Back to Journals » Journal of Pain Research » Volume 10
A randomized, Phase IIb study investigating oliceridine (TRV130), a novel µ-receptor G-protein pathway selective (µ-GPS) modulator, for the management of moderate to severe acute pain following abdominoplasty
Authors Singla N , Minkowitz HS , Soergel DG, Burt DA , Subach RA, Salamea MY, Fossler MJ, Skobieranda F
Received 25 March 2017
Accepted for publication 4 July 2017
Published 6 October 2017 Volume 2017:10 Pages 2413—2424
DOI https://doi.org/10.2147/JPR.S137952
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Katherine Hanlon
Neil Singla,1 Harold S Minkowitz,2 David G Soergel,3 David A Burt,3 Ruth Ann Subach,3 Monica Y Salamea,3 Michael J Fossler,3 Franck Skobieranda3
1Lotus Clinical Research, Pasadena, CA, 2Memorial Hermann Memorial City Medical Center, Houston, TX, 3Trevena, Inc, King of Prussia, PA, USA
Background: Oliceridine (TRV130), a novel µ-receptor G-protein pathway selective (µ-GPS) modulator, was designed to improve the therapeutic window of conventional opioids by activating G-protein signaling while causing low β-arrestin recruitment to the µ receptor. This randomized, double-blind, patient-controlled analgesia Phase IIb study was conducted to investigate the efficacy, safety, and tolerability of oliceridine compared with morphine and placebo in patients with moderate to severe pain following abdominoplasty (NCT02335294; oliceridine is an investigational agent not yet approved by the US Food and Drug Administration).
Methods: Patients were randomized to receive postoperative regimens of intravenous oliceridine (loading/patient-controlled demand doses [mg/mg]: 1.5/0.10 [regimen A]; 1.5/0.35 [regimen B]), morphine (4.0/1.0), or placebo with treatment initiated within 4 hours of surgery and continued as needed for 24 hours.
Results: Two hundred patients were treated (n=39, n=39, n=83, and n=39 in the oliceridine regimen A, oliceridine regimen B, morphine, and placebo groups, respectively). Patients were predominantly female (n=198 [99%]) and had a mean age of 38.2 years, weight of 71.2 kg, and baseline pain score of 7.7 (on 11-point numeric pain rating scale). Patients receiving the oliceridine regimens had reductions in average pain scores (model-based change in time-weighted average versus placebo over 24 hours) of 2.3 and 2.1 points, respectively (P=0.0001 and P=0.0005 versus placebo); patients receiving morphine had a similar reduction (2.1 points; P<0.0001 versus placebo). A lower prevalence of adverse events (AEs) related to nausea, vomiting, and respiratory function was observed with the oliceridine regimens than with morphine (P<0.05). Other AEs with oliceridine were generally dose-related and similar in nature to those observed with conventional opioids; no serious AEs were reported with oliceridine.
Conclusion: These results suggest that oliceridine may provide effective, rapid analgesia in patients with moderate to severe postoperative pain, with an acceptable safety/tolerability profile and potentially wider therapeutic window than morphine.
Keywords: TRV130, acute pain, analgesic, opioid, biased ligand
Introduction
Conventional opioids that act at the µ receptor, including morphine, hydromorphone, and fentanyl, are the standard of care for the management of moderate to severe acute postoperative pain;1–5 however, opioid-related adverse events (ORAEs), including gastrointestinal (GI) effects, respiratory effects, and sedation, are common and may impede effective patient care.6–10 Nausea and vomiting, while not life-threatening, are a major cause of distress to patients.9,11–13 Signs of respiratory depression may be observed in up to 26.9% of patients being managed for acute postoperative pain.7,8 Finally, ORAEs may increase length of stay, rehospitalization rates, and cost of care.14–18 To prevent ORAEs, opioid dosing may be purposely limited, leading to suboptimal pain management.19,20 Undertreated acute postoperative pain has been associated with the development of chronic postoperative pain21–23 and long-term opioid use.24 Thus, improved management of acute postoperative pain may reduce the risk of transformation of acute pain into chronic pain. In current practice guidelines, multimodal approaches that combine analgesics with different mechanisms, including regimens of opioids, non-opioids, and various adjuvant analgesics, are recommended to manage postoperative pain and limit ORAEs.25 However, even with multimodal approaches, ORAEs remain a critical clinical issue.19,26,27
The therapeutic windows of conventional opioids – the separation of doses that produce desired therapeutic effects and doses that produce adverse effects – are derived in part from their receptor pharmacology.28–31 At the cellular level, conventional opioids bind to µ receptors and non-selectively activate two intracellular signaling pathways: the G-protein pathway, associated with analgesia, and the β-arrestin pathway, associated with ORAEs and feedback inhibition of G-protein-mediated analgesia.32–37 This dual pathway mechanism of action may explain why, in many cases, the occurrence of adverse events (AEs) limits the analgesic effectiveness of conventional opioids. The therapeutic window of some conventional opioids may also be affected by their complex metabolic pathways, which result in the accumulation of active metabolites.38–40 Thus, an unmet need exists for a predictable and powerful analgesic with improved safety and tolerability compared with currently available therapies.
