Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 16
A Real World Study to Assess the Effectiveness of Switching to Once Daily Closed Triple Therapy from Mono/Dual Combination or Open Triple Therapy in Patients with Chronic Obstructive Pulmonary Disease
Authors Huang WC, Chen CY, Liao WC, Wu BR , Chen WC , Tu CY, Chen CH , Cheng WC
Received 2 March 2021
Accepted for publication 20 May 2021
Published 3 June 2021 Volume 2021:16 Pages 1555—1568
DOI https://doi.org/10.2147/COPD.S308911
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Richard Russell
Wei-Chun Huang,1,2 Chih-Yu Chen,1 Wei-Chih Liao,1,2 Biing-Ru Wu,1,3,4 Wei-Chun Chen,1,3,4 Chih-Yen Tu,1,2 Chia-Hung Chen,1,2 Wen-Chien Cheng1– 4
1Division of Pulmonary and Critical Care, Department of Internal Medicine, China Medical University Hospital, Taichung, Taiwan; 2School of Medicine, College of Medicine, China Medical University, Taichung, Taiwan; 3Ph.D. Program in Translational Medicine, National Chung Hsing University, Taichung, Taiwan; 4Rong Hsing Research Center for Translational Medicine, National Chung Hsing University, Taichung, Taiwan
Correspondence: Wen-Chien Cheng; Chia-Hung Chen
Division of Pulmonary and Critical Care, Department of Internal Medicine, China Medical University Hospital, No. 2, Yude Road, North District, Taichung City, 40402, Taiwan
Email [email protected]; [email protected]
Objective: This real world study evaluated the effectiveness of switching to closed triple therapy from mono/dual combination or open triple therapy in patients with chronic obstructive pulmonary disease (COPD).
Methods: We conducted this retrospective study at a single medical center from December 2014 to September 2020. Patients with COPD who were stepped up to triple therapy were enrolled. We analyzed the duration from initial COPD management to open or closed triple therapy and identified the clinical predictors of the patients who needed triple therapy early. We also evaluated the effectiveness of triple therapy after switching from initial management, and closed triple therapy after switching from open triple therapy.
Results: A total 115 COPD patients who were stepped up to triple therapy from initial treatment were analyzed. The duration from initial treatment to triple therapy was 22.4 months. The baseline peripheral blood eosinophil counts of the patients who switched to triple therapy early (n=63, less than 22 months) and those who switched to triple therapy later (n=52, more than 22 months) were similar (489.6 vs 434.5 cells/uL; p=0.589). After univariate and multivariate analysis, the patients who were older had more acute exacerbations (AEs) in the previous year, asthma and COPD overlap (ACO), and initial dual bronchodilator therapy were stepped up to triple therapy early. The FEV1 of the patients was significantly increased after switching to open triple therapy from mono bronchodilator therapy. In addition, switching from initial or open triple therapy to closed triple therapy significantly reduced the incidence of AEs.
Conclusion: COPD patients with high blood eosinophilia, older age, more AEs in the previous year, ACO, and initial dual bronchodilator therapy were stepped up to triple therapy early. Triple therapy showed improvements in lung function of most patients switching from mono bronchodilator therapy. After switching to closed triple therapy further reduced the incidence of AEs.
Keywords: COPD, chronic obstructive pulmonary disease, closed triple therapy, open triple therapy, ACO, asthma COPD overlap
Introduction
Triple inhaled therapy for chronic obstructive pulmonary disease (COPD) is recommended for patients who have clinically significant symptoms and a high risk of frequent exacerbations who are already being treated with inhaled glucocorticoid (ICS)/long-acting β2-agonist (LABA) or long-acting muscarinic antagonist/long-acting β2-agonist (LAMA/LABA) according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) management strategy.1
Most studies evaluating the efficacy of “open triple” therapy, defined as the addition of a LAMA to ICS/LABA, have not only demonstrated improvements in lung function, but also improvements in health status, rescue medication, and risk of acute exacerbations (AEs) compared to ICS/LABA or single LAMA therapy.2–7 Several studies have investigated two different ICS/LABA/LAMA combinations in a single inhaler: BDG/FF/G and FF/VI/UMEC, also called “closed triple therapy”. Closed triple therapy has been shown to be better at reducing exacerbations and improving quality of life compared to LABA/LAMA, and also to be better than ICS/LABA at reducing exacerbations and improving lung function, symptoms, and quality of life, especially in ICS responsive diseases.8–12
However, few studies have compared the effectiveness between open triple and closed triple therapy. Ferguson et al reported that once daily closed triple therapy with FF/VI/UMEC resulted in similar improvements in pulmonary function, health status, and safety profile as twice-daily multiple inhaler triple therapy with BUD/FOR+TIO.13 Compared with closed inhaled triple therapy, open inhaled triple therapy requires the use of multiple inhalers several times a day. Simplifying inhaler treatment regimens can improve adherence to treatment in subjects with COPD, thereby improving health outcomes. Although adherence to COPD therapy has been reported to be high in conventional randomized control trials (RCTs) (up to 90%), it is lower in usual practice (10–40%).14 The effectiveness of treatment as assessed in real world studies can complement conventional RCTs by offering a comprehensive overview of therapy in the setting of usual clinical practice. There is a lack of real world data on the differences in quality of life, lung function, and annual exacerbation rate between patients receiving open triple inhaled therapy and closed triple inhaled therapy. Therefore, the aim of this real world study was to evaluate the effectiveness of triple inhaled therapy after switching from initial management and once daily closed triple inhaled therapy after switching from open triple inhaled therapy. We also analyzed the duration from initial COPD management to open or closed triple inhaled therapy and identified the clinical predictors of patients who needed triple inhaled therapy early.
