Back to Journals » Infection and Drug Resistance » Volume 16
A Simple Disk Stacking Plus Micro-Elution Method for Rapid Detection of the Synergistic Effect of Aztreonam and Ceftazidime/Avibactam Against Metallo-β-Lactamase Producing Enterobacterales
Authors Liu Z , Hang X, Yan T, Chu W , Gong Z, Liu Y , Dai Y, Yang M, Li J, Zhou Q
Received 22 December 2022
Accepted for publication 6 March 2023
Published 15 March 2023 Volume 2023:16 Pages 1537—1543
DOI https://doi.org/10.2147/IDR.S402275
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Suresh Antony
Zhou Liu,1,* Xiubing Hang,1,* Tao Yan,1 Wenwen Chu,1 Zhen Gong,1 Yanyan Liu,2– 4 Yuanyuan Dai,5 Min Yang,6 Jiabin Li,2– 4 Qiang Zhou1
1Department of Laboratory Medicine, The Second Affiliated Hospital of Anhui Medical University, Hefei, Anhui, People’s Republic of China; 2Department of Infectious Diseases, The First Affiliated Hospital of Anhui Medical University, Hefei, Anhui, People’s Republic of China; 3Anhui Center for Surveillance of Bacterial Resistance, Hefei, Anhui, People’s Republic of China; 4Institute of Bacterial Resistance, Anhui Medical University, Hefei, Anhui, People’s Republic of China; 5Department of Laboratory Medicine, Anhui Provincial Hospital, Hefei, Anhui, People’s Republic of China; 6Department of Intensive Care, The Second Affiliated Hospital of Anhui Medical University, Hefei, Anhui, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Min Yang, Department of Intensive Care, The Second Affiliated Hospital of Anhui Medical University, Furong Road No. 678, Hefei, Anhui, 230032, People’s Republic of China, Email [email protected] Qiang Zhou, Department of Laboratory Medicine, The Second Affiliated Hospital of Anhui Medical University, Furong Road No. 678, Hefei, People’s Republic of China, Email [email protected]
Purpose: To establish and evaluate a simple disk stacking plus micro-elution (DSE) method that can be routinely performed to rapidly detect the synergistic effect between aztreonam (ATM) and ceftazidime/avibactam (CZA) against metallo-β-lactamase (MBL)-producing carbapenem-resistant Enterobacterales (CRE).
Methods: The DSE method was established, and a total of 32 MBL-producing CRE isolates collected from multiple centers were tested for ATM-CZA synergy. The results obtained after 8 h of incubation were compared with those obtained by a reference checkerboard assay (CBA) after 18~24 h. Reproducibility experiments were completed on three separate days.
Results: The reproducibility study showed that the results of the DSE method were precise. Compared with CBA, the DSE method exhibited excellent performance, with 92.8% sensitivity, 100.0% specificity 93.8% categorical agreement, 0.0% very major error, 0.0% major error, and 6.2% minor error over three days of testing.
Conclusion: The DSE method is a simple, rapid and practical method for ATM-CZA combination testing. Further evaluation should be completed to improve its clinical application.
Keywords: aztreonam, ceftazidime/avibactam, metallo-β-lactamases, Enterobacterales, antimicrobial combinations, clinical method
Introduction
The prevalence of clinical isolates of carbapenem-resistant Enterobacterales (CRE) has been on the rise, representing an increasingly urgent public health concern.1,2 The most common resistance mechanism of CRE is the production of carbapenemase, mainly including class A Klebsiella pneumoniae carbapenemase (KPC), class B metallo-β-lactamases (MBLs), and class D oxacillinase (OXA)-48.2 Among them, the activity of KPC and OXA-48 can be effectively inhibited by avibactam, a novel β-lactamase inhibitor. However, MBLs, including mainly New Delhi MBL (NDM), imipenem hydrolyzing MBL (IMP) and Verona integron-encoded MBL (VIM), are worrisome because they can hydrolyze most of β-lactams with the exception of aztreonam (ATM), and are not inhibited by any therapeutically utilized β-lactamase inhibitors.3,4 Although ATM is capable of evading hydrolysis by MBLs, its usefulness as a of monotherapy against MBL-producing CRE is limited because these strains often co-produce serine β-lactamase, which can hydrolyze ATM. Thus, a combination of ATM and ceftazidime/avibactam (CZA) has become an attractive combination with synergistic activity against MBL-producing CRE and currently has the most clinical data of any available antibiotic to support its use.4–7 On the other hand, decreased susceptibility to ATM-CZA among MBL-producing CRE has already been observed and determined to be at least in part due to a small insertion into in PBP3 that impacts the binding of ATM, ceftazidime, and other β-lactams.8 Therefore, a practical and convenient in vitro method for use in the clinic to assess the efficacy of the above synergistic effect is needed.