Oliceridine (TRV130) is a novel µ-receptor G-protein pathway selective (µ-GPS) modulator that activates G-protein signaling while causing low β-arrestin recruitment to the µ receptor. In accord with its low β-arrestin recruitment, oliceridine differs from morphine in its impact on receptor phosphorylation and internalization.41,42 Also, unlike morphine, oliceridine has no known active metabolites. In studies of healthy volunteers, oliceridine demonstrated equal or greater analgesia with less reduction in respiratory drive and less severe nausea than morphine.43 Recently, the results of an adaptive design, randomized, double-blind, placebo-, and active-controlled Phase II study of oliceridine in patients experiencing moderate to severe acute pain following bunionectomy were reported.44 Bolus doses of intravenous oliceridine that produced statistically significant reductions in pain intensity compared with morphine did so with acceptable safety and tolerability.44 Oliceridine is an investigational agent that has not been approved by the US Food and Drug Administration. In this study, we present findings from a Phase IIb study conducted to assess the efficacy, safety, and tolerability of oliceridine compared with morphine and placebo, each administered via patient-controlled analgesia (PCA), in patients with moderate to severe pain following abdominoplasty.
Methods
Study design
This was a randomized, double-blind, two-part Phase IIb study in patients with moderate to severe acute postoperative pain following abdominoplasty (NCT02335294). The primary objective was to evaluate the analgesic efficacy of intravenous oliceridine compared with placebo administered “as-needed” via PCA. Secondary objectives were to evaluate the analgesic efficacy of as-needed oliceridine compared with as-needed morphine (as an active control) via PCA and to evaluate the safety and tolerability of oliceridine compared with morphine and placebo.
The study was conducted at a single center in the US (Lotus Clinical Research, Pasadena, CA, USA). The study protocol was approved by an Institutional Review Board (Aspire IRB; http://aspire-irb.com) and complied with the International Conference on Harmonisation Guidance for Industry and the Declaration of Helsinki.45,46 All the participants provided written informed consent.
Patients
At screening, males or females aged 18–65 years who planned to undergo abdominoplasty without any additional collateral procedures were enrolled. At that time, patients were excluded if they were classified as P3 or worse on the American Society of Anesthesiologists Physical Status Classification System, had clinically significant medical conditions or a history of such conditions, or had previously participated in an oliceridine clinical study. In the immediate postoperative period, patients were eligible to enroll if they had recovered from the intraoperative anesthetic and analgesic regimen to a degree to which they were considered by investigators to be sufficiently lucid to complete the required questionnaires. They were also required to have a score of ≥5 on an 11-point numeric pain rating scale (NPRS) and moderate or severe pain on a 4-point categorical rating scale (with categories of none, mild, moderate, or severe) within 4 hours after the end of surgery. Patients who had undergone prolonged surgeries (>2.5 hours) or surgeries that deviated from surgical or anesthetic protocols and those who had surgical or postsurgical complications were excluded from the study. Patients were required to meet all of these preoperative (screening) and postoperative inclusion and exclusion criteria to be eligible for the study and receive study medication.
The general anesthetic regimen was standardized to reduce variability and consisted of fentanyl and propofol, with or without volatile anesthetics or muscle relaxants. Opioids other than fentanyl were prohibited; at least 20 minutes must have elapsed between the final dose of fentanyl (either intraoperative or postoperative) and the initiation of study medication. Other prohibited medications included intraoperative or postoperative steroids, nonsteroidal anti-inflammatory drugs, intravenous acetaminophen, regional or neuraxial anesthesia, long-acting local anesthetics, and prophylactic anti-emetics.
