of responders maintained response 1 year after treatment.28
Patient-Related Predictors of Response
Of the patient-related factors hypothesized as influential determinants of clinical response age is frequently reported. Several studies have found that age is inversely correlated with antidepressant response, that is, younger adults with depression tend to benefit more from TMS treatment compared to their older adult counterparts.13,29–35 On the other hand, a few studies have failed to replicate these findings.36–38 In a review by Sabesan et al,39 the authors note a similar treatment effect among younger and older adults.
These inconsistent findings can be explained in part by aging-related changes. Researchers report that smaller frontal gray matter volumes were associated with a poorer response to TMS.34,40 Magnetic pulses are attenuated by distance so the distance between the scalp (where the coil is placed) and the cortex is a key variable for providing effective treatment. Atrophy affects the brain such that it increases the distance between the scalp and the cortex, thus reducing the treatment effect of the magnetic pulses. This is of special importance seeing as many studies use a figure-of-eight coil, which produces relatively focal stimulation only penetrating to a depth of about 2 cm. Therefore, older adults in some of the earlier studies may have failed to find benefit from rTMS because the DLPFC was never stimulated in the first place or perhaps only partially.
Moreover, many of these earlier studies did not use the standard dose of 120% of the resting motor threshold.40 Under these conditions, the right treatment location may have been stimulated, but the dose was insufficient to produce any meaningful response. Advances in brain mapping techniques, such as MRI-guided TMS, will increase treatment target identification and improvements in coil design will help to ensure that the treatment location is stimulated, and that the stimulation delivered is of a sufficient dose.
The brain’s plasticity during younger age has been linked to higher rates of response among younger adults. Younger adults, that is to say, the younger brain, may be more responsive to TMS than the older brain. The obverse of this is that there might be a delayed effect in older adults. Older adults might require more time to respond to stimulation than what has generally been allocated in clinical trials. Along these lines, it has been proposed that initially non-responsive patients could benefit from a larger number of pulses than responders.34,41 More pulses per session was associated with positive TMS outcomes according to Sackeim et al,38 with patients who averaged 4000 or more pulses per session seeing greater benefit.
Research has also identified a link between clinical response and treatment refractoriness, noting less treatment‐refractory patients as being more likely to respond to rTMS.33,37,42,43 Otherwise stated, a lower degree of medication resistance in the current or recent episode predicts superior antidepressant response to rTMS. This is in line with copious research demonstrating a strong negative correlation between treatment refractoriness and response to antidepressant medication.37,44
A greater antidepressant effect has been observed among women.38,41,45,46 The superior response seen in women has been attributed to a combination of factors – the type of depressive symptoms women typically manifest relative to men,47 gender differences in cranio-facial anatomy,48 and estrogen levels.45,48 Upon closer examination, the variation in treatment response between genders might not be due to gender alone but moderated by age.38,41,45 Female hormonal fluctuations across the lifecycle could partly explain age as a moderator. Evidence suggests, for example, that the pre-menopausal stage, as compared with post-menopause, is associated with improved response to rTMS.45,46
In terms of neurobiological predictors, brain derived neurotrophic factor49–52 and pre-treatment regional cerebral blood flow51,53–56 have been suggested, but further investigation with high-quality studies is required.
The most effective intervention for treatment refractory depression is electroconvulsive therapy (ECT) but for several reasons – be it tolerability, side effects, or nonresponse – patients with previous exposure to ECT might seek out rTMS as a treatment strategy. Taking that into account, determining whether history of ECT is an independent predictor of TMS response is clinically important. According to one retrospective analysis, the data revealed no significant difference between patients with previous ECT exposure versus those with no history of ECT and TMS outcomes57 (see also Fitzgerald et al).21
A group of researchers conducted an open-label study with high refractory MDD patients (at least three failed adequate trials of different antidepressants) and found 10 Hz modulation of corticomotor excitability to be a positive predictor of antidepressant response to DLPFC rTMS.58 They then followed up with another study, further substantiating this hypothesis.59 However, given the open-label nature of these studies and the fact that plasticity was assessed in the motor cortex as opposed to the DLPFC where treatment was applied, further evidence is needed.
Findings relating to depressive symptoms associated with the antidepressant properties of TMS are mixed.60 Researchers have found symptoms such as psychomotor retardation36,61 and sleep disturbance36 among responders, but the data is scarce. Cognitiveaffective symptoms of depression, especially loss of interest, were identified as potential predictor variables by Rostami et al.13 A possible explanation for this depressive symptom subtype, as opposed to a somatic subtype, has to do with the DLPFC as the treatment target. Owing to the fact that the prefrontal cortex plays a critical role in emotion regulation, the likelihood that a patient will benefit from rTMS over the DLPFC might be higher for a patient with cognitive-affective symptoms than one with a more somatic symptom profile.
