Back to Journals » Breast Cancer: Targets and Therapy » Volume 15
A Randomized, Double-Blind, Placebo-Controlled Trial to Evaluate the Therapeutic Effect of Magnesium-L-Threonate Supplementation for Persistent Pain After Breast Cancer Surgery
Authors Ni Y, Deng F, Yu S, Zhang J, Zhang X, Huang D, Zhou H
Received 22 March 2023
Accepted for publication 14 July 2023
Published 25 July 2023 Volume 2023:15 Pages 495—504
DOI https://doi.org/10.2147/BCTT.S413435
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Harikrishna Nakshatri
Yuncheng Ni,1 Fang Deng,1 Shanzi Yu,1 Jianping Zhang,1 Xiaoxue Zhang,1 Dong Huang,1,2 Haocheng Zhou1,2
1Department of Pain, the Third Xiangya Hospital and Institute of Pain Medicine, Central South University, Changsha, People’s Republic of China; 2Hunan Key Laboratory of Brain Homeostasis, Central South University, Changsha, People’s Republic of China
Correspondence: Haocheng Zhou, Department of Pain, the Third Xiangya Hospital and Institute of Pain Medicine, Central South University, Changsha, People’s Republic of China, Email [email protected]
Purpose: Post-mastectomy pain syndrome is a common yet debilitating neuropathic complication after breast cancer procedures, resulting in significantly reduced quality of life. Recently, emerging evidence has supported the therapeutic effect of magnesium administration in chronic pain. However, the role of magnesium supplementation in development of chronic pain after breast cancer surgery remains less known. The aim of this study was to evaluate therapeutic effect of magnesium supplementation on persistent pain after breast cancer procedure.
Patients and Methods: This was a randomized, double-blind, placebo-controlled clinical trial. A total of 109 patients who underwent breast cancer procedure received magnesium-L-threonate (n = 48) or placebo (n = 61) for 12 weeks. Chronic pain incidence, short form of the McGill Pain Questionnaire (SF-MPQ), Generalized Anxiety Disorder Scale (GAD-7), Patient Health Questionnaire-9 (PHQ-9), Pittsburgh Sleep Quality Index (PSQI), and Telephone Interview for Cognitive Status (TICS) were evaluated at 3- and 6-month follow-up.
Results: About 31% (15 out of 48) of patients reported chronic pain after magnesium supplementation, and 26% (16 out of 61) of the control group at 6-month follow-up respectively. Total scores of SF-MPQ were significantly increased in the control group 6 months after surgical intervention (mean difference, 1.475; 95% CI, − 2.730 to − 0.2211), but NOT in the magnesium treated group (mean difference, 1.250; 95% CI, − 2.775 to 0.2748). No significant differences were found between two cohorts on SF-MPQ, GAD-7, PHQ-9, PSQI, or TICS at each timepoint.
Conclusion: Oral supplementation of magnesium-L-threonate did not effectively prevent the development of persistent pain in breast cancer survivors, nor provide sufficient pain relief over placebo. We did not observe improvement of pain, mood, sleep disorder, or cognitive function after 12-week magnesium supplementation. Future study may focus on magnesium combined with other effective anti-neuropathic pain treatment.
Keywords: breast cancer surgery, chronic pain, magnesium-l-threonate, neuropathic pain, pain management
Introduction
The incidence of post-mastectomy pain syndrome (PMPS) has been estimated to range from 20–50% in previous reports,1 more than half of whom suffer moderate to severe pain after breast cancer treatment.1–4 PMPS is characterized by its neuropathic pain symptoms, such as persistent pain and multiple forms of sensory deficiency (eg, allodynia, hyperpathia, aftersensations, burning, or sensory loss), which is consistent with other types of post-surgical pain.4,5 Despite surgical injury, adjuvant chemotherapy and radiotherapy, younger age, preoperative pain condition, and axillary lymph node dissection may also increase the risk of PMPS,4–6 which requires ongoing treatment and significantly reduces quality of life in patients with breast cancer.
