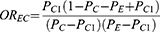Back to Journals » Clinical Epidemiology » Volume 16
A Study to Evaluate the Effectiveness and Safety of Prephase Steroid Treatment before Remission Induction Chemotherapy in Patients with Pediatric Acute Lymphoblastic Leukemia Using Common Data Model-Based Real-World Data: A Retrospective Observational Study
Authors Choi Y , Kim BK, Won JH, Yoo JW, Choi W, Jung S, Kim JY, Choi IY, Chung NG, Lee JW, Choi JY, Kang HJ, Lee H
Received 5 January 2024
Accepted for publication 9 April 2024
Published 22 April 2024 Volume 2024:16 Pages 293—304
DOI https://doi.org/10.2147/CLEP.S454263
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Lars Pedersen
Yoona Choi,1,2,* Bo Kyung Kim,3,4,* Jung-Hyun Won,2,5 Jae Won Yoo,6 Wona Choi,7 Surin Jung,7 Jae Yoon Kim,7,8 In Young Choi,7 Nack-Gyun Chung,6 Jae Wook Lee,6 Jung Yoon Choi,3,4 Hyoung Jin Kang,3,4,9 Howard Lee1,2,5,10,11
1Department of Applied Bioengineering, Graduate School of Convergence Science and Technology, Seoul National University, Seoul, Republic of Korea; 2Center for Convergence Approaches in Drug Development, Graduate School of Convergence Science and Technology, Seoul National University, Seoul, Republic of Korea; 3Department of Pediatrics, Seoul National University Hospital, Seoul, Republic of Korea; 4Seoul National University Cancer Research Institute, Seoul, Republic of Korea; 5Department of Molecular Medicine and Biopharmaceutical Sciences, Graduate School of Convergence Science and Technology, Seoul National University, Seoul, Republic of Korea; 6Department of Pediatrics, Seoul St. Mary’s Hospital, College of Medicine, the Catholic University of Korea, Seoul, Republic of Korea; 7Department of Medical Informatics, College of Medicine, the Catholic University of Korea, Seoul, Republic of Korea; 8Department of Biomedicine & Health Sciences, the Catholic University of Korea, Seoul, Republic of Korea; 9Department of Pediatrics, Seoul National University College of Medicine, Seoul, Republic of Korea; 10Advanced Institutes of Convergence Technology, Suwon, Republic of Korea; 11Department of Clinical Pharmacology and Therapeutics, Seoul National University Hospital, Seoul, Republic of Korea
*These authors contributed equally to this work
Correspondence: Howard Lee, Department of Clinical Pharmacology and Therapeutics, Seoul National University Hospital, 101 Daehak-ro, Jongno-gu, Seoul, 03080, Republic of Korea, Email [email protected] Hyoung Jin Kang, Department of Pediatrics, Seoul National University College of Medicine, 101 Daehak-ro, Jongno-gu, Seoul, 03080, Republic of Korea, Email [email protected]
Background: Rapid reduction of leukemic cells in the bone marrow during remission induction chemotherapy (RIC) can lead to significant complications such as tumor lysis syndrome (TLS). We investigated whether prephase steroid treatment before RIC could decrease TLS incidence and improve overall survival in pediatric patients with acute lymphoblastic leukemia (ALL).
Methods: Data were extracted from the Common Data Model databases in two tertiary-care hospitals in Seoul, South Korea. Patients were classified into the treated or untreated group if they had received RIC with prephase steroid treatment ≥ 7 days before RIC in 2012– 2021 or not, respectively. Stabilized Inverse Probability of Treatment Weighting (sIPTW) was applied to ensure compatibility between the treated and untreated groups. The incidence of TLS within 14 days of starting RIC, overall survival (OS), and the incidence of adverse events of special interest were the primary endpoints. Multiple sensitivity analyses were performed.
Results: Baseline characteristics were effectively balanced between the treated (n=308.4) and untreated (n=246.6) groups after sIPTW. Prephase steroid treatment was associated with a significant 88% reduction in the risk of TLS (OR 0.12, 95% CI: 0.03– 0.41). OS was numerically greater in the treated group than in the untreated group although the difference was not statistically significant (HR 0.64, 95% CI 0.25– 1.64). The treated group experienced significantly elevated risks for hyperbilirubinemia and hyperglycemia. The reduction in TLS risk by prephase steroid treatment was maintained in all of the sensitivity analyses.
Conclusion: Prephase steroid treatment for ≥ 7 days before RIC in pediatric patients with ALL reduces the risk of TLS, while careful monitoring for toxicities is necessary. If adequately analyzed, real-world data can provide crucial effectiveness and safety information for proper management of pediatric patients with ALL, for whom prospective randomized studies may be difficult to perform for ethical and practical reasons.