Methods
The present systematic review was performed in line with the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement. Search terms used were “predictors transcranial magnetic stimulation.” Based on a literature search conducted using the PubMed database in December of 2022, a total of 375 records on TMS involving humans published in English from 1986 until December 5, 2022, were retrieved. Additional relevant articles (n=18) were obtained by scanning the reference lists of articles identified in the initial search. A PRISMA study selection flow diagram has been included (Figure 1).
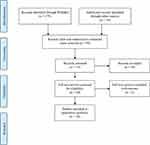 |
Figure 1 PRISMA flow diagram. Notes: PRISMA figure adapted from Liberati A, Altman D, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Journal of clinical epidemiology. 2009;62(10). Creative Commons.62 |
Inclusion criteria were randomized sham-controlled studies in which at least five sessions of TMS were administered over a limited number of days with stimulus parameters comparable to those of conventional TMS and studies with well-described outcome measures (ie, valid rating scales), clearly defined stimulus parameters, response and remission rates, as well as statistical analyses. The author included studies with male or female patients 18 years of age and older for indications of MDD. Studies which investigated forms of depression caused by injury or disease were not included. Studies did not meet inclusion criteria if they evaluated forms of depression with organic factors as the origin (eg, studies investigating post-stroke depression were excluded). Only studies that stimulated over the prefrontal cortex were included. Open-label and naturalistic studies were excluded.
Selection and Quality Assessment
One reviewer evaluated articles identified as a result of the search. Studies were initially screened for relevance in the title and abstract. The remaining full-text articles were reviewed. Studies were assessed for eligibility and risk of bias using the recommendations of the Cochrane Collaboration: sequence generation, allocation concealment, blinding of subjects, personnel and outcome assessors, incomplete outcome data, selective outcome reporting and other sources of bias. The final papers chosen for inclusion were also searched to ensure other eligible trials were not missed.
Outcome Measures and Data Collected
The main outcome measure was response, generally defined as a 50% or greater reduction in scores from pretreatment to post-treatment as assessed by an appropriate psychometric scale, such as the Hamilton Depression Rating Scale (HDRS), Montgomery-Asberg Depression Rating Scale (MADRS), or the Beck Depression Inventory (BDI). Remission of symptoms, as determined by the change in scores according to a predetermined threshold set by each study, was also a meaningful outcome here. Study data were collected on data collection forms, which recorded reference data, randomization, treatment arms, inclusion and exclusion criteria, TMS treatment parameters, demographic information, as well as numbers of participants in the active and control groups.
Results
The search strategy identified 375 published articles. Once the “clinical trial” filter was applied in PubMed, 314 articles were removed. The author reviewed titles and abstracts of the remaining 61 articles and excluded papers that were not clinical trials, implemented treatment methods vastly dissimilar to conventional TMS or which reported Supplementary Data (see Supplementary Material) from trials published elsewhere. The full text of the remaining 20 articles was assessed for eligibility. After studies were excluded on the basis of study design, randomization, indication, and study blinding, the search strategy yielded 18 randomized sham-controlled trials8,9,16,31,35,63–75 that enrolled 1316 patients (see Table 1).
 |
Table 1 Characteristics of the 18 Randomized Controlled Trials Identified by the Search Strategy |
Of the 18 studies identified, two studies reported age as associated with outcome.35,69 Using a p < 0.05 level of significance, Aguirre et al35 showed that age correlated with improvement of depressive symptoms at the end of 4 weeks of treatment (r=−0.683, p=0.002). In the study conducted by Loo et al,69 the mean age for responders and non-responders who received active TMS was 43.2 ± 13 and 53.3 ± 13 (p=0.032), respectively.
One study reported that treatment refractoriness is implicated in treatment response,8 whereby patients with a moderate level of treatment resistance to medication benefitted from TMS. In that study, patients had an average of 1.6 failed medication trials for the current episode8 and the primary endpoint – change in MADRS scores – established significance for the active treatment group (p=0.057).
The linear regression model performed by Baeken et al72 revealed that duration of the current depressive episode was significant in the model (p=0.03). Yesavage et al16 reported that, in their study of United States veterans, treatment effect was moderated by comorbid posttraumatic stress disorder (PTSD), that is, veterans with comorbid PTSD displayed the least improvement. With a p value of 0.03, PTSD comorbidity was significantly correlated with rates of remission.16
Eijndhoven et al74 performed analysis and found an inverse association between change in HDRS score and the Dutch measure for quantification of treatment resistance in depression, however this was not statistically significant relative to sham (p>0.1).