Materials and Methods
Study Design
This retrospective study enrolled all patients who visited the Division of Pulmonary and Critical Care Medicine, China Medical University Hospital, Taiwan between December 2014 and September 2020. The study was approved by the China Medical University Hospital Institutional Review Board (CMUH108-REC3-119), and the need for informed consent was waived due to the observational and retrospective design. The data were anonymized and confidentiality was maintained.
Patients and Data Collection
The inclusion criteria for this study were: (1) patients aged 40 years old and above on the day of presentation; (2) diagnosis of COPD based on the clinical symptoms (chronic cough, sputum production, and dyspnea on exertion), a risk factor for the development of COPD, and the presence of air-flow limitation (presence of post-bronchodilator FEV1/FVC < 0.7); (3) patients who were stepped up to inhaled open or closed triple therapy from mono therapy (LABA or LAMA) or dual combination therapy (ICS/LABA or LABA/LAMA). Patients were excluded if they were being treated with once daily closed triple therapy at the beginning of management, or if they had never been treated with triple therapy (Figure 1).
The open triple therapy regimen included ICS/LABA combination therapy (FF/VI 100 μg/25 μg via a Relvar ElliptaTM; BUD/FOR 160 μg/4.5 μg via a Symbicort TurbuhalerTM or RapihalerTM; FP/SAL 250 μg/50 μg via a Seretide AccuhalerTM or EvohalerTM; BDP/FOR 100 μg/6 μg via a Foster NEXThalerTM or metered dose inhaler) plus a LAMA (TIO 5 μg via a Spiriva RespimatTM; UMEC 55 μg via Incruse Ellipta) or dual bronchodilator treatment with a LAMA and LABA (UMEC/VI 55 μg/22 μg via an Anoro ElliptaTM; TIO/OLO 2.5 μg/2.5 μg via a Spiolto RespimatTM; IDA/GLY 110 μg/50 μg via an Ultibro BreezhalerTM) plus ICS (ciclesonide 160 μg via an Alvesco metered dose inhaler). The only closed triple therapy regimen used in this study was VI/UMEC/FF 25 μg/55 μg/100 μg via a Trelegy ElliptaTM. All patients participated in healthcare case management to improve health outcomes and healthcare quality.
Treatment Assessment
We evaluated the effectiveness of open and closed triple therapy from the initial management of COPD, and once daily closed triple therapy after switching from open triple therapy. The effectiveness of management was assessed according to lung function, the incidence rates of moderate/severe AEs every year, and health-related quality of life. The quality of life was assessed using the COPD Assessment Test (CAT) and Modified British Medical Research Council (mMRC) questionnaire.1 We recorded the duration from initial COPD management to open or closed triple therapy, and identified the clinical predictors of patients who needed triple therapy early.
The clinical data of the patients included age, sex, body height (BH), body weight (BW), body mass index (BMI), smoking status, history of asthma, pulmonary function tests (PFTs), CAT score, mMRC score, AEs in the previous year, GOLD “ABCD” group, absolute eosinophil count, and treatment history.
Moderate or severe exacerbations were defined according to the number of emergency department visits or hospitalizations due to COPD. The GOLD “ABCD” groups were defined according to the patient’s symptoms and their history of exacerbations as follows: Group A: one or fewer exacerbations per year, no hospitalization, mMRC 0–1, CAT < 10; Group B: one or fewer exacerbations per year, no hospitalization, mMRC ≥ 2, CAT ≥ 10; Group C: two or more exacerbation per year, one or more exacerbation with hospitalization, mMRC 0–1, CAT < 10; Group D: two or more exacerbations per year, one or more exacerbation with hospitalization, mMRC ≥ 2, CAT ≥ 10.1 Reversible airway was defined as an increase of ≥ 12% and ≥ 200 mL as an absolute value compared with baseline in either FEV1 or FVC after inhalation of salbutamol.