Study procedures
This exploratory study was divided into two stages, separated by an interim analysis. Since oliceridine had not been administered by PCA previously, the interim analysis was incorporated to adjust the oliceridine PCA regimen if required. No predefined stopping rules were established for futility or for efficacy at the interim analysis. Self-administration of study medication via PCA was used to optimize the treatment for each patient, reflecting the as-needed dosing most commonly used with postoperative analgesics. All treatment regimens were blinded and volume-matched and consisted of intravenous loading doses and patient-controlled demand doses with a 6-minute lockout interval after any demand dose. In the first stage of the study, the clinician was given the option to up-titrate the demand dose in a blinded fashion, but this option was eliminated at the interim analysis because it was rarely used and added operational complexity to the study.
In each part of the study, eligible patients were randomized to postoperative regimens of intravenous oliceridine, morphine, or volume-matched placebo, in a 2:2:1 ratio, beginning when postoperative pain became moderate or severe in intensity, as defined by a score of ≥5 on an 11-point NPRS and by report of moderate or severe pain on a 4-point categorical rating scale (with categories of none, mild, moderate, or severe) within 4 hours after the end of surgery. As-needed study treatment was administered in a double-blind manner and continued for 24 hours after initial dosing. In the first part of the study (stage I), the oliceridine regimen consisted of two 0.75 mg loading doses separated by 10 minutes, followed by 0.10 mg demand doses (oliceridine regimen A). In the second part of the study (stage II), following the interim analysis, the oliceridine demand dose was increased from 0.10 to 0.35 mg (oliceridine regimen B). The morphine treatment regimen consisted of two 2 mg loading doses separated by 10 minutes followed by 1 mg demand doses in both stage I and stage II.
Oral rescue analgesics were available as necessary (and their use recorded) for patients whose pain was not adequately treated with study medication. Inadequate pain control was defined as a score ≥4 on the NPRS. First-line rescue medication was ibuprofen (400 mg orally every 6 hours as needed); second-line rescue medication was oxycodone (5 mg orally every 2 hours as needed). Patients whose pain was not adequately controlled with study and rescue analgesic medication were discontinued from the study.
Measures
Pain intensity was measured using the NPRS at baseline, and at 5, 10, 15, 30, and 45 minutes; at 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 16, 18, 20, and 24 hours following the first dose of study medication; prior to any rescue analgesic use; and prior to early discontinuation. The NPRS is an 11-point scale from 0 to 10, with higher scores indicating greater pain intensity. A categorical, 5-point pain relief scale (“none,” “a little,” “some,” “a lot,” and “complete”) was assessed at the same time points, excluding baseline. Efficacy was also assessed utilizing the two-stopwatch technique: patients were given two running stopwatches at the initiation of study medication and instructed to stop the first when they felt perceptible pain relief and the second when they experienced meaningful pain relief. Use of the stopwatches was discontinued in patients who did not achieve perceptible or meaningful pain relief within 6 hours.
Safety assessments included spontaneous AE reporting, vital sign measurements, physical examinations, electrocardiography, and clinical laboratory assessments conducted throughout the pre-surgery, surgery, immediate post-surgery, and randomized treatment periods. Other prespecified assessments evaluated opioid-related safety and tolerability, including spontaneous reports of well-recognized ORAEs. Upper GI effects (vomiting and nausea) and respiratory effects (hypoxia, bradypnea, decreased respiratory effort, or hypoventilation) were also captured.
Efficacy endpoints
The primary endpoint was the model-based, time-weighted average change in NPRS over 24 hours (TWA NPRS 0–24) versus placebo. This endpoint was calculated as the area under the 0–24 hour pain intensity curve divided by 24 hours, minus the baseline pain intensity. Secondary endpoints included: change from baseline in TWA NPRS over different time intervals; pain intensity difference (PID) at each scheduled time point and the sum of PID (SPID); pain intensity score at each scheduled time point; categorical assessments of pain relief at scheduled time points; time to first meaningful pain relief; proportion of patients using rescue analgesics; and total number of doses of rescue analgesics used over 0–24 hours.