One strategy to prevent the chronicity of neuropathic pain is to block the N-methyl-d-aspartate receptors (NMDARs) of the sensory neurons located at the dorsal horn of the spinal cord,7,8 which are blocked by magnesium ions under physiological state.9 In contrast, consistent nerve lesion and pathological inflammation can induce hyperactivity of postsynaptic depolarization and remove magnesium blockage, resulting in central sensitization of pain transmission.10 Thus, magnesium administration may contribute to restoring the physiological functions of NMDARs, to attenuate the phenomenon of central sensitization, which is clinically relevant with manifestations of allodynia and hyperalgesia in patients with PMPS.11,12 However, the therapeutic effect of magnesium supplement on persistent pain caused by breast cancer procedure remains uncertain.
Recently, emerging evidence has demonstrated that a novel magnesium compound, Magnesium-L-Threonate (MLT) at a daily dosage of 1800 mg for 8 weeks, can achieve cognitive benefits in the elderly with or without Alzheimer’s Disease,13 which shares common therapeutic mechanism of NMDARs antagonists for neuropathic pain.14 Additionally, magnesium supplementation restores intracellular free Mg2+ and natural killer activating receptor NKG2D in natural killer and CD8+ T cells,15 which may further provide immune benefits in breast cancer survivors.
Thus, we hypothesized that blockage of NMDARs through MLT supplementation may attenuate neurophysiological disorders after breast tumor surgery, with regard to painful and cognitive co-morbidity. In this study, we primarily aimed to investigate the analgesic effect of MLT administration on persistent pain after breast cancer procedure. In addition, we evaluated the effect of MLT on cognitive function in patients who have undergone breast tumor surgery.
Materials and Methods
Study Design and Ethics Statement
This was a randomized, double-blind, placebo-controlled clinical trial, which was conducted in accordance with the guidance of the Helsinki Declaration and approved by the Ethics Committee of The Third Xiangya Hospital, Central South University, China (NO. 19076). This clinical trial was registered in Chinese Clinical Trial Registry (ChiCTR1900023820), and written informed consent was obtained from all patients. Data Safety Monitoring Board was appointed independently to the researchers and participants in this clinical trial, mainly to supervise the clinical safety.
Participants
A total of 526 female patients who were scheduled to undergo breast procedure for primary unilateral breast cancer were initially screened. Participants were excluded for: (1) aged below 18 or over 70 years old; (2) pregnant or breastfeeding; (3) drug abuse or psychiatric disease history; (4) severe respiratory, cardiovascular, hepatic, renal, or autoimmune disease, other type of neoplasm; (5) previous administration of analgesic, antiepileptic, antidepressant medications, or drugs which may influence the metabolism of magnesium (diuretics, proton pump inhibitor, calcium channel blocker, penicillamine, antacid containing magnesium and corticosteroids); (6) allergy to MLT; (7) were currently participating in other clinical trials; (8) unwillingness to take part in the study.
Randomization and Intervention
Random numbers were automatically generated in a 1:1 ratio using the SAS software (Cary, NC, USA), which were sequentially labeled with packs containing MLT or the placebo. The randomized, labeled packs were then allocated to participants according to the sequence of procedure date. There were five boxes containing MLT (Wuxi Naomisu Bio pharmaceutical Technology Co., Ltd, 0.6 g per cachet) in each pack, and one box contained sixty cachets (45.9 mg magnesium per cachet). The placebo contained the same ingredients but no MLT. Neither the packs nor the cachets had identifications to distinguish the placebo from MLT. Each patient was assigned one pack in order after recruitment, and was required to take 1.2 g MLT (91.8 mg magnesium) or placebo once a day for a 12-week treatment period. The investigators and participants were blinded to the contents of the pack.
Assessments and Data Collection
After initial recruitment, enrolled participants provided demographic, clinical, and laboratory data at baseline. The primary outcome was incidence of persistent pain after breast cancer procedure, and pain severity assessed at different time points (1-, 3-, and 6-month) after breast procedure with the short form of the McGill Pain Questionnaire (SF-MPQ).16 The secondary clinical evaluations included Generalized Anxiety Disorder Scale (GAD-7), Patient Health Questionnaire-9 (PHQ-9), Pittsburgh Sleep Quality Index (PSQI), and Telephone Interview for Cognitive Status (TICS). One independent researcher (F.D.) who was blinded to the allocation and intervention conducted the follow-up and data collection with a standard questionnaire containing the observational index mentioned previously. A 6-month efficacy and safety end-point was set in this study.