Both active treatments in Fitzgerald et al63 were significantly different from sham (p<0.005 for both). A higher baseline agitation score on the Clinical Outcomes in Routine Evaluation scale was associated with an increased response (p=0.004) but only predicted the response for the low-frequency TMS group (p=0.01, r2=0.29).
For several studies, treatment response appeared to be unrelated to the demographic and clinical characteristics recorded.9,31,65,68,71
Though no predictors of response were identified by Avery et al,9 active TMS was superior to sham. In the active group, higher response (Fisher’s p = 0.008) and remission rates (Fisher’s p = 0.033) were observed, compared with sham.9
Based on the results in the study by Mosimann et al,31 there was no correlation between age and depression rating or episode duration, nor did active stimulation differ from sham.
With 9 out of 28 subjects in the active group classified as responders and 3 out of 29 in the sham group classified as responders (p=0.06), the data from Hausmann et al64 suggest that TMS is not effective as an adjuvant to medication.
Data from 14 studies in this review demonstrated the efficacy of TMS,7,9,16,31,63,65–69,71–73,75 while the remaining four studies reported null findings – that no benefit is derived from rTMS.35,64,70,74
Discussion
Based on the results provided by these studies, age emerges as one of the strongest candidate predictor variables associated with rTMS response. For the Aguirre et al study,35 no other variable besides age correlated with change in HDRS scores. Similarly, the two-tailed t-tests run by Loo et al69 only found age to be significant. Thus, in both cases it was a bivariate correlation. That notwithstanding, the study conducted by Dai et al73 was able to establish the effectiveness of TMS in an elderly sample in which the mean age was 68.2. Indeed, this was a sample of inpatients so the results might not translate to other populations.
Treatment refractoriness is another possible predictor variable that holds promise.8,74 The most compelling evidence came from a multi-site trial with a large sample size (n=301).8 According to the data, a lower degree of medication resistance predicts a response.8 Patients who had one medication failure exhibited greater improvement in depression rating scale scores, compared to patients who had two or more medication failures. Of note, in their analyses no other predictor variables interacted with treatment resistance.8
Treatment refractoriness, as it was defined by most studies included in this review, is taken to mean a failure to respond to two adequate trials of antidepressant medication. This is consistent with previous research that has attempted to formalize an operational definition for treatment-resistant depression.76 Treatment refractoriness as a predictor of TMS response hangs together with the literature on antidepressant medication, in that the more stubborn the depression, the less likely one is to find relief.37,44 As a rule, “treatment resistance portends treatment resistance.”76 It is important to keep in mind, however, that a patient refractory to medication may not necessarily display the same degree of resistance to TMS. Indeed, as we learn more about the mechanisms of action of rTMS from those of antidepressants, we will be better suited to explain the variability in response between these interventions.
As researchers hone-in on effective treatment parameters, patients will receive better treatment, potentially leading to patients responding who may not have responded previously using older, less precise methods. A reduction in the number of partial and non-responders is therefore to be expected.
Once a patient has responded to an initial acute course of rTMS, the treatment plan that follows may involve long-term maintenance therapy with TMS. TMS maintenance therapy spanning several years has been shown to be safe and effective.77 For older adults in this situation, it may do well to incorporate the occasional brain mapping session to control for atrophy, though it is hard to say how frequently such sessions should occur. Including more brain mapping sessions would greatly increase the overall costs associated with TMS treatment but, on the other hand, the economic burden associated with MDD78 may far outweigh the addition of a few brain mapping sessions. If it means a life where one’s depression is in partial or full remission so that one can go about his or her daily life, the added costs may be well worth it.79
A common criticism brought against TMS clinical trials is the perceived lack of a suitable sham method. Despite researcher’s best efforts to make sham stimulation indistinguishable from active stimulation – largely by orienting the magnetic field away from the treatment location (usually at a 45- or 90-degree angle) – it has been shown that these forms of sham can be active.80 Based on that, one might argue that the integrity of TMS clinical trials is compromised. Through meta-analyses, however, it turns out that this is not the case. Overall, studies had acceptable levels of blinding integrity and the use of angulation versus sham coil in trials produced similar results.81
All the studies included in this review had serious limitations. First, small and homogeneous samples limit generalizability. Many early studies did not include patients over 65 years of age,40 which greatly restricts drawing conclusions from the data. Take for instance the study conducted by Rumi et al.66 It was determined that TMS accelerated the onset and augmented the therapeutic response to Amitriptyline,66 yet, they had a relatively young sample. The mean age of the active and sham arm was 39.3 and 38.9, respectively.66 Therefore, the results must be interpreted in that particular context.