Treatment-emergent adverse events (TEAEs)
Safety was assessed by monitoring for TEAEs throughout the study. The Medical Dictionary for Regulatory Activities (Version 17) was used to classify all AEs with respect to system organ class and preferred term. The prevalence of the following pre-specified ORAEs was also assessed: nausea, vomiting, and respiratory effects (including clinically apparent and persistent hypoventilation, respiratory depression, or hypoxia).
Study population and statistical analyses
Efficacy was assessed in the modified intention-to-treat population, which was defined as all randomized subjects who met entry criteria, received any study medication, and had at least one post-baseline NPRS score. The safety and tolerability analysis population consisted of all patients who received study medication.
A last observation carried forward approach was used to impute missing NPRS data from the time of study treatment discontinuation (because of lack of efficacy) or from the time of first use of rescue analgesic (for those who used rescue analgesic). Analysis of continuous efficacy variables employed an analysis of covariance model with treatment as the experimental factor and baseline pain intensity as a covariate. Stopwatch times used the Kaplan–Meier method and log-rank P-values. Categorical pain relief comparisons used the Cochran–Mantel–Haenszel (CMH) test. For categorical endpoints, the number and percentage of patients were reported for each category of response and statistical comparisons utilized a CMH test. Data for the morphine and placebo groups were similar across the two stages of the study (Supplementary materials) and were combined for analysis. No alpha adjustment was made because no plans were established to stop the study based on efficacy at the interim analysis.
TEAEs occurring in ≥10% of any treatment group were summarized descriptively. Post hoc statistical analyses were performed for the above-described pre-specified ORAEs to compare their prevalence between oliceridine and morphine treatment groups.
Results
Patient disposition, demographics, and baseline characteristics
Overall, 200 patients were enrolled in the study; 39, 39, 83, and 39 patients were treated with oliceridine regimen A, oliceridine regimen B, morphine, and placebo, respectively (Figure S1). Of these, 186 (93%) completed the study. Following treatment initiation, 14 patients were discontinued early from the study: nine patients were discontinued for lack of therapeutic effect, including two patients each in the oliceridine regimen A, morphine, and placebo groups and three patients in the oliceridine regimen B group; and five patients were discontinued for AEs, including one patient with a respiratory AE in the oliceridine regimen A group, two patients with respiratory AEs in the morphine group, one patient with a respiratory AE and sedation in the morphine group, and one patient with an abnormal T-wave on electrocardiogram in the morphine group.
Demographics and baseline characteristics were similar among treatment groups (Table 1). Patients were aged 19–62 years, with a mean of 38.2 years, and were predominantly female (n=198; 99%). Mean weight and body mass index were 71.2 kg and 26.7 kg/m2, respectively. Mean baseline NPRS intensity was 7.7. The mean cumulative doses of oliceridine over the 24-hour study period were 7.6 and 14.8 mg for regimens A and B, respectively; the mean cumulative dose of morphine was 26.4 mg.
Efficacy
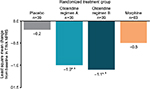
For the primary endpoint analysis, oliceridine regimens A and B produced statistically significant reductions in pain (TWA NPRS 0–24 hours), relative to placebo, of 2.3 points (P=0.0001) and 2.1 points (P=0.0005), respectively (Figure 1). The efficacy of the oliceridine regimens was similar to that of morphine, which reduced pain by 2.1 points relative to placebo (P<0.0001). At the 24-hour primary time point, changes in TWA NPRS from baseline (standard error [SE]) for oliceridine regimen A, oliceridine regimen B, and morphine were –3.7 (0.42), –3.5 (0.41), and –3.5 (0.28), respectively, compared with –1.4 (0.41) for placebo. Mean SPID (SD) with oliceridine regimens A and B was –92.7 (60.96) and –84.5 (55.76), respectively, compared with –83.3 (64.34) and –34.0 (62.43) with morphine and placebo, respectively.
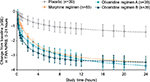
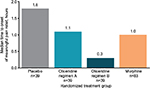
To depict single-dose efficacy, the reduction in pain was evaluated at 5 minutes following the second loading dose (Figure 2). Changes in TWA NPRS at 5 minutes were –1.0 and –1.1 for oliceridine regimens A and B, respectively, compared with –0.5 for morphine and –0.2 for placebo (P=0.0016, 0.0004, and 0.135 for oliceridine regimen A, oliceridine regimen B, and morphine, respectively, versus placebo). Median time to meaningful pain relief was 1.1 and 0.3 hours for oliceridine regimens A and B, respectively, compared with 1.0 and 1.8 hours for morphine and placebo, respectively (Figure 3).