Statistical Analysis
Based on previous data,17,18 about one third of breast cancer patients reported persistent pain condition after surgical intervention. We considered patients with pain relief more than 50% as responders in our previous report.19 Sample size estimation was calculated based on these data for Type I error (alpha) of 0.05, and power (beta) of 90% to detect a clinically relevant change in pain relief with a sample size per group of 50 patients. Assuming a drop-out rate of 20%, we thus planned to enroll 120 patients.
Descriptive analysis was used to capture the clinical features of participants. Results were presented as mean ± standard deviation. The Shapiro–Wilk test was used to calculate the normality of each variable. Chi-squared test, Fisher’s exact test, Student’s t-test, or Mann–Whitney U-test was used to compare the clinical features between MLT and placebo group when appropriate. To compare the therapeutic effect between MLT and placebo, two-way ANOVA followed by post-hoc multiple pair-wise comparison Bonferroni correction was performed to evaluate the changes of SF-MPQ, GAD-7, PHQ-9, PSQI, and TICS at each timepoint. A two-sided p value <0.05 was considered statistically significant for all testing. Data analysis was processed with Prism v8 (GraphPad, San Diego, CA, United States).
Results
Cohort Features
A total of 526 patients who were scheduled to undergo breast tumor resection were initially screened for eligibility, and 146 participants who received breast cancer procedure consented to participate in the study from July 2019 to December 2020. Four patients were lost at 1-month follow-up, and there were 25 withdrawals at 3-month follow-up and 21 at six-month interview respectively. Two patients in the MLT subgroup were excluded for postoperative wound infection. Consequently, there were 48 patients assigned to the MLT intervention group, and 61 patients were enrolled in the placebo group respectively. The flowchart of patient selection is shown in Figure 1.
 |
Figure 1 Flow chart of participant screening and selection. |
The mean age of participants was about 47.3 ± 8.4 years old in the MLT-treated group, and 45.6 ± 9.6 in the control group respectively. About one fifth of participants (20 out of 109 participants) presented with symptomatic pain (VAS ≥ 3/10) preoperatively, but no significant difference was found in preoperative pain scores. The general features of patients are shown in Table 1.
 |
Table 1 Summary of general features of participants |
Comparison of the Therapeutic Effect of MLT versus Placebo on Persistent Pain After Breast Cancer Procedure
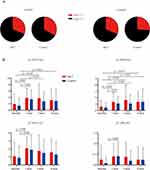
As shown in Figure 2A, about one third of patients (16 out of 48) reported symptomatic pain at 3-month follow-up in the MLT group, and the incidence of persistent pain after breast cancer procedure was 31% (19 out of 61) in the control group respectively (p = 0.839). Similarly, the incidence of symptomatic pain was not significantly (p = 0.67, Fisher’s exact testing) attenuated after MLT treatment (31%, 15/48) compared with placebo (26%, 16/61) at 6-month follow-up.
To further evaluate the pain intensity, we applied SF-MPQ for the qualitative aspect of pain in three dimensions of pain experience. In general, total pain scores were significantly increased one month after breast surgery in both MLT (p = 0.002, two-way ANOVA with Bonferroni correction) and placebo group (p < 0.001, two-way ANOVA with Bonferroni correction). Likewise, both groups presented with enhanced pain scores at 3- and 6-month follow-up compared with baseline. However, no statistical difference was found between groups at each interview point (Figure 2B). The change of Pain Rating Index (PRI) was consistent with total SF-MPQ scores at each timepoint of follow-up. In contrast, the alternation of VAS or Pain Intensity (PPI) index was not that significantly different after surgical intervention.
Effects of MLT Supplementation on Anxious, Depressive Symptoms, and Sleep Disorder in Breast Cancer Survivors
Despite sensory impairment, anxiety, depression and sleep disorder frequently coexist with chronic pain. To examine the therapeutic effect of MLT on affective dysfunction, a 7-item anxiety scale (GAD-7) was used to evaluate the anxiety symptoms, and PHQ-9 for depression respectively. Inconsistent with pain severity, the anxiety scale gradually decreased after breast tumor surgery, however, no significant changes were found between MLT and control group (Figure 3A). In shown in Figure 3B, we did not observe any improvement in depressive symptoms in either cohort. Both MLT and control group presented with slight but not statistical reduction of sleep quality, as demonstrated by the increasing trend of PSQI scoring (Figure 3C).