Keywords: pediatric acute lymphoblastic leukemia, tumor lysis syndrome, remission induction chemotherapy, prephase steroid treatment, common data model
Introduction
Remission induction chemotherapy (RIC) is the primary treatment for pediatric patients with acute lymphoblastic leukemia (ALL), which aims to quickly reduce the number of leukemic cells in the bone marrow.1 RIC typically involves a combination of vincristine, steroid, L-asparaginase, and an anthracycline, and has been shown to be highly successful in achieving complete remission in >95% of children.1–4 However, rapid reduction of leukemic cells caused by RIC may result in several critical complications such as tumor lysis syndrome (TLS), febrile neutropenia, and systemic infections.1 Of them, TLS is a life-threatening condition, with a prevalence of 5% to 26% in pediatric ALL patients who receive RIC.5 TLS can lead to increased morbidity, including acute renal failure, arrhythmia, seizures, and mortality unless appropriately managed.
Prephase treatment with steroids such as prednisolone or dexamethasone a week before initiating multi-agent RIC is one way to prevent TLS in pediatric patients with ALL. This strategy was employed by the Berlin-Frankfurt-Münster (BFM) group in the 1980s to gradually reduce the initial leukemic burden and lower the risk of toxicity.6 The BFM group also suggested prephase steroid treatment could provide information on risk stratification based on the response to it.7 However, prephase steroid treatment has not been widely accepted as a preferred prognostic indicator due to the availability of more accurate indicators, including minimal residual disease (MRD) at the end of remission induction.8 Furthermore, with supportive cares improving in recent decades to manage TLS and the use of urate oxidase, such as rasburicase,9,10 the potential benefits of prephase steroid treatment in reducing RIC-related toxicity remain unclear.
Based on this understanding, we conducted a retrospective observational study using real-world data to evaluate the effectiveness and safety of prephase steroid treatment before RIC in patients with pediatric ALL. The objective of our study was to evaluate whether prephase steroid treatment prior to RIC reduces the incidence of TLS and other adverse events and increases overall survival when compared with RIC without prephase steroid treatment.
Methods
Data Source
This multi-center retrospective observational study utilized the electronic medical record (EMR) databases from two university-affiliated tertiary-care hospitals in Seoul, South Korea: Seoul National University Hospital (SNUH) and The Catholic University of Korea College of Medicine, Seoul St. Mary’s Hospital (CUMC). We used the data extracted, transformed, and loaded into the Common Data Model databases (CDMs) in accordance with the Observational Medical Outcomes Partnership Common Data Model (OMOP CDM, version 5.3.1) specifications. Patient records from January 2012 to December 2021 were de-identified and data on demographics, conditions, drug exposures, measurements, and hospital visits were extracted from the CDMs of SNUH and CUMC.
Study Population
Eligible patients were children aged <18 years who had been diagnosed with ALL between January 1, 2012 and December 31, 2021, and received RIC consisting of vincristine, prednisolone, L-asparaginase with or without daunorubicin. The RIC regimen, lasting 4 to 5 weeks, typically included prednisone at 60 mg/m2 daily, orally; vincristine at 1.5 mg/m2, IV, once a week; L-Asparaginase at 6000 U/m2, IV, three times per week; and daunorubicin at either 25 mg/m2 weekly or 45 mg/m2 on Day 1, IV. These patients were divided into two groups based on whether they received prephase steroid treatment before RIC or not: treated and untreated (Figure 1). The prephase treatment involved prednisone at 60 mg/m2, administered orally daily. The treated group was defined as patients who received prephase steroid treatment at least 7 days before RIC, whereas the untreated group comprised those who didn’t receive any prephase steroid treatment or received it within 7 days before the start of RIC.
 |
Figure 1 Study Design: Treatment and Follow-Up Durations for Treated vs. Untreated Groups. |
Effectiveness and Safety Assessments
The primary outcome was the incidence of TLS within 14 days of starting RIC in both groups. TLS was defined as a diagnosis of TLS or the presence of two or more of the following laboratory findings as per the Cairo-Bishop definition of laboratory TLS:11 1) hyperuricemia (serum uric acid ≥8.0 mg/dL), 2) hyperkalemia (serum potassium ≥6.0 mmol/L), 3) hyperphosphatemia (serum phosphorous ≥6.5 mg/dL), 4) hypocalcemia (serum calcium ≤7 mg/dL). The secondary outcome was overall survival (OS).
For safety assessment, we compared the risk of adverse events (AEs) between the treated and untreated groups. We focused on the following AEs of special interest (AESI): hyperbilirubinemia, liver dysfunction, hyperglycemia, hyperlipidemia, pancreatitis, and hypertension. These AESIs were chosen due to their common occurrence and significant clinical implications related to steroids during RIC.
With the exception of hyperglycemia and hypertension, AEs were diagnosed using respective laboratory test results obtained within a 28-day period from the initiation of RIC, which met or exceeded grade 2 as defined by the Common Terminology Criteria for Adverse Events (CTCAE) version 5.0. Hyperglycemia was diagnosed using the criteria of American Diabetes Association.12 Hypertension was defined as a new prescription, i.e., not given in the 30 days prior to RIC, of amlodipine, carvedilol, or captopril, within 28 days after the initiation of RIC. We chose these three antihypertensive agents because they were commonly given to pediatric patients when they developed hypertension at SNUH and CUMC. A comprehensive list detailing the laboratory tests and associated definitions for each AE of interest is provided in Supplementary Table 1.