Rescue analgesic use was similar for all active treatment groups. The proportion of patients using rescue analgesics was 31% with oliceridine regimen A, 21% with oliceridine regimen B, and 25% with morphine, compared with 64% with placebo (P<0.0005 for all three active treatment groups versus placebo). For patients using rescue analgesics, the mean number of rescue doses was 1.7, 1.6, 1.7, and 2.2, in the oliceridine regimen A, oliceridine regimen B, morphine, and placebo groups, respectively.
Safety and tolerability
Compared with morphine, oliceridine was generally well-tolerated. TEAEs associated with oliceridine were largely opioid-related in nature; the most frequently reported events were nausea, vomiting, and headache (Table 2).One serious AE (anemia) was reported in a patient in the morphine group.
In post hoc analyses of pre-specified ORAEs, statistically significant differences were observed in the tolerability of oliceridine versus morphine. Lower percentages of patients treated with oliceridine experienced nausea (41% and 46% with oliceridine regimens A and B; 72% with morphine; P<0.01, for oliceridine regimens A and B versus morphine) and vomiting (15% with both oliceridine regimens; 42% with morphine; P<0.01, for oliceridine regimens A and B versus morphine). A lower percentage of patients receiving oliceridine also experienced respiratory effects (15% and 31% with oliceridine regimens A and B, respectively) than patients receiving morphine (53%; P<0.05, for oliceridine regimens A and B versus morphine).
No clinically significant changes from baseline were reported in vital signs, physical examination findings, or hematology or chemistry parameters. Clinically significant abnormal electrocardiograms were reported in two patients (one patient in the placebo group and one patient in the morphine group).
Discussion
The dual signaling profile of conventional opioids (activating both G protein and β-arrestin signaling32–34) is associated with analgesia but also with AEs that greatly restrict their clinical utility. The most important and prevalent of these AEs are nausea, vomiting, and respiratory effects. AEs associated with conventional opioids frequently have a substantial impact on the treatment of postoperative pain, with potential adverse effects on patient safety, pain control, hospital length of stay, rehospitalization rates, and overall cost of care.11,12,14–18,20 With conventional opioids, clinicians often face the challenge of finding the optimal balance between pain control and ORAEs, an exercise that can be particularly relevant in high-risk patients (eg, patients with chronic obstructive pulmonary disease, obesity, renal impairment, sleep apnea, or advanced age4,47,48). A novel therapy with opioid-like efficacy and improved safety and tolerability would therefore satisfy an important unmet medical need.
The results of this Phase IIb study demonstrated that oliceridine produced rapid and meaningful relief of moderate to severe acute pain and was generally well tolerated. This study was the first in the oliceridine development program to deliver study medication on an as-needed basis and via PCA device. Earlier studies of oliceridine investigated efficacy, safety, and tolerability using fixed-dose designs to assess the dose–response and duration of effect of oliceridine.44 The flexible dosing of study medication utilized in this trial reflects the as-needed dosing most commonly used with postoperative analgesics in the real-world setting. Inherent to this design, each patient determined their individual balance of benefit–risk, weighing such factors as baseline pain intensity, on-treatment pain intensity, and any experienced side effects, making their experience in this study clinically relevant.44 The results of this study demonstrated that when patients self-titrated to adequate analgesic levels, a favorable AE profile was observed with oliceridine. In addition to use of flexible dosing of study medication, the evaluation of oliceridine against the active comparator morphine is a strength of the clinical trial design and enhances the evaluation of efficacy as well as safety. Finally, the study design was also noteworthy for its use of the abdominoplasty pain model: a predictable, low variability model of pain due to soft-tissue surgery or injury.49–52
Efficacy analyses demonstrated that oliceridine treatment produced statistically significant analgesia compared with placebo over the 24-hour treatment period. Notably, pain relief was rapid, with statistically significant differences compared with placebo at 5 minutes following the first loading dose. Analgesia with oliceridine was similar to morphine over 24 hours, and analgesia occurred more rapidly with oliceridine, with greater reductions from baseline in TWA NPRS at both the 5- and 30-minute time points. The results of this study are consistent with results from the recent randomized, placebo- and active-controlled Phase II study in patients with acute pain following bunionectomy.44 In the earlier study, oliceridine 3 mg demonstrated superior efficacy to morphine, with average reduction in NPRS of 6 points from a baseline score of 7 points following the first dose of study medication. Similar to the current study, pain relief was rapid: 2- and 3-mg doses of oliceridine produced greater categorical pain relief than morphine (P<0.005) following the first dose, with meaningful pain relief occurring in less than 5 minutes.