In addition to inadequate samples, generalizing study findings are restricted by study design and methodological differences. Stimulation protocols differ markedly between studies with respect to total number of pulses, treatment duration, stimulation at different motor thresholds, site of stimulation, intervals, and duration of trains. Often enough, parameters are not held constant within the same study. Unless a study is designed to incorporate flexible dosing rather than a fixed dose, attention must be given to controlling for treatment parameters because improvements in mood could be due to more stimulation as opposed to different frequencies, motor thresholds, or sites of stimulation.
Conventional rTMS is based on the paradigmatic clinical trial that was carried out by O’Reardon et al,8 and yet, very few studies after 2008 use these evidence-based treatment parameters. Instead, most studies in this review implemented a lower number of pulses per session and treatment sessions. These reasons alone could account for much of the futility observed in many studies. The main weakness of this review is the use of a single reviewer, which may have introduced bias in the initial search of studies. The current study also did not include treatment methods such as intermittent theta burst stimulation or deep transcranial magnetic stimulation, as they were deemed too dissimilar from conventional rTMS.
The current systematic review was concerned with determining and analyzing patient-related predictive factors for therapeutic response to TMS in major depressive disorder. At present, there are very few randomized sham-controlled studies that address this question. Of those studies that do, drawing comparisons between them is problematic because of the differences in study design. Having said that, there is accumulating evidence pointing to age35,69 and treatment refractoriness8 as potential predictor variables. The data in support of these variables, however, are not robust enough to warrant excluding a patient from TMS treatment.
Given the heterogeneity in the presentation of depression combined with the limited understanding of the mechanisms of action of TMS, it is not surprising that the identification of reliable patient-specific predictors of response to TMS has been challenging. Even so, this endeavor is an important one because not only will it aid clinicians in identifying suitable candidates for treatment but also it will elucidate the place of TMS in the stock of interventions for depressive disorders. For these reasons, further research is warranted.
Disclosure
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors. The author reports no conflicts of interest in this work.
Reference
1. Groppa S, Oliviero A, Eisen A, et al. A practical guide to diagnostic transcranial magnetic stimulation: report of an IFCN committee. Clin Neurophysiol. 2012;123(5):858–882. doi:10.1016/j.clinph.2012.01.010
2. Barker AT, Jalinous R, Freeston IL. Non-invasive magnetic stimulation of human motor cortex. Lancet. 1985;325:1106–1107. doi:10.1016/S0140-6736(85)92413-4
3. Conca A, Koppi S, Konig P, et al. Transcranial magnetic stimulation: a novel antidepressive strategy? Neuropsychobiology. 1996;34:204–207. doi:10.1159/000119312
4. Grunhaus L, Dannon PN, Gershon AA. Transcranial magnetic stimulation: a new tool in the fight against depression. Dialogues Clin Neurosci. 2002;4(1):93–103. doi:10.31887/DCNS.2002.4.1/lgrunhaus
5. George MS, Wassermann EM, Williams WA, et al. Daily repetitive transcranial magnetic stimulation (rTMS) improves mood in depression. Neuroreport. 1995;6(14):1853–1856. doi:10.1097/00001756-199510020-00008
6. Pascual-Leone A, Rubio B, Pallardó F, et al. Rapid-rate transcranial magnetic stimulation of left dorsolateral prefrontal cortex in drug-resistant depression. Lancet. 1996;348:233–237. doi:10.1016/S0140-6736(96)01219-6
7. Klein E, Kreinin I, Chistyakov A, et al. Therapeutic efficacy of right prefrontal slow repetitive transcranial magnetic stimulation in major depression: a double-blind controlled study. Arch Gen Psychiatry. 1999;56(4):315–320. doi:10.1001/archpsyc.56.4.315
8. O’Reardon JP, Solvason HB, Janicak PG, et al. Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: a multisite randomized controlled trial. Biol Psychiatry. 2007;62(11):1208–1216. doi:10.1016/j.biopsych.2007.01.018