Statistical Analysis
To ensure the baseline compatibility between the treated and untreated groups in the distributions of covariates that may affect both the probability of treatment assignment and outcome, stabilized inverse probability of treatment weighting (sIPTW) based on propensity score (PS) was used.13 We derived PS using a logistic regression model, where the following baseline characteristics were incorporated as covariates: age at ALL diagnosis, sex, presence of Down syndrome, risk group, prophylactic use of rasburicase to prevent TLS before RIC, RIC regimen (either consisting of vincristine, prednisolone, and L-asparaginase, with or without daunorubicin), and baseline laboratory test results. The laboratory tests included white blood cell count (WBC), hemoglobin, platelet count, absolute neutrophil count (ANC), uric acid, phosphorus, potassium, calcium, blood urea nitrogen (BUN), creatinine, and lactate dehydrogenase (LDH). Risk groups were categorized based on the age at diagnosis of ALL and WBC count at the baseline: infants (<1 year old regardless of baseline WBC), standard risk (1‒10 years old and baseline WBC count ≤50000/μL), and high risk (>10 years old or baseline WBC count >50000/μL).
Then, we determined a stabilized weight for sIPTW, where inverse probability weights (i.e., 1/PS for treated patients and 1/(1-PS) for untreated patients) were normalized by the marginal probability of treatment in the entire sample. This aimed to make the pseudo population maintain a similar sample size to the original population, while avoiding excessively high weights for certain individuals due to extremely small PS or 1-PS values.13 We evaluated a standardized mean difference (SMD) in each covariate before and after adjustment using sIPTW to check the balance of the covariate between the treated and untreated groups. The SMD with a value of ≤0.1 considered to be balanced.14 The MatchIt package in R was used for PS generation.15
To investigate the association between prephase steroid treatment and clinical outcomes, we developed a logistic regression model to estimate the odds ratios (OR) of TLS and AESI between the treated and untreated groups. For the logistic regression, we adopted a backward elimination approach to iteratively remove covariates that were not statistically significant, ensuring the retention of only the most relevant predictors in the final model.16
Additionally, we utilized a Cox’s proportional hazard model, adjusted for age at ALL diagnosis, sex, presence of Down syndrome, risk group, and premedication with rasburicase, RIC regimen, and baseline laboratory test results using sIPTW, to estimate a hazard ratio (HR) for the association between the prephase steroid treatment and overall survival (OS). Kaplan-Meier survival curves, weighted by the sIPTW, were plotted for the two groups, and the differences in the curves were assessed using the Wald test. All statistical analyses were performed using R (ver 4.1.3; R Foundation, Vienna, Austria), after patient-level data from both hospitals were combined for analysis.
Sensitivity Analysis
We performed several sensitivity analyses to assess the robustness of the study findings. First, we examined the outcomes using different PS-based strategies besides sIPTW including PS matching without weights, PS matching with different model specifications such as caliper and trimming, and IPTW without stabilization.13,17
Second, we analyzed the effect of varying the duration of prephase steroid treatment on treatment outcome. In the main analysis, the duration of prephase steroid treatment was defined as at least 7 days before the initiation of RIC, as specified by the BFM group.6 However, there were patients in both hospitals who received steroids for less than 7 days, but at least 3 days before the start of the RIC. Therefore, we performed a sensitivity analysis to determine whether reducing the cut-off duration of prephase steroid treatment to 3 or 5 days would result in similar results. Each of these variations was scrutinized using the array of PS-based strategies including PS matching without weights, PS matching applying different model specifications, IPTW, and sIPTW.
Third, we conducted a sensitivity analysis to assess the confounding effect of a potential, but unmeasured, prognostic factor for TLS in pediatric ALL, i.e., immunophenotype (B-cell ALL or T-cell ALL), due to the absence of its information in our study. 18 To this end, we employed a rule-out approach, which assumes a hypothetical unmeasured confounder that is strongly associated with both the exposure (i.e., prephase steroid treatment) and the outcome (i.e., TLS). We calculated the strengths of the association between the exposure and potential unmeasured confounders (OREC) and between confounders and the outcome (RRCD) required to explain the observed increased risk of TLS associated with prephase steroid treatment (apparent relative risk, ARR), assuming there is no true association between prephase steroid treatment and TLS.19 The equations used are given (1) and (2), respectively:
OREC: Association between exposure and potential unmeasured confounder
PC: Prevalence of confounder
PC1: Prevalence of confounder in the exposed
PE: Prevalence of exposure
ARR: Apparent relative risk
RRCD: Association between potential unmeasured confounders and disease outcome
If the estimated effect of an unmeasured confounder is implausible or unlikely to exist, then it provides evidence that the observed association is less likely to be explained by unmeasured confounding.