In this study, oliceridine demonstrated a favorable safety and tolerability profile, with no reports of serious AEs. Statistically significant differences in the prevalence of nausea, vomiting, and respiratory effects were seen between the oliceridine and morphine groups, with a lower prevalence in patients treated with either oliceridine regimen than in those treated with morphine. These results will need to be confirmed in additional well-controlled trials.
The differentiated performance characteristics of oliceridine may be a consequence of biased ligand pharmacology. As a µ-GPS, oliceridine activates the µ receptor in a differential manner, with a preference toward the G-protein pathway (associated with analgesia) over the β-arrestin pathway (associated with ORAEs and inhibition of G-protein-mediated analgesia). Additionally, unlike morphine, oliceridine has no known active metabolites; the latter property may allow for more predictable performance, in terms of analgesic efficacy and dose-related ORAEs, that is directly linked to the plasma concentration of the parent compound.
This study had some notable limitations. First, this study was performed at a single center. Additional multicenter studies to confirm these findings are warranted and are ongoing. Second, all but two of the study participants were female, which is not unexpected for the abdominoplasty surgical model. However, it is noteworthy that in the Phase II bunionectomy study,44 which enrolled a higher proportion of males, no statistically significant sex effect or sex-by-treatment interaction was seen. In addition, a preliminary analysis of the Phase I and II pharmacokinetics data showed no clinically relevant differences in oliceridine exposure due to sex. The results with oliceridine, although promising, are preliminary and will need to be confirmed.
In conclusion, oliceridine is a novel µ-GPS with differential receptor signaling compared with conventional opioids. In this study, oliceridine produced analgesia similar to morphine with an improved safety and tolerability profile. These results suggest that oliceridine, by activating G-protein-mediated analgesia and mitigating β-arrestin-mediated ORAEs, may have a wider therapeutic window and offer effective, rapid analgesia with fewer ORAEs. If confirmed, its use may allow for increased flexibility in dosing for analgesia, particularly in high-risk patient populations. The promising findings from this study (conducted in a predictable, low variability model of pain due to soft-tissue surgery or injury) and in a previous Phase II bunionectomy study (hard tissue model) demonstrate the potential of intravenous oliceridine in the management of postoperative pain that warrants further testing in Phase III clinical trials.
Acknowledgments
Medical writing support was provided by Kevin Wang of Xelay Acumen (San Mateo, CA, USA) and Donna McGuire of Engage Scientific Solutions (Philadelphia, PA, USA) and data analytical support by Cory Mekelburg of Xelay Acumen, which were funded by Trevena, Inc (King of Prussia, PA, USA).
The results of this study were presented at the American Society of Regional Anesthesia 41st Annual Regional Anesthesiology and Acute Pain Medicine Meeting (March 31 to April 2, 2016, New Orleans, LA, USA).
Author contributions
All the authors contributed to the conception or design of the study; contributed to the acquisition, analysis, or interpretation of the data; contributed to the drafting and revision of the manuscript for important intellectual content; agreed to be accountable for the work; and provided final approval of this version to be published.
Disclosure
This study was supported by Trevena, Inc (King of Prussia, PA, USA). Neil Singla is an employee of Lotus Clinical Research, LLC, contracted by the sponsor to conduct the study; Dr Singla has also received consulting fees from Trevena, Inc. Harold S Minkowitz has received investigator fees and consulting fees from Trevena, Inc, AcelRx, and The Medicines Company. Ruth Ann Subach was an employee of Trevena, Inc, during the conception/conduct of the study and owns Trevena, Inc, stock. David G Soergel, David A Burt, Monica Y Salamea, Michael J Fossler, and Franck Skobieranda are employees of the study’s sponsor, Trevena, Inc. The authors report no other conflicts of interest in this work.