After the starting of primary treatment, patients underwent regular (every three to six months) follow-up at our Institute. The upgrade or switching treatment strategy was not only based on the GOLD management guidelines,1 but also clinical physician judgment included decline of trough FEV1, CAT scores deterioration, and experience of acute exacerbation. In addition, the patient’s preference, adherence and satisfaction were also taken into consideration.
Statistical Analysis
All continuous variables were reported as median and interquartile range (IQR; 25th and 75th percentiles) or mean with standard deviation. Differences in continuous variables were compared using the Mann–Whitney U-test or the independent t-test. Categorical variables were reported as the number of patients and percentages. Differences in categorical variables were examined using Fisher’s exact test or the chi-square test. Univariate and multivariate logistic regression analyses were used to identify independent predictors among the patients with early escalation to triple therapy. Differences in the duration from initial COPD management to triple therapy were analyzed using Kaplan-Meier log-rank analysis. The effectiveness of triple therapy switching from other therapy were also analyzed using paired t-test or Wilcoxon signed-rank test. All tests of significance were two sided, and a P value ≤ 0.05 was considered to be statistically significant. All statistical analyses were performed using MedCalc for Windows version 18.10 (MedCalc Software, Ostend, Belgium).
Results
A total 115 patients with COPD were stepped up to triple therapy from our hospital (Figure 1A). The duration from initial treatment of COPD to triple therapy was 22.4 (95% CI, 17.9–26.5) months (Figure 2A). The patients were divided into two groups according to the duration from initial treatment of COPD to triple therapy as more than 22 months (Group 1), and less than 22 months (Group 2). The baseline clinical characteristics of these 115 patients are summarized in Table 1.
More patients in Group 2 were GOLD Group D compared to those in Group 1 (46.0% vs 30.8%, p = 0.036), and more patients in Group 1 had reversible airway obstruction after bronchodilator therapy compared to Group 2 (46.2% vs 25.4%, p = 0.021). More patients in Group 2 were initially treated with dual bronchodilator therapy compared to Group 1 (54.3% vs 23.1%, p = 0.003). The duration from initial treatment to triple therapy was shortest among the patients who were initially treated with dual bronchodilator therapy (median, 13.1 months; 95% CI 9.1–18.8 months) followed by the those who were initially treated with ICS/LABA (median, 26.3 months; 95% CI 17.5–36.5 months), and those who were initially treated with a mono bronchodilator (28.2 months; 95% CI 23.3–42.8 months; p < 0.001) (Figure 2B). The baseline characteristics of the patients according to initial treatment for COPD are shown in Table S1. The patients who were initially treated with dual bronchodilator therapy appeared to have worse lung function with significant air trapping compared to the other initial treatment strategies (Table S1).
There were no significant differences in baseline characteristics between Group 1 and Group 2 including age, sex, BMI, smoking status, initial lung function, eosinophil count, CAT score, mMRC score, and comorbidities. However, the patients in Group 2 seemed to be older (67.9 vs 64.6, p = 0.075), higher smoking status (pack-years) (53.6 vs 43.1, p = 0.089), higher rate of AEs in the previous year (0.60 vs 0.36, p = 0.071), and lower FEV1(%) (45.8 vs 51.5, p = 0.066) compared to those in Group 1. The baseline peripheral blood eosinophil counts between the two groups were similar (489.6 vs 434.5 cells/uL; p = 0.589).
Clinical Predictors of Early Initiation of Triple Therapy
Univariate and multivariate logistic regression analyses were conducted to identify predictors of early initiation of triple therapy, including baseline age, smoking status (pack-years), AEs in the previous year, initial pulmonary function (FEV1%), COPD “ABCD” group, airway reversibility, asthma and COPD overlap (ACO), and initial treatment (Table 2). Multivariate analysis revealed that an older age, more AEs in the previous year, ACO, and initial treatment with dual bronchodilator therapy were associated with stepping up to triple therapy early. The duration from initial management to triple therapy was longer in the patients with a reversible obstructive airway.