Lastly, we performed a negative control analysis to examine the presence of any unmeasured residual confounding.20 We selected medicines commonly administered to pediatric patients, regardless of ALL diagnosis, that were neither associated with prephase steroid treatment nor with the occurrence of TLS. Specifically, we chose acetaminophen (an antipyretic analgesic) and chlorpheniramine (an antihistamine) given within 7 days prior to the first RIC, as negative controls. We evaluated whether the use of each drug was associated with an increased risk of TLS. If no association was found statistically significant in each analysis, the main findings of the present study were not likely affected by residual confounding.
Results
Study Population and Baseline Characteristics
To start, a total of 571 (250 and 321 from SNUH and CUMC, respectively) pediatric patients with ALL were included. Because of the variations in treatment protocols between the two hospitals, 83.8% (269 of 321) of patients at CUMC received prephase steroid treatment prior to RIC, whereas only 1.6% (4 of 250) did at SNUH.
Before sIPTW, the treated and untreated groups comprised 273 and 298 patients, respectively. The treated group exhibited significantly higher baseline levels of platelets (mean ± standard deviation: 102.08 ± 81.33 vs 86.19 ± 71.62, p=0.013), potassium (4.21 ± 0.46 vs 3.78 ± 0.64, p<0.001), and LDH (1771.24 ± 3164.41 vs 1049.31 ± 1649.71, p=0.001) (Table 1). The treated group also had a significantly lower proportion of patients who received prophylactic rasburicase to prevent TLS before RIC (2.2% vs 8.0%, p=0.003) and a larger proportion who were administered with a RIC regimen consisting of 4 agents (vincristine, prednisolone, L-asparaginase, and daunorubicin) (99.6% vs 58.5%, p<0.001) (Table 1).
 |
Table 1 Baseline Demographic and Clinical Characteristics of Patients Before and After sIPTW |
After sIPTW, a pseudo-population was created with 248.7 and 308.9 patients for the treated and untreated groups, respectively. Patient baseline characteristics became successfully balanced between the treated and untreated groups maintaining an SMD of at ≤0.1 except for phosphorous (SMD: 0.179) and proportion who were administered with a RIC regimen consisting of 4 agents (SMD: 0.216) (Table 1).
Effectiveness of Prephase Steroid Treatment
Prephase steroid treatment was associated with a significant 88% reduction in the risk of TLS (OR 0.12, 95% CI: 0.03–0.41, p=0.004) (Figure 2). Significant covariates in the final logistic regression model following backward elimination were age at ALL diagnosis, sex, WBC, hemoglobin, platelet, phosphorus, potassium, BUN, creatinine, risk group, and premedication with rasburicase. The associations of these covariates with the risk of TLS are provided in Supplementary Table 2.
 |
Figure 2 Odds Ratios for Tumor Lysis Syndrome Associated with Prephase Steroid Treatment Before Remission Induction Chemotherapy. |
The median follow-up duration was 53.7 months (interquartile range 23.3–84.9) and the median OS was not reached in either group. Although statistically not significant, the treated group showed a numerically higher OS than the untreated group (HR 0.64, 95% CI 0.25–1.64, p=0.352) (Figure 3). The 5-year survival probability was 90.0% and 86.5% for the treated and untreated groups, respectively (p=0.397) (Figure 3). Deaths within 30 days following the initiation of RIC were observed in one patient in the treated group and two patients in the untreated group. Of these, one patient in the untreated group experienced TLS prior to death. However, due to the nature of the CDM database, the causality between TLS and death could not be determined due to the limited data available.
 |
Figure 3 Kaplan-Meier Survival Curve for Overall Survival. |
Safety of Prephase Steroid Treatment
The treated group exhibited a higher incidence of AESI than the untreated group. Hyperbilirubinemia emerged as the predominant AESI in both the treated (63.2%) and untreated groups (39.9%), with a significantly higher risk in the treated group than in the untreated group (OR 2.98, 95% CI 1.60‒5.54, p-value=0.001) (Figure 4). Additionally, the treated group showed a statistically significant increase in the risk of hyperglycemia (OR 2.61, 95% CI 1.20‒5.69, p-value=0.016) although the incidence of hyperglycemia was much lower than that of hyperbilirubinemia (13.8% and 6.2% in the treated and untreated groups, respectively, Figure 4). While the risks for liver dysfunction, pancreatitis, and hyperlipidemia were elevated in the treated group, these increases were not statistically significant (Figure 4). However, most AESIs were generally manageable because the proportion of patients with grade 3 or higher was ≤10% in the treated group for all events, except for liver dysfunction (31.1%) (Supplementary Figure 1).
 |
Figure 4 Odds Ratios for Adverse Events of Special Interest Associated with Prephase Steroid Treatment Before Remission Induction Chemotherapy. |
In contrast, the incidence of hypertension was significantly lower in the treated group than in the untreated group (OR 0.08, 95% CI 0.02‒0.33, p-value=0.001) (Figure 4).