Recently, Khan et al evaluated the performance of four ATM-CZA combination testing methods, including broth disk elution, disk stacking, strip stacking and strip crossing. Among them, the most accurate methods were disk elution (100% sensitivity and specificity), followed by strip crossing (95.8~100% sensitivity, 100% specificity) and strip stacking (87.5~100% sensitivity, 100% specificity), the disk stacking method had the lowest performance, with only 42.7% sensitivity and 100% specificity.9 As it is the simplest method and is valuable in low-resource settings, the disk stacking was modified in this study, and the disk stacking plus micro-elution (DSE) method was established and evaluated. The DSE method is accurate and can be routinely performed in clinical laboratories to rapidly detect the synergistic effect of ATM-CZA against MBL-producing CRE.
Materials and Methods
Bacterial Isolates
A total of 32 MBL-producing CRE and 5 non-MBL-producing CRE isolates, all recovered from blood culture samples, including 14 Escherichia coli, 10 Klebsiella pneumoniae, 5 Enterobacter cloacae complex, 2 Klebsiella oxytoca, and 1 Citrobacter freundii were included in this study. All strains were retrospectively collected randomly from clinical laboratories in three tertiary hospitals [the First Affiliated Hospital of Anhui Medical University (n=18), The Second Affiliated Hospital of Anhui Medical University (n=9), and the Anhui Provincial Hospital (n=5)] between January 2018 and December 2021 in Anhui Province, Eastern China (Table S1). The strains were stored at −70°C and subcultured on Columbia blood agar prior to testing. Species identification was performed using mass spectrometry (BRUKER, Bremen, Germany). The minimum inhibitory concentrations (MICs) of ATM (Yuanye, Shanghai, China) and CZA [ceftazidime (Solarbio, Beijing, China) and avibactam (Yuanye, Shanghai, China)] were determined by the broth microdilution method, using the Clinical and Laboratory Standards Institution (CLSI) guidelines.10 Carbapenemase-encoding genes (including blaKPC, blaNDM, blaIMP, blaVIM, and blaOXA-48) and common extended-spectrum β-lactamase (ESBL) encoding genes (including blaCTX-M, blaSHV, blaTEM) were screened by general PCR with custom primers and DNA sequencing, as described previously.11 The standard strain E. coli ATCC25922 was used as the quality control.
The Disk Stacking Plus Micro-Elution (DSE) Method
The DSE method was performed as follows (Figure 1a): first, an MH agar (MHA) plate was inoculated with a suspension of the tested isolate (0.5 McFarland) for the routine disk diffusion procedure; second, two ATM (30 μg) disks (Oxoid, Basingstoke, UK) and two CZA (10/4 μg) disks (MAST GROUP LTD, Merseyside, UK) were placed on the inoculated MHA. After ensuring that the four disks were evenly distributed, one CZA disk was stacked on top of one ATM disk, and one ATM disk was stacked on top of one CZA disk. Next, 20 μL of sterile saline solution was added to the ATMCZA and CZAATM stacking disks. Finally, the MHA plate was incubated at 35°C for 8 h. The inhibition zones of the ATM and ATMCZA disks were measured, and the results were interpreted according to the ATM breakpoint from the rapid antimicrobial susceptibility testing (RAST) guidelines in the CLSI document.10 The inhibition zones of CZA and CZAATM against E. coli and K. pneumoniae were measured, and the results were interpreted according to the European Committee on Antimicrobial Susceptibility Testing (EUCAST) RAST guidelines, while the inhibition zones against other Enterobacterales species were interpreted according to the EUCAST RAST guidelines for K. pneumoniae because they were more stringent than those for E. coli.12 The results from DSE were interpreted as follows: if an isolate was resistant to ATM and CZA but susceptible to ATMCZA and CZAATM, it was categorized as “synergy”; if an isolate was susceptible to ATM and CZA but resistant to ATMCZA and CZAATM, it was categorized as “antagonism”; all other situations were categorized as “no interaction”.
Checkerboard Assay, Reproducibility Study and Data Analysis
Synergy between ATM and CZA was assessed by using a microtiter plate checkerboard assay (CBA) as the reference method.13 In brief, 96-well microtiter plates (Sigma-Aldrich China, Inc.) were set-up with an orthogonal 2-fold dilution series of ATM and ceftazidime, with avibactam at a constant concentration of 4 mg/L. The plates were incubated at 35°C for 18~24h and visually inspected for turbidity to determine growth. Fractional inhibitory concentration index (FICI) values were calculated and interpreted as follows:14 synergy for FICI ≤ 0.5; no interaction for 0.5<FICI ≤ 4; antagonism for FICI>4. A reproducibility study was completed by independently testing all the isolates on three separate days by the CBA and DSE methods. The sensitivity and specificity of the DSE were calculated using the CBA as the reference. The categorical agreement (CA), very major error (VME), major error (ME), and minor error (MIE) values were evaluated as follows: CA was defined if the results of DSE were consistent with those of CBA. VME was defined if the result of DSE showed synergy but CBA showed antagonism. ME was defined if the result of DSE showed antagonism but CBA showed synergy. MIE was defined if the result of DSE showed no interaction but CBA showed synergy.