 |
Table 2 Clinical Characteristics to Predict COPD Patient with Stepping Up to Triple Therapy Early |
The Reasons for Switching Triple Therapy
Fifty-eight patients (58/115, 50.4%) received ICS/LABA plus LAMA, 29 patients (29/115, 25.2%) received dual bronchodilator therapy (LABA/LAMA) plus ICS, and 28 patients (28/115, 24.3%) were directly switched to closed triple therapy from our hospital (Figure 1A and B). The reasons for switching therapy are reported in Figure 3. The reasons for 115 patients switching from initial management to triple therapy were as follows: a decline in trough FEV1 (53/115, 46.1%), higher CAT scores (14/115, 12.2%), and acute exacerbation (13/115, 11.3%). Fifteen patients (15/115, 13.1%) had at least two reasons for switching treatment, whereas twenty patients (20/115, 17.4%) stepped up to triple therapy due to no clinical improvement (Figure 3A). On the other hand, fifty-six patients (56/102, 54.9%) switched from open triple therapy to once daily closed triple therapy (47 from our hospital and 9 from another hospital; 30 with ICS/LABA plus LAMA and 26 with LABA/LAMA plus ICS) (Figure 1B). The reasons for switching the therapy strategy were as follows: increased CAT scores (18/56, 32.1%), acute exacerbation (11/56, 19.6%), and a decline in trough FEV1 (7/56, 12.5%). Six patients (6/56, 10.7%) had at least two reasons for switching treatment, whereas fourteen patients (14/56, 25%) switched to closed triple therapy due to adherence concerns (Figure 3B).
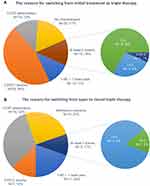 |
Figure 3 (A) The reasons for the 115 patients switching from initial management to triple therapy. (B) The reasons for the 56 patients switching from open to closed triple therapy. |
The Effectiveness of Open Triple Therapy Compared to Once Daily Closed Triple Therapy
The mean annualized rate of moderate/severe exacerbations was 0.47 and 0.45 in the previous year before and after switching to open triple therapy from initial management, respectively (Δ = −0.027, p = 0.787) (Figure 4A). However, the mean annualized rate of moderate/severe exacerbations was lower in the patients who switched from open triple to closed triple therapy (0.38 vs 0.25, Δ = −0.132, p = 0.047) (Figure 4B). We further analyzed the effectiveness of inhaled triple therapy not only from single or multiple inhalers but also from different pre-treatment status. The effectiveness of triple therapy might be influenced by single or multiple devices and pre-treatment status. Most patients who received dual bronchodilator therapy as initial treatment switched to closed triple (16/28, 57.1%) and LABA/LAMA plus ICS (15/29, 51.7%). In contrast, most patients who received a mono bronchodilator with or without ICS (43/58, 74.1%) as initial therapy switched to ICS/LABA plus LAMA (p = 0.003) (Table 3). In total, 56 patients received closed triple therapy. Thirty patients (30/56, 53.6%) switched from ICS/LABA plus LAMA, and 26 patients (26/56, 46.4%) switched from LABA/LAMA plus ICS. (Figure 1)
 |
Table 3 The Shifting Treatment Strategies from the Different Initial Treatments |
There was no symptom improvement after switching to open triple therapy. The CAT scores were 10.02 and 9.61 before and after switching to ICS/LABA plus LAMA, respectively (Δ = −0.407, p = 0.325) (Figure 5A). The mMRC scores were 1.98 both before and after switching to ICS/LABA plus LAMA (Δ = 0, p = 1.00) (Figure 5C). The patients switching to LABA/LAMA plus ICS also did not have symptom control. The CAT scores were 9.16 and 9.56 before and after switching to LABA/LAMA plus ICS, respectively (Δ = 0.400, p = 0.613) (Figure 5B). The mMRC scores were 1.60 and 1.96 before and after switching to LABA/LAMA plus ICS, respectively (Δ = 0.360, p = 0.107) (Figure 5D). The shift from open to closed inhaled triple therapy provided similar symptom control regardless of pre-treatment status (Figure 6). The CAT scores of patients switching from ICS/LABA plus LAMA to closed triple therapy seemed to be higher than before (9.36 vs 10.66, Δ = 1.305, p = 0.019).
A significant improvement in lung function (FEV1) was observed in patients who were stepped up to ICS/LABA plus LAMA from initial COPD management. The trough FEV1 values were 1.14 L and 1.25 L before and after switching to ICS/LABA plus LAMA, respectively (p = 0.046) (Figure 7A). However, there was no significant improvement of lung function in patients switching to LABA/LAMA plus ICS (1.37 vs 1.37, p = 0.178) (Figure 7B). There was also no significant difference in lung function improvement between patients before and after receiving once daily closed triple therapy (Figure 7C and D).