Sensitivity Analysis
Results from the sensitivity analyses were consistent across various PS-based strategies and different cut-offs for the duration of prephase steroid treatment. The OR estimates consistently indicated a significant reduction in the TLS risk in the treated group compared with the untreated group, with ORs ranging from 0.09 to 0.26 (Figure 2).
Furthermore, potential confounding effect by immunophenotype, an unmeasured prognostic factor, was ruled out. Given that the prevalence of T-cell type in pediatric ALL is approximately 15%,21,22 and the prevalence of RIC with prephase steroid treatment is assumed to be equal to that of RIC without prephase steroid treatment (50%), OREC was negative (<-0.01), an implausible value, when RRCD >2.1.19 In a prior study, the OR of TLS between T-cell and B-cell ALLs was 4.7 (95% CI 2.6–8.8).18 Using an equation reported previously,23 this OR corresponds to an RR of 3.4, which exceeds 2.1. Therefore, an OR of 0.12 for TLS in our primary analysis was unlikely to be influenced by an unmeasured confounder such as immunophenotype.
Our primary results were unaffected by residual confounding either. In the sensitivity analysis using acetaminophen and chlorpheniramine administered within 7 days prior to the first RIC as negative controls, ORs for TLS were close to unity and statistically insignificant, i.e., 1.04 (95% CI 0.48–2.24, p-value=0.939) and 1.72 (95% CI 0.45–6.54, p-value=0.421), respectively. Therefore, the association between TLS risk and prephase steroid treatment was unlikely to be attributed to residual confounding.
Discussion
This study shows that prephase steroid treatment administered for ≥7 days prior to RIC in patients with ALL is effective for reducing the risk of TLS, while careful monitoring for toxicities is necessary. The evidence is that the treated group exhibited a significantly lower risk of TLS than the untreated group (OR 0.12, 95% CI: 0.03–0.41, p=0.004) (Figure 2) and numerically increased overall survival, although it failed to reach statistical significance (HR 0.64, 95% CI 0.25–1.64, p=0.352) (Figure 3). At the same time, it should be also noted that the treated group experienced significantly elevated risks for a majority of AESIs such as hyperbilirubinemia (OR 2.98, 95% CI 1.60‒5.54, p-value=0.001) and hyperglycemia (OR 2.61, 95% CI 1.20‒5.69, p-value=0.016) (Figure 4).
The treatment effect of prephase steroid treatment in reducing the risk of TLS was consistent even when redefining the cut-off duration to <7 days such as 3 or 5 days (Figure 2). This suggests that a minimum of 3 days of prephase steroid treatment before RIC might effectively reduce TLS. This observation is in line with the National Comprehensive Cancer Network (NCCN) guidelines, which recommend 3‒7 days of prephase steroid treatment as prophylaxis for pediatric ALL patients at specific risk for TLS, different from the 7-day duration suggested by the BFM group.24 However, the OR estimates in our finding increased as the duration of prephase steroid treatment was shortened from 7 days to 5 and 3 days across similar PS-based strategies (e.g., with sIPTW, 0.12 for ≥7 days, 0.15 for ≥5 days, and 0.23 for ≥3 days). Future research is needed to ascertain whether shorter prephase steroid treatments offer effects comparable to treatments lasting at least 7 days.
While prephase steroid treatment significantly lowered the risk of TLS, it didn’t lead to a significant increase in overall survival (Figure 3). This could be because the hospitals effectively managed TLS, preventing it from becoming fatal. A single-center study from Pakistan, a developing country, reported that patients receiving one week of prephase steroid treatment before RIC experienced significantly lower mortality within five weeks after treatment than those who received upfront RIC without prephase steroid treatment (9% vs 13%, p=0.023).25 On the contrary, in our study, only three patients died in the first month, with just one of them having TLS. Our mortality data were restricted to deaths reported within the participating hospitals, potentially omitting fatalities that occurred outside these institutions. However, given that most ALL patients undergoing RIC remain hospitalized for the majority of the initial month, the likelihood of overlooking fatalities during this timeframe appears minimal.
Prephase steroid treatment did not lower the risk of most of toxicities other than TLS and rather increased the risk for certain AESIs, such as hyperbilirubinemia and hyperglycemia (Figure 4). This discrepancy might arise because the underlying mechanisms for the AESIs observed in our study differ from those of TLS, which is primarily linked to rapid and extensive leukemic cell death. For example, hyperbilirubinemia might result from hepatotoxicity or drug interactions following the administration of cytotoxic chemotherapy agents like vincristine or daunorubicin.26 Furthermore, steroids themselves can induce adverse events such as hyperglycemia, hypertension, and pancreatitis.27 While it’s challenging to conclusively attribute the elevated risk of these AESIs to prephase steroid treatment, given a myriad of potential causes and the inherent complexity of these AESIs compared with TLS, different protocols for managing adverse events and laboratory test frequencies between SNUH and CUMC might have also played a role. Nevertheless, our findings emphasize the importance of vigilant monitoring for certain AESIs, particularly hyperbilirubinemia and hyperglycemia, during prephase steroid treatment followed by RIC.