Oral Administration of MLT Did Not Attenuate Cognitive Function in Patients Who Underwent Breast Cancer Surgery
After 12-week treatment of MLT or placebo, participants were interviewed via telephone and the cognitive function was evaluated by the TICS. About 71% (34 out of 48) of participants accomplished the TICS testing, and 54 (88%) in the control group respectively. The average TICS scoring was 32 in general, and the cognitive conditions were almost the same between control and MLT-treated cohorts (Figure 3D).
Discussion
Patients with breast cancer commonly report persistent suffering from pain after surgical intervention, nevertheless, management of PMPS remains relatively less focused-on compared with postoperative anti-tumor treatment, such as chemo-, radio-, and hormone-therapy. Given its neuropathic features, we assumed that supplementation of magnesium may provide alternative pain relief via blockage of NMDARs, which is crucial in the development of sensory sensitization. However, our latest data from this clinical trial did NOT support the therapeutic effect of MLT in patients who underwent breast cancer procedure, neither pain nor cognitive symptoms were significantly attenuated after magnesium administration for twelve weeks.
Consistent with principle of neuropathic pain therapy, a multimodal strategy should be considered in pain management after breast cancer procedure, including non-pharmacological and pharmacological intervention.20 Physical rehabilitation is commonly recommended in breast cancer survivors at early postoperative phase,21 providing not only pain relief, but also improving shoulder motion, lymphedema, fatigue, physical functioning, anxiety, and sleep disorder.22 Oral medication may be required for those who need long-term control of symptoms, as one third of patients reported ongoing moderate to severe pain.17
It has been well-accepted that the first-line medical agents for neuropathic pain remain tricyclic antidepressants, selective serotonin-norepinephrine reuptake inhibitors, anti-epileptic, topical capsaicin and lidocaine patch.23 Despite the general consensus, only a few clinical trials have examined the indication of these anti-neuropathic medications for persistent pain after breast cancer procedure. To date, data from limited randomized studies have proven the role of amitriptyline, venlafaxine, and gabapentin in treating pain in breast cancer survivors.24–26 However, little evidence supports the prophylactic usage for PMPS. Actually, it is essentially needed to prevent the development of chronic pain after surgical intervention, rather than symptomatic control.
Recently, emerging evidence has indicated the potential benefits of NMDARs antagonists in reducing chronic post-surgical pain,27,28 and ketamine has been most evaluated. However, its clinical application remains limited due to the neuropsychiatric side effect and addictive concerns. Alternatively, the therapeutic function of magnesium in chronic pain has been recently noticed,12,29,30 and may serve as blockage of NMDARs to prevent the development PMPS. In this study, we reported clinically relevant data about magnesium administration in prevention of persistent pain after breast cancer procedure. In addition, we compared MLT with placebo, a novel compound containing magnesium, which has been demonstrated to attenuate neuropathic pain-like behaviors in rodent model of chemotherapy, by restoring magnesium deficiency and normalization of tumor necrosis factor-α/nuclear factor-κB pathway.31 Moreover, cognitive symptoms were partially attenuated after 8-week MLT treatment,13 which also commonly affects patients with chronic pain. Thus, another goal was to determine the effect of MLT on cognitive function after breast cancer surgery and adjuvant chemotherapy in this study, which significantly increases risk of cognitive impairment in breast cancer survivors.32
Against our expectation, we did not observe any significant reduction of chronic pain incidence after 12-week treatment of MLT (Figure 2). Similarly, pain scoring assessed by SF-MPQ was not improved in the magnesium-treated cohort at 3-, and 6-month follow-up. Despite distinct etiology, a parallel, two-arm randomized clinical trial conducted by Pickering et al33 demonstrated similar and negative data, that 4-week treatment of magnesium chloride (6x419 mg daily) did not significantly attenuate neuropathic pain compared with placebo. Another two trials tested the effect of magnesium on neuropathic pain with intravenous approach,34,35 however, only one achieved short-term relief from pain with limited sample size.34 Despite drug delivery method, other parameters may also influence the pharmacokinetics, such as dosage, administration interval, composition, and adjuvant analgesic agents may also contribute to the therapeutic effect.36,37 Nevertheless, the optimal strategy of magnesium therapy for chronic pain remains to be further investigated.