Discussion
To the best of our knowledge, this is the first real world study to evaluate the effectiveness of switching to once daily closed triple therapy from open triple therapy in the same patient. Our results showed an improvement in pulmonary function (FEV1) under triple inhaled therapy after switching from mono bronchodilator therapy. In addition, the patients who switched to once daily closed triple inhaled therapy had a significantly lower rate of AEs after switching from initial therapy or open triple inhaled therapy. Our results also indicated that the patients with high blood eosinophilia, who were older, had more AEs in the previous year, ACO, and initial treatment with LABA/LAMA were stepped up to triple therapy early.
Several previous studies have reported that triple inhaled therapy could improve lung function and improve symptom control in COPD patients compared with dual combination therapy.9,15,16 A meta-analysis of triple therapy in COPD which enrolled 13 RCTs including 15,519 patients found that triple therapy improved trough FEV1, reduced AE rates, and did not increase cardiovascular risk compared with ICS/LABA combination therapy.7 Among these RCTs, some patients were treated with closed triple therapy, and the others were treated with open triple therapy.8,9,11,15–17 In the current study, the patients who were stepped up to open triple therapy with ICS/LABA plus LAMA had improvements in lung function, but not in those who stepped up to LABA/LAMA plus ICS. The reasons may be explained by the influence of different pre-treatment statuses. Most patients shifted to ICS/LABA plus LAMA from a mono bronchodilator with or without ICS (43/58, 74.1%), and most patients shifted to LABA/LAMA plus ICS from dual bronchodilators (15/29, 51.7%). One study also reported that patients who added a second bronchodilator to a single bronchodilator to receive dual bronchodilation achieved the greatest benefit. This change demonstrated the potential impact of prior medication on therapy results.18 The effectiveness of inhaled triple therapy may be influenced by pre-treatment status.
Besides, the AE rate and quality of life were also not improved after shifting to open triple therapy. The difference between our results and the previous RCTs may be explained by not only the prior medication, but also a low adherence rate to inhaled therapy, which has been reported to range from 50–80% in real world COPD studies.19,20 In addition, adherence to COPD therapy is higher in conventional RCTs than in real world daily practice (10–40%).14 Therefore, the IMPACT and FULFIL conventional RCTs were unable to fully evaluate the effectiveness in a usual practice setting.21 Once daily closed triple therapy appeared to increase the adherence rate compared to open triple therapy in the present real world study setting.
Before the introduction of closed triple therapy, patients under inhaled triple therapy may have had to use multiple inhalers several times a day.22,23 However, the previous RCTs on closed triple therapy (IMPACT and FULFIL) did not compare FF/UMEC/VI with open triple therapy under conditions of low adherence and high critical error rates in daily practice.14 Ferguson et al reported that once daily single-inhaler (FF/UMEC/VI) closed triple inhaled therapy provided similar overall improvements in health status, weighted mean FEV1, and similar safety profile compared with multiple-inhaler triple therapy (BUD/FOR plus once daily TIO).13 These results indicate that closed triple therapy with FF/UMEC/VI is a suitable option for patients who want to simplify their treatment regimen and increase adherence to inhaled therapy. The INTREPID trial was designed to provide evidence of the effectiveness of FF/UMEC/VI in patients with COPD managed in routine healthcare systems.24 The results of the current study showed that switching to once daily closed triple therapy from open triple therapy in the same patient reduced the rate of AEs. However, there was no improvement in pulmonary function or quality of life after switching to closed triple therapy from open triple therapy. The CAT scores of patients switching from ICS/LABA plus LAMA to closed triple therapy seems to be higher than before. Further real world effectiveness studies are therefore needed to investigate closed triple therapy with a single inhaler versus multiple-inhaler triple therapies.