There has been few studies on the treatment effect of prephase steroid treatment since its introduction by the BFM group in the 1980s.6 This may be due to the diminished significance of the initial benefits associated with prephase steroid treatment as proposed by the BFM group. One of the primary benefits of prephase steroid treatment was that the response after one week of prednisone could serve as a prognostic factor for ALL. Patients categorized as having a prednisone poor response accounted for around 10% of ALL cases and exhibited a significantly higher relapse risk than those with a prednisone good response.28 Subsequent BFM studies, including BFM-83, 86, and 90, reaffirmed the prednisone response as a significant prognostic factor in pediatric ALL.7,28,29 However, the MRD during and post-induction has since emerged as the most important predictor of ALL relapse.30 Moreover, a discrepancy arose between MRD and prednisone response: a subset of prednisone poor response patients with low MRD exhibited favorable outcomes.31 Consequently, the prednisone response is no longer universally recognized as a pivotal prognostic indicator.
The more significant benefit of prephase steroid treatment in recent days was its ability to significantly reduce toxicity, especially TLS, which motivated our study. However, as there have been substantial advances in supportive care for toxicity management over recent decades such as vigorous hydration and urine alkalinization,11 prephase steroid treatment has been mostly left to each hospital’s preference.8 In particular, rasburicase has been recognized as an effective prophylactic and therapeutic agent for TLS since the early 2000s. The NCCN recommends both prephase steroid treatment for 3‒7 days and prophylactic rasburicase for pediatric ALL patients with specific risk factors.24 In South Korea, the utilization of rasburicase has increased after its reimbursement for patients with high risk of TLS by the national insurance in 2018. However, in our study spanning 2012‒2021, only around 5% of the sIPTW-adjusted population were premedicated with rasburicase, which makes it challenging to conduct subgroup analysis about the impact of premedication with rasburicase on TLS risk reduction. Future studies comparing the effectiveness of prephase steroid treatment and that of premedication with rasburicase, or determining the optimal patient profile for each treatment, would be valuable. Nevertheless, our study holds significance in that it shows the effectiveness in reduction of the risk of TLS across all TLS risk levels in pediatric ALL patients. Furthermore, in settings with limited access to rasburicase or other supportive care, our findings may have greater impact.
Our study has several strengths. First, to our knowledge, our study represents the first evidence comparing the effectiveness and safety of prephase steroid treatment prior to RIC among a large cohort of pediatric ALL patients across multiple sites in real-world settings. The prephase steroid treatment hasn’t been supported by evidence from a randomized-controlled trial (RCT) that demonstrates its superiority over no prephase steroid treatment since its first introduction. Due to the limited patient population and ethical considerations related to pediatric clinical trial, conducting an RCT on this topic will remain challenging in the future as well. Thus, our results provide critical real-world evidence on prephase steroid treatment. Second, the large number of patients enrolled in our study is another strength. SNUH and CUMC are major tertiary hospitals, which manage approximately one-third of all pediatric ALL patients in South Korea. Third, we adjusted for numerous potential confounders using sIPTW to enhance the validity of our findings by controlling for confounding effects. Previous studies indicated that age (either ≥10 or <1), a WBC count of ≥ 50×109/L, an LHD level ≥ 2 × upper limit normal and T-cell immunophenotype could significantly elevate the risk of TLS in pediatric patients with ALL.18,32 In our research, age, WBC, LDH were incorporated as covariates in sIPTW (Table 1). Although we couldn’t include immunophenotype as a covariate due to data limitations, we successfully showed that our results were not significantly influenced by this factor using the rule-out approach, a part of our sensitivity analysis. Additionally, we ascertained that our results were unaffected by residual confounding through the negative control analysis. Our findings also remained consistent across additional sensitivity analyses that examined the effects of varying durations of prephase steroid treatment and employing diverse PS-based strategies (Figure 2). Lastly, this study also highlights the value of RWD in conducting observational studies in pediatric patients with ALL. Specifically, the OMOP CDM utilized in this study provides the same concepts from EMRs in a standardized and structured way irrespective of hospitals.33 This standardization can significantly diminish the often lengthy and labor-intensive process of data pre-processing and preparation, offering particular advantages for multi-site studies.