Ethics Statement
All the CRE strains were isolated from clinical specimens and generated as part of routine clinical laboratory procedures. This study complied with the Declaration of Helsinki and the Ethics Committee of Anhui Medical University exempted this study from review because it focused only on bacteria.
Results and Discussion
Of the CRE strains tested, 30 isolates (93.8%) produced NDM, one (3.1%) produced IMP-4, and one (3.1%) co-produced KPC-2 and IMP-4. Screening for ESBL genes showed the existence of ESBL genes in 31 (96.9%) of the isolates, including CTX-M (23, 71.9%), SHV (14, 43.8%), and TEM (9, 28.1%) type β-lactamases (Table S1). Their rates of resistance to ATM and CZA were 90.6% and 100.0%, respectively (Table 1). According to the CBA, the ATM-CZA combination showed a synergistic effect against 90.6% of MBL-producing CRE isolates. The DSE method exhibited excellent performance compared with the CBA, as most of the synergistic effect could be detected (Tables 1 and S2), with 92.8% sensitivity and 100.0% specificity.
 |
Table 1 Comparison of Results Between the DSE Method and Checkerboard Assay |
Although the sensitivity of the DSE method was slightly lower than that of the disk elution method (a 100.0% sensitivity and specificity reported in Khan et al’s study),9 it is worth noting that the results of the DSE method can be determined within 8 h, while the result of the disk elution method should be read visually after 16~20 h of incubation. According to our practice, an obvious inhibition zone could be observed and measured after incubation at 35°C for 8 h, because of the fast growth rate of Enterobacterales (Figure 1b). Recently, EUCAST has defined a methodology for disk diffusion RAST, which was originally used in the performed directly in positive blood culture bottles, with the breakpoint of CZA to E. coli and K. pneumoniae for short incubations of 4, 6, and 8 h,12 while CLSI has also defined the RAST with the breakpoint of ATM for short incubations of 8~10 h.10 Therefore, considering the above two guidelines, the incubation time setting for this study was 8 h. In the study by Khan et al, the inhibition area of Enterobacterales could also be observed after incubation at 35°C for 8 h when the strip stacking and strip crossing methods were carried out.9 However, neither CLSI nor EUCAST has a standard for the interpretation of relevant results at the time point of 8 h, therefore, the results of the strip stacking and strip crossing methods cannot be determined as quickly as those of the DSE method.
As a user-friendly method, disk elution is mainly used to determine the susceptibility to colistin, and the volume of MH broth as the eluent is usually 10 mL.15,16 In this study, 20 μL of sterile saline was used as the eluent and assisted in the diffusion of the agent contained in the upper disk to the agar. Because the absorption capacity of a single commercial disk is 20 μL,17 this micro-eluent does not have an adverse effect on the bottom disk.
To further evaluate the stability and repeatability of the tests, a reproducibility study was performed for the DSE method with CBA as the reference. Except for the isolates ECO5061 and ECO8096, the results of DSE were consistent with those of CBA, and the above results were stable appeared in all three independent tests (Tables 1 and S2). As the results of strains ECO5061 and ECO8096 detected by DSE showed no interaction but those detected by CBA showed synergy, both results were defined as MIE (Tables 1 and S2). According to the β-lactamase detection results in this study, the two isolates did not have any peculiar characteristics (Table S1), and further research will be conducted to determine whether there are other resistance mechanisms. Taken together, the results of the DSE method were repeatable and precise, with 93.8% CA, 0.0% VME, 0.0% ME, and 6.2% MIE over three days of testing (Table 2).
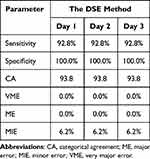 |
Table 2 Evaluation of the Qualitative and Reproducible Performance of the DSE Method |
Conclusion
In summary, we describe a simple, rapid and practical method for use in clinical microbiology laboratories to perform ATM-CZA combination testing. Although the strains included in this study were limited in number, the results were encouraging and exhibited excellent reproducibility. Further evaluation in a large multicentre validation study with more CRE isolates and other organisms should be performed to further improve the clinical application potential of the DSE method.