In a United Kingdom retrospective study, approximately one third of all COPD patients received triple therapy. Of these patients, 25% received triple therapy 1 year after a diagnosis, 50% within 3 years post diagnosis, and 100% within 8 years post diagnosis.25 In the present study, 27.8% (115/413) of our cohort were stepped up to triple therapy, which is consistent with similar studies conducted in Japan (21%)26 and the United Kingdom (33%).25 The median duration from initial management to triple therapy was 22.4 months. Triple therapy is seldom the initial therapy for COPD, and only 4.3% of the patients were treated with triple therapy at the beginning of the clinical course in the United Kingdom cohort.25
Vanfleteren et al recommended triple therapy as the initial treatment under the following conditions: (1) patients who are diagnosed with COPD for the first time because of severe exacerbations; and (2) patients who are diagnosed with severe airflow limitation (FEV1 < 50%), symptomatic, frequent moderate (≥ 2) or severe exacerbations in the previous year, and peripheral eosinophilia (> 300/μL).27 In the current study, we included patients with high peripheral blood eosinophil counts (489.6 and 434.5 cells/uL in the two study groups, respectively). A previous study also indicated that triple therapy may be considered as first-line treatment in patient with at least two moderate to severe exacerbation in the previous year, reduced lung function (FEV1 ≤ 42%), and more symptoms (CAT score ≥ 18).28 These results are similar to the current study. We demonstrated that COPD patients with high blood eosinophilia who were older, had more AEs in the previous year, ACO, and initial dual bronchodilator therapy were stepped up to triple therapy early. However, the patients with reversible airway obstruction seemed to have a longer duration to triple therapy. This may be because the patients had mild COPD or ACO. Initial dual bronchodilator therapy is always used in patients with poor lung function, which means that our cohort had more severe COPD or ACO. The clinical features of ACO include more respiratory symptoms, exertional dyspnea, persistent partially reversible airway obstruction, and periodic exacerbations.29 A previous study reported that adding UMEC to FF/VI provided a greater improvement in lung function for ACO, indicating that triple therapy is a suitable initial or early treatment.30 The 2020 Global Initiative for Asthma strategy document (GINA) recommended adding TIO to patients with asthma who have airflow limitation even if they are already being treated with combination therapy with ICS+LABA.31 RCTs on COPD or asthma usually include highly selected patient populations with limited comorbidities and concomitant medications, which may make it difficult to extrapolate the results directly to usual daily practice, especially in patients with ACO. Our real world observational study included more complex and heterogeneous COPD patients to reflect usual practice.
There are several limitations to this study. First, this was a retrospective study performed in a single medical center with relatively few patients and some degree of selection bias. Following the study inclusion criteria, the COPD patients with high eosinophilia and without initial closed triple therapy were included. The number of patients was not sufficient to perform adjusted analysis. However, this subgroup of COPD patients was really needed to step up to triple therapy. Therefore, multivariate analysis was performed to reduce the magnitude of bias. Second, the decision to treat patients with initial inhaled therapy and switch from open triple to once daily closed triple therapy was determined by primary care physician. Despite that, the treatment strategies were not only based on clinical physician judgment. They included a decline of trough FEV1, higher CAT scores, and acute exacerbation, the patient’s preference and adherence, and also the GOLD management guidelines. Third, we did not discuss the impact of the different types of inhalers, which may have influenced the selection of medications based on patient preference. Fourth, all patients received once daily closed inhaled triple therapy with a Trelegy Ellipta. Other closed triple inhaled therapy devices were not available at our department. Whether other closed triple therapies can provide similar results is uncertain. Fifth, we did not report the adherence rate of open or closed inhaled triple therapy. However, the patients included our study had regular follow-up at our clinic with an interval of 3 months. We provided health education and counseling services to the patients in every visit. Finally, cigarette smoking was not clearly defined in our study. Smoking status should be defined as an ex-smoker or current smoker because it significantly affects the outcomes of patients with COPD. Despite these limitations, the novelty and strength of this study were that we reported the treatment pathway to triple therapy in the same cohort and evaluated treatment efficacy before and after triple therapy, independently from single or multiple devices and pre-treatment status. We reported the benefits that single inhaler triple therapy provided in our clinical practice.
Conclusion
In conclusion, this study showed that COPD patients with high blood eosinophilia who were older, had more frequent AEs in the previous year, ACO, and initial dual bronchodilator therapy were stepped up to triple therapy early. The lung function (FEV1) of the patients was significantly improved after switching to triple therapy from initial single bronchodilator therapy. In addition, switching from initial or open triple to once daily closed triple inhaled therapy significantly reduced the incidence of AEs.
Abbreviations
COPD, chronic obstructive pulmonary disease; ICS, inhaled glucocorticoid; LABA, long-acting β2-agonist; LAMA, long-acting muscarinic antagonist; GOLD, Global Initiative for Chronic Obstructive Lung Disease; ACO, asthma and COPD overlap; AE, acute exacerbation; mMRC, Modified Medical Research Council; CAT, COPD Assessment Test; FF/VI, fluticasone furoate/vilanterol; BUD/FOR, budesonide/formoterol; FP/SAL, fluticasone propionate/salmeterol; BDP/FOR, beclometasone dipropionate/formoterol; TIO, tiotropium; UMEC, umeclidinium; OLO, olodaterol; IND, indacaterol; GLY, glycopyrronium; RCT, randomized controlled trial; GINA, Global Initiative for Asthma strategy document; Dual, Dual bronchodilators.