In addition to neuropathic pain, magnesium treatment has been applied in different types of chronic pain management. Recently, a systematic literature review summarized its validations well in chronic pain syndromes,12 and emerging usage in complex regional pain syndrome (CRPS), chronic low back pain, and headache disorder. Given that CRPS shares common symptoms with PMPS due to its neuropathic pain phenotype, likely, our finding is consistent with previously reported negative data in CRPS-1 cases.38,39
Mood dysfunction, depression and anxiety are most commonly seen in chronic pain state, which may contribute to the exacerbation of pain perception in cancer survivors.40 In a population-based longitudinal study, the estimated prevalence of comorbid anxiety-depression was about 9%.41 In this study, we noticed a gradually attenuated trend of anxiety symptoms after cancer procedure, as demonstrated by the decreasing GAD-7 scoring (Figure 3A). This result was consistent with previous data that surgical treatment was a major relieving factor of anxiety.42 However, the anxiety symptoms were not significantly attenuated by magnesium treatment. In contrast, no changes were found in depressive symptoms, but sleep disorder was worsened in the breast cancer survivors. Thus, we assume that sleep disorder but not anxiety nor depression was more closely associated with pain experience in patients who underwent breast cancer procedure.
About 20–30% of survivors may develop cognitive impairment after breast surgery and adjuvant treatment.43 Given that adjuvant chemotherapy was applied routinely in this study, the risk factor of cognitive deficits may be greater in terms of attention, concentration, working memory, and executive function.44 The mean score of TICS was about 32.2, which was relatively low and close to the level of amnestic mild cognitive impairment.43 One limitation of this study was that we did not compare the cognitive condition with baseline, thus, we may not conclude that the cognitive dysfunction was induced by breast cancer therapy. Recent evidence suggests cognitive benefits of magnesium supplementation,45 however, we did not find significant improvement of cognitive status after 12-week administration of MLT compared with control group. Indeed, we may consider magnesium supplementation as an essential mineral nutrient,46 rather than an independent agent providing pain relief. Thus, a multimodal pain control strategy, that combines distinct interacting drugs with or without other interventional therapy (ie, neuromodulation), is of great value in long-term management of neuropathic pain syndrome.
Conclusion
In this randomized, double-blind, placebo-controlled clinical trial, our data could not demonstrate the superior efficiency of MLT supplementation over placebo, in prevention of pain chronicity or attenuation of pain severity. Neither emotional, nor cognitive benefit was achieved after one 12-week magnesium treatment. Future study may focus on magnesium combined with other effective anti-neuropathic pain treatment.
Data Sharing Statement
The data are available upon reasonable request from the corresponding author.
Acknowledgments
This research was funded by National Natural Science Foundation of China, (81901146 to H.Z.), Excellent Youth Foundation of Hunan Scientific Committee, Key Laboratory of Hunan Province grants (2018TP1009 to H.Z. and D.H.), and Huizhiyucai Project of the Third Xiangya Hospital, Central South University.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Tait RC, Zoberi K, Ferguson M, et al. Persistent post-mastectomy pain: risk factors and current approaches to treatment. J Pain. 2018;19(12):1367–1383.
2. Monib S, Abdelaziz MI. Epidemiology and predictive factors for persistent breast pain following breast-conserving surgery. Cureus. 2021;13(3):e14063.
3. Lin ZM, Li MH, Zhang F, et al. Thoracic paravertebral blockade reduces chronic postsurgical pain in breast cancer patients: a randomized controlled trial. Pain Med. 2020;21(12):3539–3547.
4. Wang L, Cohen JC, Devasenapathy N, et al. Prevalence and intensity of persistent post-surgical pain following breast cancer surgery: a systematic review and meta-analysis of observational studies. Br J Anaesth. 2020;125(3):346–357.
5. Mercado LA, Liu R, Bharadwaj KM, et al. Association of intraoperative opioid administration with postoperative pain and opioid use. JAMA Surg. 2023;e232009.
6. Miaskowski C, Topp K, Conley YP, et al. Perturbations in neuroinflammatory pathways are associated with paclitaxel-induced peripheral neuropathy in breast cancer survivors. J Neuroimmunol. 2019;335:577019.