9. Avery DH, Holtzheimer PE, Fawaz W, et al. A controlled study of repetitive transcranial magnetic stimulation in medication-resistant major depression. Biol Psychiatry. 2006;59(2):187–194. doi:10.1016/j.biopsych.2005.07.003
10. Berlim MT, van den Eynde F, Tovar-Perdomo S, Daskalakis ZJ. Response, remission and drop-out rates following high-frequency repetitive transcranial magnetic stimulation (rTMS) for treating major depression: a systematic review and meta-analysis of randomized, double-blind and sham-controlled trials. Psychol Med. 2014;44(2):225–239. doi:10.1017/S0033291713000512
11. Carpenter LL, Janicak PG, Aaronson ST, et al. Transcranial magnetic stimulation (TMS) for major depression: a multisite, naturalistic, observational study of acute treatment outcomes in clinical practice. Depress Anxiety. 2012;29(7):587–596. doi:10.1002/da.21969
12. Connolly KR, Helmer A, Cristancho MA, Cristancho P, O’Reardon JP. Effectiveness of transcranial magnetic stimulation in clinical practice post-FDA approval in the United States: results observed with the first 100 consecutive cases of depression at an academic medical center. J Clin Psychiatry. 2012;73(4):e567–e573. doi:10.4088/JCP.11m07413
13. Rostami R, Kazemi R, Nitsche MA, Gholipour F, Salehinejad MA. Clinical and demographic predictors of response to rTMS treatment in unipolar and bipolar depressive disorders. Clin Neurophysiol. 2017;128(10):1961–1970. doi:10.1016/j.clinph.2017.07.395
14. Pandya M, Altinay M, Malone DA, et al. Where in the brain is depression? Curr Psychiatry Rep. 2012;14(6):634–642. doi:10.1007/s11920-012-0322-7
15. Brunoni AR, Chaimani A, Moffa AH, et al. Repetitive transcranial magnetic stimulation for the acute treatment of major depressive episodes: a systematic review with network meta-analysis [published correction appears in JAMA Psychiatry. 2017 Apr 1;74(4):424]. JAMA Psychiatry. 2017;74(2):143–152. doi:10.1001/jamapsychiatry.2016.3644
16. Yesavage JA, Fairchild JK, Mi Z, et al. Effect of repetitive transcranial magnetic stimulation on treatment-resistant major depression in US veterans: a randomized clinical trial. JAMA Psychiatry. 2018;75(9):884–893. doi:10.1001/jamapsychiatry.2018.1483
17. Lam RW, Chan P, Wilkins-Ho M, et al. Repetitive transcranial magnetic stimulation for treatment-resistant depression: a systematic review and metaanalysis. Can J Psychiatry. 2008;53(9):621–631. doi:10.1177/070674370805300909
18. Fitzgerald PB, Hoy K, Daskalakis ZJ, Kulkarni J. A randomized trial of the anti-depressant effects of low- and high-frequency transcranial magnetic stimulation in treatment-resistant depression. Depress Anxiety. 2009;26(3):229–234. doi:10.1002/da.20454
19. Fitzgerald PB, Daskalakis ZJ. The effects of repetitive transcranial magnetic stimulation in the treatment of depression. Expert Rev Med Devices. 2011;8(1):85–95. doi:10.1586/erd.10.57
20. Berlim MT, Van den Eynde F, Daskalakis ZJ. A systematic review and meta-analysis on the efficacy and acceptability of bilateral repetitive transcranial magnetic stimulation (rTMS) for treating major depression. Psychol Med. 2013;43(11):2245–2254. doi:10.1017/S0033291712002802
21. Fitzgerald PB, Hoy KE, Singh A, et al. Equivalent beneficial effects of unilateral and bilateral prefrontal cortex transcranial magnetic stimulation in a large randomized trial in treatment-resistant major depression. Int J Neuropsychopharmacol. 2013;16(9):1975–1984. doi:10.1017/S1461145713000369
22. Chen JJ, Liu Z, Zhu D, et al. Bilateral vs. unilateral repetitive transcranial magnetic stimulation in treating major depression: a meta-analysis of randomized controlled trials. Psychiatry Res. 2014;219(1):51–57. doi:10.1016/j.psychres.2014.05.010
23. Aaronson ST, Carpenter LL, Hutton TM, et al. Comparison of clinical outcomes with left unilateral and sequential bilateral Transcranial Magnetic Stimulation (TMS) treatment of major depressive disorder in a large patient registry. Brain Stimul. 2022;15(2):326–336. doi:10.1016/j.brs.2022.01.006
24. Lefaucheur JP. Transcranial magnetic stimulation. Handb Clin Neurol. 2019;160:559–580. doi:10.1016/B978-0-444-64032-1.00037-0
25. Grimm S, Beck J, Schuepbach D, et al. Imbalance between left and right dorsolateral prefrontal cortex in major depression is linked to negative emotional judgment: an fMRI study in severe major depressive disorder. Biol Psychiatry. 2008;63(4):369–376. doi:10.1016/j.biopsych.2007.05.033