Ethics Approval and Consent to Participate
The study (CMUH108-REC3-119) was approved by the China Medical University Hospital Institutional Review Board, and the need for informed consent was waived due to the observational and retrospective design. The data were anonymized, and confidentiality was maintained. This study was conducted in accordance with the Declaration of Helsinki.
Consent for Publication
All authors have reviewed and approved the manuscript for publication.
Funding
There is no funding to report.
Disclosure
No conflicts exist for the specified authors.
The abstract of this paper was presented at the 2020 Annual Congress of Taiwan Society of Pulmonary and Critical Care Medicine and Taiwan Society of Thoracic Surgeons as a poster presentation with interim findings.
References
1. Singh D, Agusti A, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease: the GOLD science committee report 2019. Eur Respir J. 2019;53(5):1900164. doi:10.1183/13993003.00164-2019
2. Singh D, Brooks J, Hagan G, Cahn A, O’Connor BJ. Superiority of “triple” therapy with salmeterol/fluticasone propionate and tiotropium bromide versus individual components in moderate to severe COPD. Thorax. 2008;63(7):592–598. doi:10.1136/thx.2007.087213
3. Jung KS, Park HY, Park SY, et al. Comparison of tiotropium plus fluticasone propionate/salmeterol with tiotropium in COPD: a Randomized Controlled Study. Respir Med. 2012;106(3):382–389. doi:10.1016/j.rmed.2011.09.004
4. Hanania NA, Crater GD, Morris AN, Emmett AH, O’Dell DM, Niewoehner DE. Benefits of adding fluticasone propionate/salmeterol to tiotropium in moderate to severe COPD. Respir Med. 2012;106(1):91–101. doi:10.1016/j.rmed.2011.09.002
5. Siler TM, Kerwin E, Singletary K, Brooks J, Church A. Efficacy and safety of umeclidinium added to fluticasone propionate/salmeterol in patients with COPD: results of two randomized, double-blind studies. COPD. 2016;13(1):1–10. doi:10.3109/15412555.2015.1034256
6. Lee SD, Xie CM, Yunus F, et al. Efficacy and tolerability of budesonide/formoterol added to tiotropium compared with tiotropium alone in patients with severe or very severe COPD: a Randomized, Multicentre Study in East Asia. Respirology. 2016;21(1):119–127. doi:10.1111/resp.12646
7. Calzetta L, Cazzola M, Matera MG, Rogliani P. Adding a LAMA to ICS/LABA therapy: a meta-analysis of triple combination therapy in COPD. Chest. 2019;155(4):758–770. doi:10.1016/j.chest.2018.12.016
8. Lipson DA, Barnacle H, Birk R, et al. FULFIL trial: once-daily triple therapy for patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2017;196(4):438–446. doi:10.1164/rccm.201703-0449OC
9. Lipson DA, Barnhart F, Brealey N, et al. Once-daily single-inhaler triple versus dual therapy in patients with COPD. N Engl J Med. 2018;378(18):1671–1680. doi:10.1056/NEJMoa1713901
10. Papi A, Vestbo J, Fabbri L, et al. Extrafine inhaled triple therapy versus dual bronchodilator therapy in chronic obstructive pulmonary disease (TRIBUTE): a double-blind, parallel group, randomised controlled trial. Lancet. 2018;391(10125):1076–1084. doi:10.1016/S0140-6736(18)30206-X
11. Singh D, Papi A, Corradi M, et al. Single inhaler triple therapy versus inhaled corticosteroid plus long-acting beta2-agonist therapy for chronic obstructive pulmonary disease (TRILOGY): a double-blind, parallel group, randomised controlled trial. Lancet. 2016;388(10048):963–973. doi:10.1016/S0140-6736(16)31354-X
12. Vestbo J, Papi A, Corradi M, et al. Single inhaler extrafine triple therapy versus long-acting muscarinic antagonist therapy for chronic obstructive pulmonary disease (TRINITY): a double-blind, parallel group, randomised controlled trial. Lancet. 2017;389(10082):1919–1929. doi:10.1016/S0140-6736(17)30188-5
13. Ferguson GT, Brown N, Compton C, et al. Once-daily single-inhaler versus twice-daily multiple-inhaler triple therapy in patients with COPD: lung function and health status results from two replicate randomized controlled trials. Respir Res. 2020;21(1):131. doi:10.1186/s12931-020-01360-w