Author Contributions
All authors made a significant contribution to the work reported, whether in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by the Anhui Province University Natural Science Research Major Project of China (No. KJ2021ZD0029), Anhui Translational Medicine Research Fund Project (No. 2021zhyx-C47), and Scientific Research Fund of Anhui Medical University (No.2022xkj172).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Hansen GT. Continuous evolution: perspective on the epidemiology of carbapenemase resistance among enterobacterales and other gram-negative bacteria. Infect Dis Ther. 2021;10(1):75–92. doi:10.1007/s40121-020-00395-2
2. Jean SS, Harnod D, Hsueh PR. Global Threat of carbapenem-resistant gram-negative bacteria. Front Cell Infect Microbiol. 2022;12:823684. doi:10.3389/fcimb.2022.823684
3. Karaiskos I, Galani I, Souli M, Giamarellou H. Novel β-lactam-β-lactamase inhibitor combinations: expectations for the treatment of carbapenem-resistant Gram-negative pathogens. Expert Opin Drug Metab Toxicol. 2019;15(2):133–149. doi:10.1080/17425255.2019.1563071
4. Tan X, Kim HS, Baugh K, et al. Therapeutic options for metallo-β-lactamase-producing enterobacterales. Infect Drug Resist. 2021;14:125–142. doi:10.2147/IDR.S246174
5. Marshall S, Hujer AM, Rojas LJ, et al. Can ceftazidime-avibactam and aztreonam overcome β-lactam resistance conferred by metallo-β-lactamases in Enterobacteriaceae? Antimicrob Agents Chemother. 2017;61(4):e02243–16. doi:10.1128/AAC.02243-16
6. Falcone M, Daikos GL, Tiseo G, et al. Efficacy of ceftazidime-avibactam plus aztreonam in patients with bloodstream infections caused by metallo-β-lactamase-producing enterobacterales. Clin Infect Dis. 2021;72(11):1871–1878. doi:10.1093/cid/ciaa586
7. Yu W, Xiong L, Luo Q, et al. In vitro activity comparison of ceftazidime-avibactam and aztreonam-avibactam against bloodstream infections with carbapenem-resistant organisms in China. Front Cell Infect Microbiol. 2021;11:780365. doi:10.3389/fcimb.2021.780365
8. Sadek M, Juhas M, Poirel L, Nordmann P. Genetic features leading to reduced susceptibility to aztreonam-avibactam among metallo-β-lactamase-producing Escherichia coli isolates. Antimicrob Agents Chemother. 2020;64(12):e01659–20. doi:10.1128/AAC.01659-20
9. Khan A, Erickson SG, Pettaway C, Arias CA, Miller WR, Bhatti MM. Evaluation of susceptibility testing methods for aztreonam and ceftazidime-avibactam combination therapy on extensively drug-resistant gram-negative organisms. Antimicrob Agents Chemother. 2021;65(11):e0084621. doi:10.1128/AAC.00846-21
10. CLSI. Performance Standards for Antimicrobial Susceptibility Testing.
11. Liu Z, Gu Y, Li X, et al. Identification and Characterization of NDM-1-producing hypervirulent (Hypermucoviscous) Klebsiella pneumoniae in China. Ann Lab Med. 2019;39(2):167–175. doi:10.3343/alm.2019.39.2.167
12. EUCAST. Zone diameter Breakpoint Tables for rapid antimicrobial susceptibility testing (RAST) directly from blood culture bottles. Version 5.0; 2022. Available from: http://www.eucast.org.
13. Bhatnagar A, Ransom EM, Machado MJ, et al. Assessing the in vitro impact of ceftazidime on aztreonam/avibactam susceptibility testing for highly resistant MBL-producing Enterobacterales. J Antimicrob Chemother. 2021;76(4):979–983. doi:10.1093/jac/dkaa531
14. Odds FC. Synergy, antagonism, and what the chequerboard puts between them. J Antimicrob Chemother. 2003;52(1):1. doi:10.1093/jac/dkg301
15. Simner PJ, Bergman Y, Trejo M, et al. Two-site evaluation of the colistin broth disk elution test to determine colistin in vitro activity against gram-negative bacilli. J Clin Microbiol. 2019;57(2):e01163–18. doi:10.1128/JCM.01163-18
16. Humphries RM, Green DA, Schuetz AN, et al. Multicenter evaluation of colistin broth disk elution and colistin agar test: a report from the clinical and laboratory standards institute. J Clin Microbiol. 2019;57(11):e01269–19. doi:10.1128/JCM.01269-19
17. Shang H, Wang YS, Sheng ZY. National Guide to Clinical Laboratory Procedures. Beijing: People′s medical publishing house; 2015:574–578.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