7. Inquimbert P, Moll M, Latremoliere A, et al. NMDA receptor activation underlies the loss of spinal dorsal horn neurons and the transition to persistent pain after peripheral nerve injury. Cell Rep. 2018;23(9):2678–2689.
8. Deng M, Chen SR, Pan HL. Presynaptic NMDA receptors control nociceptive transmission at the spinal cord level in neuropathic pain. Cell Mol Life Sci. 2019;76(10):1889–1899.
9. Kirkland AE, Sarlo GL, Holton KF. The role of magnesium in neurological disorders. Nutrients. 2018;10(6):730.
10. Cohen SP, Mao J. Neuropathic pain: mechanisms and their clinical implications. BMJ. 2014;348:f7656.
11. Khoury AL, Keane H, Varghese F, et al. Trigger point injection for post-mastectomy pain: a simple intervention with high rate of long-term relief. NPJ Breast Cancer. 2021;7(1):123.
12. Park R, Ho AM, Pickering G, Arendt-Nielsen L, Mohiuddin M, Gilron I. Efficacy and safety of magnesium for the management of chronic pain in adults: a systematic review. Anesth Analg. 2020;131(3):764–775.
13. Wroolie TE, Chen K, Watson KT, et al. An 8-week open label trial of l-Threonic Acid Magnesium Salt in patients with mild to moderate dementia. Personal Med Psychiat. 2017;4(6):7–12.
14. Liu J, Chang L, Song Y, Li H, Wu Y. The Role of NMDA Receptors in Alzheimer’s Disease. Front Neurosci. 2019;13:43.
15. Chaigne-Delalande B, Li FY, O’Connor GM, et al. Mg2+ regulates cytotoxic functions of NK and CD8 T cells in chronic EBV infection through NKG2D. Science. 2013;341(6142):186–191.
16. Yang X, Zhu R, Zhang J, et al. First-in-human Phase I studies of YJ001 spray applied to local skin in healthy subjects and patients with diabetic neuropathic pain. Expert Opin Investig Drugs. 2023;1–10.
17. Schreiber KL, Zinboonyahgoon N, Flowers KM, et al. Prediction of Persistent Pain Severity and Impact 12 Months After Breast Surgery Using Comprehensive Preoperative Assessment of Biopsychosocial Pain Modulators. Ann Surg Oncol. 2021;28(9):5015–5038.
18. Vilholm OJ, Cold S, Rasmussen L, Sindrup SH. The postmastectomy pain syndrome: an epidemiological study on the prevalence of chronic pain after surgery for breast cancer. Br J Cancer. 2008;99(4):604–610.
19. Han R, Guo G, Ni Y, et al. Clinical efficacy of short-term peripheral nerve stimulation in management of facial pain associated with herpes zoster ophthalmicus. Front Neurosci. 2020;14:574713.
20. Khan JS, Ladha KS, Abdallah F, Clarke H. Treating persistent pain after breast cancer surgery. Drugs. 2020;80(1):23–31.
21. Harris SR, Schmitz KH, Campbell KL, McNeely ML. Clinical practice guidelines for breast cancer rehabilitation: syntheses of guideline recommendations and qualitative appraisals. Cancer. 2012;118(8 Suppl):2312–2324.
22. Redemski T, Hamilton DG, Schuler S, Liang R, Michaleff ZA. Rehabilitation for women undergoing breast cancer surgery: a systematic review and meta-analysis of the effectiveness of early, unrestricted exercise programs on upper limb function. Clin Breast Cancer. 2022;22(7):650–665.
23. Fornasari D. Pharmacotherapy for neuropathic pain: a review. Pain Ther. 2017;6(Suppl 1):25–33.
24. Chappell AG, Yuksel S, Sasson DC, Wescott AB, Connor LM, Ellis MF. Post-mastectomy pain syndrome: an up-to-date review of treatment outcomes. JPRAS Open. 2021;30:97–109.
25. Birkinshaw H, Friedrich CM, Cole P, et al. Antidepressants for pain management in adults with chronic pain: a network meta-analysis. Cochrane Database Syst Rev. 2023;5(5):CD014682.
26. de Miguel-Jimeno JM, Forner-Cordero I, Zabalza-Azparren M, Matute-Tobias B. Postmastectomy pain syndrome in our region: characteristics, treatment, and experience with gabapentin. Rev Neurol. 2016;62:258–266.