Our study had several limitations. First, our data were sourced from only two sites with heterogeneous treatment preferences, i.e., one preferring prephase steroid treatment before RIC and the other not. Although we adopted various strategies to adjust for both known and unknown confounders between the treated and untreated groups, we couldn’t incorporate site into the covariates due to the imbalance in treatment allocation between the sites. Therefore, biases may still exist in our findings, as there could be other confounders in treatment practices which we haven’t accounted for. Additionally, the limited number of sites may restrict the generalizability of our findings. Thus, future studies are encouraged to assess clinical outcomes across a broader range of sites, ideally with comparable treatment allocation, and spanning different countries and healthcare systems. Second, we were unable to evaluate several pivotal clinical outcomes other than TLS due to the intrinsic constraints of retrospective data collection. For example, we couldn’t assess clinical TLS, which is defined as TLS that requires clinical interventions in addition to meeting the criteria of laboratory TLS used in this study.11 Such clinical TLS can include severe cases leading to complications like cardiac arrhythmias, seizures, or renal failure that requires continuous renal replacement therapy. However, the limited data elements in the CDMs did not allow us to phenotype clinical TLS. Likewise, we couldn’t evaluate certain steroid-induced toxicities, e.g., osteonecrosis or infections, because these conditions are also challenging to phenotype using the available data. Lastly, we were unable to address response rates and recurrence-free survival because blast counts in the bone marrow, essential for determining treatment response and detecting ALL recurrences, were missing in our data. Future studies, possibly leveraging a more extensive database or incorporating chart reviews by investigators, are crucial to further evaluate these clinical outcomes.
In conclusion, prephase steroid treatment for ≥7 days before RIC in pediatric patients with ALL reduces the risk of TLS, while careful monitoring for toxicities is necessary. If adequately analyzed, real-world data can provide crucial effectiveness and safety information for proper management of pediatric patients with ALL, for whom prospective randomized studies may be difficult to perform for ethical and practical reasons.
Ethics Approval and Patient Consent
This study was approved by the IRBs of Seoul National University Hospital (IRB No.: E-2211-089-1378) and the Catholic University of Korea College of Medicine, Seoul St. Mary’s Hospital (IRB No.: KC23ENSI0019). Obtaining informed consent was waived by the IRBs due to the retrospective nature of the study and the use of de-identified data. This study was also in accordance with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Acknowledgments
We would like to express our sincere thanks to Prof. Kwangsoo Kim of Division of Clinical Bioinformatics, Biomedical Research Institute, Seoul National University Hospital and Mr. Jun Hyeok Oh of Data Science Team, Information Convergence Institute, the Catholic University of Korea for their assistance in accessing and utilizing the CDM databases for our research. Their support was instrumental in the completion of this study. The abstract of this paper was presented at the 65th American Society of Hematology Annual Meeting & Exposition in 2023 as a poster presentation with interim findings. The poster’s abstract was published in ‘Poster Abstracts’ in Blood (2023) 142 (Supplement 1): 3791.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (Grant number: HI22C0464). Additionally, this research is partly supported by the BK21FOUR Program of the National Research Foundation of Korea (NRF) funded by the Ministry of Education (5120200513755).
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Cooper SL, Brown PA. Treatment of pediatric acute lymphoblastic leukemia. Pediatr Clin North Am. 2015;62(1):61–73. doi:10.1016/j.pcl.2014.09.006
2. Bassan R, Hoelzer D. Modern therapy of acute lymphoblastic leukemia. J Clin Oncol. 2011;29(5):532–543. doi:10.1200/JCO.2010.30.1382
3. Inaba H, Greaves M, Mullighan C. Acute lymphoblastic leukaemia. Lancet. 2013;381(9881):1943–1955. doi:10.1016/S0140-6736(12)62187-4
4. Seibel N. Treatment of acute lymphoblastic leukemia in children and adolescents: peaks and pitfalls. Hematology Am Soc Hematol Educ Program. 2008;2008(1):374–380. doi:10.1182/asheducation-2008.1.374
5. Wilson FP, Berns JS. Tumor Lysis Syndrome: New Challenges and Recent Advances. Adv Chronic Kidney Dis. 2014;21(1):18–26. doi:10.1053/j.ackd.2013.07.001
6. Schrappe M, Reiter A, Zimmermann M, et al. Long-term results of four consecutive trials in childhood ALL performed by the ALL-BFM study group from 1981 to 1995. Berlin-Frankfurt-Munster Leukemia. 2000;14:2205–2222.
7. Reiter A, Schrappe M, Ludwig WD, et al. Chemotherapy in 998 unselected childhood acute lymphoblastic leukemia patients. Results and conclusions of the multicenter trial ALL-BFM 86. Blood. 1994;84(9):3122–3133. doi:10.1182/blood.V84.9.3122.3122
8. McNeer JL, Nachman JB. The optimal use of steroids in paediatric acute lymphoblastic leukaemia: no easy answers. Br J Haematol. 2010;149(5):638–652. doi:10.1111/j.1365-2141.2010.08192.x
9. Hunger SP, Lu X, Devidas M, et al. Improved Survival for Children and Adolescents With Acute Lymphoblastic Leukemia Between 1990 and 2005: A Report From the Children’s Oncology Group. J clin oncol. 2012;30(14):1663–1669. doi:10.1200/JCO.2011.37.8018
10. Dinnel J, Moore BL, Skiver BM, Bose P. Rasburicase in the management of tumor lysis: an evidence-based review of its place in therapy. Core Evid. 2015;10:23–38. doi:10.2147/CE.S54995
11. Cairo MS, Bishop M. Tumour lysis syndrome: new therapeutic strategies and classification. Br J Haematol. 2004;127(1):3–11. doi:10.1111/j.1365-2141.2004.05094.x