14. Bourbeau J, Bartlett SJ. Patient adherence in COPD. Thorax. 2008;63(9):831–838. doi:10.1136/thx.2007.086041
15. Ferguson GT, Rabe KF, Martinez FJ, et al. Triple therapy with budesonide/glycopyrrolate/formoterol fumarate with co-suspension delivery technology versus dual therapies in chronic obstructive pulmonary disease (KRONOS): a double-blind, parallel-group, multicentre, Phase 3 randomised controlled trial. Lancet Respir Med. 2018;6(10):747–758. doi:10.1016/S2213-2600(18)30327-8
16. Siler TM, Kerwin E, Sousa AR, Donald A, Ali R, Church A. Efficacy and safety of umeclidinium added to fluticasone furoate/vilanterol in chronic obstructive pulmonary disease: results of two randomized studies. Respir Med. 2015;109(9):1155–1163. doi:10.1016/j.rmed.2015.06.006
17. Cazzola M, Ando F, Santus P, et al. A pilot study to assess the effects of combining fluticasone propionate/salmeterol and tiotropium on the airflow obstruction of patients with severe-to-very severe COPD. Pulm Pharmacol Ther. 2007;20(5):556–561. doi:10.1016/j.pupt.2006.06.001
18. Buhl R, Criee CP, Kardos P, et al. Dual bronchodilation vs triple therapy in the “real-life” COPD DACCORD Study. Int J Chron Obstruct Pulmon Dis. 2018;13:2557–2568. doi:10.2147/COPD.S169958
19. Humenberger M, Horner A, Labek A, et al. Adherence to inhaled therapy and its impact on chronic obstructive pulmonary disease (COPD). BMC Pulm Med. 2018;18(1):163. doi:10.1186/s12890-018-0724-3
20. George M. Adherence in asthma and COPD: new strategies for an old problem. Respir Care. 2018;63(6):818–831. doi:10.4187/respcare.05905
21. Halpin DM, Kerkhof M, Soriano JB, Mikkelsen H, Price DB. Eligibility of real-life patients with COPD for inclusion in trials of inhaled long-acting bronchodilator therapy. Respir Res. 2016;17(1):120. doi:10.1186/s12931-016-0433-5
22. Simeone JC, Luthra R, Kaila S, et al. Initiation of triple therapy maintenance treatment among patients with COPD in the US. Int J Chron Obstruct Pulmon Dis. 2017;12:73–83. doi:10.2147/COPD.S122013
23. Wurst KE, Punekar YS, Shukla A. Treatment evolution after COPD diagnosis in the UK primary care setting. PLoS One. 2014;9(9):e105296. doi:10.1371/journal.pone.0105296
24. Worsley S, Snowise N, Halpin DMG, et al. Clinical effectiveness of once-daily fluticasone furoate/umeclidinium/vilanterol in usual practice: the COPD INTREPID Study design. ERJ Open Res. 2019;5(4):00061–2019. doi:10.1183/23120541.00061-2019
25. Brusselle G, Price D, Gruffydd-Jones K, et al. The inevitable drift to triple therapy in COPD: an analysis of prescribing pathways in the UK. Int J Chron Obstruct Pulmon Dis. 2015;10:2207–2217. doi:10.2147/COPD.S91694
26. Miyazaki M, Nakamura H, Takahashi S, et al. The reasons for triple therapy in stable COPD patients in Japanese clinical practice. Int J Chron Obstruct Pulmon Dis. 2015;10:1053–1059. doi:10.2147/COPD.S79864
27. Vanfleteren L, Ullman A, Nordenson A, Andersson A, Andelid K, Fabbri LM. Triple therapy (ICS/LABA/LAMA) in COPD: thinking out of the box. ERJ Open Res. 2019;5(1). doi:10.1183/23120541.00185-2018
28. Cheng WC, Wu BR, Liao WC, et al. When to use initial triple therapy in COPD: adding a LAMA to ICS/LABA by clinically important deterioration assessment. Int J Chron Obstruct Pulmon Dis. 2020;15:3375–3384. doi:10.2147/COPD.S279482
29. Marron RM, Vega Sanchez ME. Asthma-COPD overlap syndrome. Chronic Obstr Pulm Dis. 2019;6(2):200–202. doi:10.15326/jcopdf.6.2.2018.0169
30. Ishiura Y, Fujimura M, Ohkura N, et al. Effect of triple therapy in patients with asthma-COPD overlap. Int J Clin Pharmacol Ther. 2019;57(8):384–392. doi:10.5414/CP203382
31. Mauer Y, Taliercio RM. Managing adult asthma: the 2019 GINA guidelines. Cleve Clin J Med. 2020;87(9):569–575. doi:10.3949/ccjm.87a.19136
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.