27. Carley ME, Chaparro LE, Choinière M, et al. Pharmacotherapy for the prevention of chronic pain after surgery in adults: an updated systematic review and meta-analysis. Anesthesiology. 2021;135(2):304–325.
28. Chaparro LE, Smith SA, Moore RA, Wiffen PJ, Gilron I. Pharmacotherapy for the prevention of chronic pain after surgery in adults. Cochrane Database Syst Rev. 2013;2013(7):CD008307.
29. Morel V, Pickering ME, Goubayon J, Djobo M, Macian N, Pickering G. Magnesium for pain treatment in 2021? State of the art. Nutrients. 2021;13(5):1397.
30. Macian N, Dualé C, Voute M, et al. Short-term magnesium therapy alleviates moderate stress in patients with fibromyalgia: a randomized double-blind clinical trial. Nutrients. 2022;14(10):2088.
31. Xu T, Li D, Zhou X, et al. Oral Application of Magnesium-L-Threonate Attenuates Vincristine-induced Allodynia and Hyperalgesia by Normalization of Tumor Necrosis Factor-α/Nuclear Factor-κB Signaling. Anesthesiology. 2017;126(6):1151–1168.
32. Van Dyk K, Ganz PA. Cancer-related cognitive impairment in patients with a history of breast cancer. JAMA. 2021;326(17):1736–1737.
33. Pickering G, Morel V, Simen E, et al. Oral magnesium treatment in patients with neuropathic pain: a randomized clinical trial. Magnes Res. 2011;24(2):28–35.
34. Brill S, Sedgwick PM, Hamann W, Di Vadi PP. Efficacy of intravenous magnesium in neuropathic pain. Br J Anaesth. 2002;89(5):711–714.
35. Felsby S, Nielsen J, Arendt-Nielsen L, Jensen TS. NMDA receptor blockade in chronic neuropathic pain: a comparison of ketamine and magnesium chloride. Pain. 1996;64(2):283–291.
36. Pickering G, Pereira B, Morel V, et al. Ketamine and magnesium for refractory neuropathic pain: a randomized, double-blind, crossover trial. Anesthesiology. 2020;133(1):154–164.
37. Yousef AA, Al-deeb AE. A double-blinded randomised controlled study of the value of sequential intravenous and oral magnesium therapy in patients with chronic low back pain with a neuropathic component. Anaesthesia. 2013;68(3):260–266.
38. Fischer SG, Collins S, Boogaard S, Loer SA, Zuurmond WW, Perez RS. Intravenous magnesium for chronic complex regional pain syndrome type 1 (CRPS-1). Pain Med. 2013;14(9):1388–1399.
39. van der Plas AA, Schilder JC, Marinus J, van Hilten JJ. An explanatory study evaluating the muscle relaxant effects of intramuscular magnesium sulphate for dystonia in complex regional pain syndrome. J Pain. 2013;14(11):1341–1348.
40. Metcalfe KA, Candib A, Giannakeas V, et al. The relationship between the predicted risk of death and psychosocial functioning among women with early-stage breast cancer. Breast Cancer Res Treat. 2021;186(1):177–189.
41. Boehmer U, Ozonoff A, Winter M, et al. Anxiety and depression in colorectal cancer survivors: are there differences by sexual orientation? Psychooncology. 2022;31(3):521–531.
42. Kim J, Cho J, Lee SK, et al. Surgical impact on anxiety of patients with breast cancer: 12-month follow-up prospective longitudinal study. Ann Surg Treat Res. 2020;98(5):215–223.
43. Pasi M, Sugita L, Xiong L, et al. Association of cerebral small vessel disease and cognitive decline after intracerebral hemorrhage. Neurology. 2021;96(2):e182–e192.
44. Carroll JE, Van Dyk K, Bower JE, et al. Cognitive performance in survivors of breast cancer and markers of biological aging. Cancer. 2019;125(2):298–306.
45. Lo K, Liu Q, Madsen T, et al. Relations of magnesium intake to cognitive impairment and dementia among participants in the Women’s Health Initiative Memory Study: a prospective cohort study. BMJ Open. 2019;9(11):e030052.
46. Shin HJ, Na HS, Do SH. Magnesium and Pain. Nutrients. 2020;12(8):2184.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.