12. American Diabetes A. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2020. Diabetes Care. 2020;43:S14–S31.
13. Chesnaye NC, Stel VS, Tripepi G, et al. An introduction to inverse probability of treatment weighting in observational research. Clin Kidney J. 2021;15(1):14–20. doi:10.1093/ckj/sfab158
14. Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28(25):3083–3107. doi:10.1002/sim.3697
15. Ho D, Imai K, King G, Stuart EA. MatchIt: Nonparametric preprocessing for parametric causal inference. J Statistical Software. 2011;42(8):1–28. doi:10.18637/jss.v042.i08
16. Bursac Z, Gauss CH, Williams DK, Hosmer DW. Purposeful selection of variables in logistic regression. Source Code Biol Med. 2008;3(1):17. doi:10.1186/1751-0473-3-17
17. Austin PC. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivariate Behav Res. 2011;46(3):399–424. doi:10.1080/00273171.2011.568786
18. Xue Y, Chen J, Gao S, et al. Clinical characteristics of tumor lysis syndrome in childhood acute lymphoblastic leukemia. Sci Rep. 2021;11(1):9656. doi:10.1038/s41598-021-88912-2
19. Schneeweiss S. Sensitivity analysis and external adjustment for unmeasured confounders in epidemiologic database studies of therapeutics. Pharmacoepidemiol Drug Saf. 2006;15(5):291–303. doi:10.1002/pds.1200
20. Lipsitch M, Tchetgen Tchetgen E, Cohen T. Negative controls: a tool for detecting confounding and bias in observational studies. Epidemiology. 2010;21(3):383–388. doi:10.1097/EDE.0b013e3181d61eeb
21. Swerdlow SH, Campo E, Harris NL, et al. WHO classification of tumours of haematopoietic and lymphoid tissues. International agency for research on cancer: Lyon, France; 2008.
22. Onciu M. Acute lymphoblastic leukemia. Hematol Oncol Clin North Am. 2009;23(4):655–674. doi:10.1016/j.hoc.2009.04.009
23. Zhang J, Yu KF. What’s the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA. 1998;280(19):1690–1691. doi:10.1001/jama.280.19.1690
24. National Comprehensive Cancer Network. Pediatric Acute Lymphoblastic Leukemia (Version 2.2023); 2023.
25. Jabbar N, Mansoor N, Nadeem K, Maqsood MS, Butt Z, Ashraf S. Prednisolone Prophase for a Week Versus Upfront Multiagent Chemotherapy in Childhood Acute Lymphoblastic Leukemia: An Analysis With Reference to Induction Mortality in a Developing Country. J Pediatr Hematol Oncol. 2020;42(3):181–184. doi:10.1097/MPH.0000000000001636
26. Grigorian A, O’Brien CB. Hepatotoxicity Secondary to Chemotherapy. J Clin Transl Hepatol. 2014;2(2):95–102. doi:10.14218/JCTH.2014.00011
27. Liu D, Ahmet A, Ward L, et al. A practical guide to the monitoring and management of the complications of systemic corticosteroid therapy. Allergy Asthma Clin Immunol. 2013;9(1):30. doi:10.1186/1710-1492-9-30
28. Riehm H, Reiter A, Schrappe M, et al. Corticosteroid-dependent reduction of leukocyte count in blood as a prognostic factor in acute lymphoblastic leukemia in childhood (therapy study ALL-BFM 83). Klin Padiatrie. 1987;199(03):151–160. doi:10.1055/s-2008-1026781
29. Schrappe M, Reiter A, Sauter S, et al. Concept and interim result of the ALL-BFM 90 therapy study in treatment of acute lymphoblastic leukemia in children and adolescents: the significance of initial therapy response in blood and bone marrow. Klin Padiatrie. 1994;206(04):208–221. doi:10.1055/s-2008-1046607
30. Borowitz MJ, Devidas M, Hunger SP, et al. Clinical significance of minimal residual disease in childhood acute lymphoblastic leukemia and its relationship to other prognostic factors: a Children’s Oncology Group study. Blood. 2008;111(12):5477–5485. doi:10.1182/blood-2008-01-132837
31. Conter V, Bartram CR, Valsecchi MG, et al. Molecular response to treatment redefines all prognostic factors in children and adolescents with B-cell precursor acute lymphoblastic leukemia: results in 3184 patients of the AIEOP-BFM ALL 2000 study. Blood. 2010;115(16):3206–3214. doi:10.1182/blood-2009-10-248146
32. Cairo MS, Coiffier B, Reiter A, Younes A, Panel TLSE. Recommendations for the evaluation of risk and prophylaxis of tumour lysis syndrome (TLS) in adults and children with malignant diseases: an expert TLS panel consensus. Br J Haematol. 2010;149(4):578–586. doi:10.1111/j.1365-2141.2010.08143.x
33. OHDSI. The Book of OHDSI: Observational Health Data Sciences and Informatics. OHDSI; 2019.
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.