26. Nicolo P, Ptak R, Guggisberg AG. Variability of behavioural responses to transcranial magnetic stimulation: origins and predictors. Neuropsychologia. 2015;74:137–144. doi:10.1016/j.neuropsychologia.2015.01.033
27. Klomjai W, Katz R, Lackmy-Vallee A. Basic principles of transcranial magnetic stimulation (TMS) and repetitive TMS (rTMS). Ann Phys Rehabil Med. 2015;58:208–213. doi:10.1016/j.rehab.2015.05.005
28. Senova S, Cotovio G, Pascual-Leone A, Oliveira-Maia AJ. Durability of antidepressant response to repetitive transcranial magnetic stimulation: systematic review and meta-analysis. Brain Stimul. 2019;12(1):119–128. doi:10.1016/j.brs.2018.10.001
29. Figiel GS, Epstein C, McDonald WM, et al. The use of rapid-rate transcranial magnetic stimulation (rTMS) in refractory depressed patients. J Neuropsychiatr Clin Neurosci. 1998;10(1):20–25. doi:10.1176/jnp.10.1.20
30. Manes F, Jorge R, Morcuende M, et al. A controlled study of repetitive transcranial magnetic stimulation as a treatment of depression in the elderly. Int Psychogeriatr. 2001;13:335. doi:10.1017/S1041610201007608
31. Mosimann UP, Schmitt W, Greenberg BD, et al. Repetitive transcranial magnetic stimulation: a putative add-on treatment for major depression in elderly patients. Psychiatry Res. 2004;126:123–133. doi:10.1016/j.psychres.2003.10.006
32. Gildengers A, Houck PH, Mulsant B. Trajectories of treatment response in late-life depression. J Clin Psychopharmacol. 2005;25(4):S8–13. doi:10.1097/01.jcp.0000161498.81137.12
33. Fregni F, Marcolin MA, Myczkowski M, et al. Predictors of antidepressant response in clinical trials of transcranial magnetic stimulation. Int J Neuropsychopharmacol. 2006;9(6):641–654. doi:10.1017/S1461145705006280
34. Jorge RE, Moser DJ, Acion L, et al. Treatment of vascular depression using repetitive transcranial magnetic stimulation. Arch Gen Psychiatry. 2008;65(3):268–276. doi:10.1001/archgenpsychiatry.2007.45
35. Aguirre I, Carretero B, Ibarra O, et al. Age predicts low-frequency transcranial magnetic stimulation efficacy in major depression. J Affect Disord. 2011;130:466–469. doi:10.1016/j.jad.2010.10.038
36. Brakemeier EL, Luborzewski A, Danker-Hopfe H, et al. Positive predictors for antidepressive response to prefrontal repetitive transcranial magnetic stimulation (rTMS). J Psychiatr Res. 2007;41(5):395–403. doi:10.1016/j.jpsychires.2006.01.013
37. Lisanby SH, Husain MM, Rosenquist PB, et al. Daily left prefrontal repetitive transcranial magnetic stimulation in the acute treatment of major depression: clinical predictors of outcome in a multisite, randomized controlled clinical trial. Neuropsychopharmacology. 2009;34(2):522–534. doi:10.1038/npp.2008.118
38. Sackeim HA, Aaronson ST, Carpenter LL, et al. Clinical outcomes in a large registry of patients with major depressive disorder treated with Transcranial Magnetic Stimulation. J Affect Disord. 2020;277:65–74. doi:10.1016/j.jad.2020.08.005
39. Sabesan P, Lankappa S, Khalifa N, et al. Transcranial magnetic stimulation for geriatric depression: promises and pitfalls. World J Psychiatry. 2015;5(2):170–181. doi:10.5498/wjp.v5.i2.170
40. Iriarte IG, George MS. Transcranial magnetic stimulation (TMS) in the elderly. Curr Psychiatry Rep. 2018;20(1):6. doi:10.1007/s11920-018-0866-2
41. Kedzior KK, Azorina V, Reitz SK. More female patients and fewer stimuli per session are associated with the short-term antidepressant properties of repetitive transcranial magnetic stimulation (rTMS): a meta-analysis of 54 sham-controlled studies published between 1997–2013. Neuropsychiatr Dis Treat. 2014;10:727–756. doi:10.2147/NDT.S58405
42. George MS, Lisanby SH, Avery D, et al. Daily left prefrontal transcranial magnetic stimulation therapy for major depressive disorder: a sham-controlled randomized trial. Arch Gen Psychiatry. 2010;67(5):507–516. doi:10.1001/archgenpsychiatry.2010.46
43. Kiebs M, Hurlemann R, Mutz J. Repetitive transcranial magnetic stimulation in non-treatment-resistant depression. Br J Psychiatry. 2019;215(2):445–446. doi:10.1192/bjp.2019.75
44. Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatr. 2006;163:1905–1917. doi:10.1176/ajp.2006.163.11.1905
45. Huang CC, Wei IH, Chou YH, Su TP. Effect of age, gender, menopausal status, and ovarian hormonal level on rTMS in treatment-resistant depression. Psychoneuroendocrinology. 2008;33(6):821–831. doi:10.1016/j.psyneuen.2008.03.006
46. Malik AM, Haque Z, Ide G, Farley A. Gender and age as factors in response and remission of depression treated with transcranial magnetic stimulation. Brain Stimul. 2016;9(5):e7. doi:10.1016/j.brs.2016.06.022
47. Albert PR. Why is depression more prevalent in women? J Psychiatry Neurosci. 2015;40(4):219–221. doi:10.1503/jpn.150205
48. Hanlon CA, McCalley DM. Sex/gender as a factor that influences transcranial magnetic stimulation treatment outcome: three potential biological explanations. Front Psychiatry. 2022;13:869070. doi:10.3389/fpsyt.2022.869070
49. Fidalgo TM, Morales-Quezada JL, Muzy GS, et al. Biological markers in noninvasive brain stimulation trials in major depressive disorder: a systematic review. J ECT. 2014;30(1):47–61. doi:10.1097/YCT.0b013e31828b34d8
50. Krstić J, Buzadžić I, Milanović SD, Ilić NV, Pajić S, Ilić TV. Low-frequency repetitive transcranial magnetic stimulation in the right prefrontal cortex combined with partial sleep deprivation in treatment-resistant depression: a randomized sham-controlled trial. J ECT. 2014;30(4):325–331. doi:10.1097/YCT.0000000000000099
51. Silverstein WK, Noda Y, Barr MS, et al. Neurobiological predictors of response to dorsolateral prefrontal cortex repetitive transcranial magnetic stimulation in depression: a systematic review. Depress Anxiety. 2015;32(12):871–891. doi:10.1002/da.22424
52. Cheng CM, Hong CJ, Lin HC, et al. Predictive roles of brain-derived neurotrophic factor Val66Met polymorphism on antidepressant efficacy of different forms of prefrontal brain stimulation monotherapy: a randomized, double-blind, sham-controlled study. J Affect Disord. 2022;297:353–359. doi:10.1016/j.jad.2021.10.077
53. Eschweiler GW, Wegerer C, Schlotter W, et al. Left prefrontal activation predicts therapeutic effects of repetitive transcranial magnetic stimulation (rTMS) in major depression. Psychiatry Res. 2000;99(3):161–172. doi:10.1016/s0925-4927(00)00062-7
54. Mottaghy FM, Keller CE, Gangitano M, et al. Correlation of cerebral blood flow and treatment effects of repetitive transcranial magnetic stimulation in depressed patients. Psychiatry Res. 2002;115(1–2):1–14. doi:10.1016/s0925-4927(02)00032-x
55. Speer AM, Willis MW, Herscovitch P, et al. Intensity-dependent regional cerebral blood flow during 1-Hz repetitive transcranial magnetic stimulation (rTMS) in healthy volunteers studied with H215O positron emission tomography: II. Effects of prefrontal cortex rTMS. Biol Psychiatry. 2003;54(8):826–832. doi:10.1016/s0006-3223(03)00324-x
56. Weiduschat N, Dubin MJ. Prefrontal cortical blood flow predicts response of depression to rTMS. J Affect Disord. 2013;150(2):699–702. doi:10.1016/j.jad.2013.04.049
57. Yuan S, Tirrell E, Gobin AP, Carpenter LL. Effect of previous electroconvulsive therapy on subsequent response to transcranial magnetic stimulation for major depressive disorder. Neuromodulation. 2020;23(3):393–398. doi:10.1111/ner.13046
58. Oliveira-Maia AJ, Press D, Pascual-Leone A. Modulation of motor cortex excitability predicts antidepressant response to prefrontal cortex repetitive transcranial magnetic stimulation. Brain Stimul. 2017;10(4):787–794. doi:10.1016/j.brs.2017.03.013
59. Hinchman CA, Fried PJ, Jannati A, Press DZ, Pascual-Leone A, Stern AP. Corticomotor plasticity as a predictor of response to high frequency transcranial magnetic stimulation treatment for major depressive disorder. J Affect Disord. 2022;303:114–122. doi:10.1016/j.jad.2022.02.005
60. May T, Pridmore S. Impact of transcranial magnetic stimulation on the symptom profile of major depressive episode. Australas Psychiatry. 2019;27(3):297–301. doi:10.1177/1039856219828134
61. Brakemeier EL, Wilbertz G, Rodax S, et al. Patterns of response to repetitive transcranial magnetic stimulation (rTMS) in major depression: replication study in drug-free patients. J Affect Disord. 2008;108(1–2):59–70. doi:10.1016/j.jad.2007.09.007
62. Liberati A, Altman DG, Tetzlaff J, et al. (2009). The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ, 339(jul21 1), b2700–b2700. doi: 10.1136/bmj.b2700
63. Fitzgerald PB, Brown TL, Marston NA, et al. Transcranial magnetic stimulation in the treatment of depression: a double-blind, placebo-controlled trial. Arch Gen Psychiatry. 2003;60(10):1002–1008. doi:10.1001/archpsyc.60.9.1002
64. Hausmann A, Kemmler G, Walpoth M, et al. No benefit derived from repetitive transcranial magnetic stimulation in depression: a prospective, single centre, randomised, double blind, sham controlled “add on” trial. J Neurol Neurosurg Psychiatry. 2004;75:320–322.
65. Rossini D, Lucca A, Zanardi R, et al. Transcranial magnetic stimulation in treatment-resistant depressed patients: a double-blind, placebo-controlled trial. Psychiatry Res. 2005;137:1–10. doi:10.1016/j.psychres.2005.06.008
66. Rumi DO, Gattaz WF, Rigonatti SP, et al. Transcranial magnetic stimulation accelerates the antidepressant effect of amitriptyline in severe depression: a double-blind placebo-controlled study. Biol Psychiatry. 2005;57(2):162–166. doi:10.1016/j.biopsych.2004.10.029
67. Anderson IM, Delvai NA, Ashim B, et al. Adjunctive fast repetitive transcranial magnetic stimulation in depression. Br J Psychiatry. 2007;190:533–534. doi:10.1192/bjp.bp.106.028019
68. Herwig U, Fallgatter A, Höppner J, et al. Antidepressant effects of augmentative transcranial magnetic stimulation: randomised multicentre trial. Br J Psychiatry. 2007;191(5):441–448. doi:10.1192/bjp.bp.106.034371
69. Loo CK, Mitchell PB, McFarquhar TF, et al. A sham-controlled trial of the efficacy and safety of twice-daily rTMS in major depression. Psychol Med. 2007;37(3):341–349. doi:10.1017/S0033291706009597
70. Mogg A, Pluck G, Eranti VS, et al. A randomized controlled trial with 4-month follow-up of adjunctive repetitive transcranial magnetic stimulation of the left prefrontal cortex for depression. Psychol Med. 2008;38:323–333. doi:10.1017/S0033291707001663
71. Pallanti S, Bernardi S, Di Rollo A, Antonini S, Quercioli L. Unilateral low frequency versus sequential bilateral repetitive transcranial magnetic stimulation: is simpler better for treatment of resistant depression? Neuroscience. 2010;167(2):323–328. doi:10.1016/j.neuroscience.2010.01.063
72. Baeken C, Vanderhasselt MA, Remue J, et al. Intensive HF-rTMS treatment in refractory medication-resistant unipolar depressed patients. J Affect Disord. 2013;151(2):625–631. doi:10.1016/j.jad.2013.07.008
73. Dai L, Wang P, Zhang P, et al. The therapeutic effect of repetitive transcranial magnetic stimulation in elderly depression patients. Medicine. 2020;99(32):e21493. doi:10.1097/MD.0000000000021493
74. van Eijndhoven PFP, Bartholomeus J, Möbius M, et al. A randomized controlled trial of a standard 4-week protocol of repetitive transcranial magnetic stimulation in severe treatment resistant depression. J Affect Disord. 2020;274:444–449. doi:10.1016/j.jad.2020.05.055
75. Akpınar K, Oğuzhanoğlu NK, Uğurlu TT. Efficacy of transcranial magnetic stimulation in treatment-resistant depression. Turk J Med Sci. 2022;52(4):1344–1354. doi:10.55730/1300-0144.5441
76. Sackeim HA. The definition and meaning of treatment-resistant depression. J Clin Psychiatry. 2001;62(Suppl 16):10–17.
77. O’Reardon JP, Blumner KH, Peshek AD, et al. Long-term maintenance therapy for major depressive disorder with rTMS. J Clin Psychiatry. 2005;66(12):1524–1528. doi:10.4088/JCP.v66n1205
78. Greenberg PE, Fournier AA, Sisitsky T, et al. The economic burden of adults with major depressive disorder in the United States (2010 and 2018). Pharmacoeconomics. 2021;39(6):653–665. doi:10.1007/s40273-021-01019-4
79. Lee JC, Blumberger DM, Fitzgerald PB, Daskalakis ZJ, Levinson AJ. The role of transcranial magnetic stimulation in treatment-resistant depression: a review. Curr Pharm Des. 2012;18(36):5846–5852. doi:10.2174/138161212803523644
80. Lisanby SH, Gutman D, Luber B, et al. Physiological effects of sham TMS: intracerebral measurements of the induced electric field and the induction of motor evoked potentials. Biol Psychiatry. 2001;49:460–463. doi:10.1016/S0006-3223(00)01110-0
81. Berlim MT, Broadbent HJ, Van den Eynde F. Blinding integrity in randomized sham-controlled trials of repetitive transcranial magnetic stimulation for major depression: a systematic review and meta-analysis. Int J Neuropsychopharmacol. 2013;16(5):1173–1181. doi:10.1017/S1461145712001691
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