References
American Society of Anesthesiologists Task Force on Acute Pain Management. Practice guidelines for acute pain management in the perioperative setting: an updated report by the American Society of Anesthesiologists Task Force on Acute Pain Management. Anesthesiology. 2012;116(2):248–273. | ||
Chou R, Gordon DB, de Leon-Casasola OA, et al. Management of postoperative pain: a clinical practice guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J Pain. 2016;17(2):131–157. | ||
Misiołek H, Cettler M, Woroń J, Wordliczek J, Dobrogowski J, Mayzner-Zawadzka E. The 2014 guidelines for post-operative pain management. Anaesthesiol Intensive Ther. 2014;46(4):221–244. | ||
Lovich-Sapola J, Smith CE, Brandt CP. Postoperative pain control. Surg Clin North Am. 2015;95(2):301–318. | ||
Veterans Health Administration. VHA/DoD clinical practice guideline for the management of postoperative pain; 2002. Available from: http://www.healthquality.va.gov/guidelines/Pain/pop/pop_fulltext.pdf. Accessed April 1, 2016. | ||
Benyamin R, Trescot AM, Datta S, et al. Opioid complications and side effects. Pain Physician. 2008;11(2 Suppl):S105–S120. | ||
Cashman JN, Dolin SJ. Respiratory and haemodynamic effects of acute postoperative pain management: evidence from published data. Br J Anaesthesia. 2004;93(2):212–223. | ||
Hagle ME, Lehr VT, Brubakken K, Shippee A. Respiratory depression in adult patients with intravenous patient-controlled analgesia. Orthop Nurs. 2004;23(1):18–27; quiz 28–29. | ||
Gan TJ, Diemunsch P, Habib AS, et al. Consensus guidelines for the management of postoperative nausea and vomiting. Anesth Analg. 2014;118(1):85–113. | ||
Wheeler M, Oderda GM, Ashburn MA, Lipman AG. Adverse events associated with postoperative opioid analgesia: a systematic review. J Pain. 2002;3(3):159–180. | ||
Lee A, Gin T, Lau AS, Ng FF. A comparison of patients’ and health care professionals’ preferences for symptoms during immediate postoperative recovery and the management of postoperative nausea and vomiting. Anesth Analg. 2005;100(1):87–93. | ||
Gregorian RS Jr, Gasik A, Kwong WJ, Voeller S, Kavanagh S. Importance of side effects in opioid treatment: a trade-off analysis with patients and physicians. J Pain. 2010;11(11):1095–1108. | ||
Eberhart LH, Morin AM, Wulf H, Geldner G. Patient preferences for immediate postoperative recovery. Br J Anaesth. 2002;89(5):760–761. | ||
Kessler ER, Shah M, Gruschkus SK, Raju A. Cost and quality implications of opioid-based postsurgical pain control using administrative claims data from a large health system: opioid-related adverse events and their impact on clinical and economic outcomes. Pharmacotherapy. 2013;33(4):383–391. | ||
Carroll NV, Miederhoff PA, Cox FM, Hirsch JD. Costs incurred by outpatient surgical centers in managing postoperative nausea and vomiting. J Clin Anesth. 1994;6(5):364–369. | ||
Coley KC, Williams BA, DaPos SV, Chen C, Smith RB. Retrospective evaluation of unanticipated admissions and readmissions after same day surgery and associated costs. J Clin Anesth. 2002;14(5):349–353. | ||
Oderda GM, Said Q, Evans RS, et al. Opioid-related adverse drug events in surgical hospitalizations: impact on costs and length of stay. Ann Pharmacother. 2007;41(3):400–406. | ||
Oderda GM, Gan TJ, Johnson BH, Robinson SB. Effect of opioid-related adverse events on outcomes in selected surgical patients. J Pain Palliat Care Pharmacother. 2013;27(1):62–70. | ||
Gan TJ, Habib AS, Miller TE, White W, Apfelbaum JL. Incidence, patient satisfaction, and perceptions of post-surgical pain: results from a US national survey. Curr Med Res Opin. 2014;30(1):149–160. | ||
Apfelbaum JL, Chen C, Mehta SS, Gan TJ. Postoperative pain experience: results from a national survey suggest postoperative pain continues to be undermanaged. Anesth Analg. 2003;97(2):534–540. | ||
Reuben SS. Chronic pain after surgery: what can we do to prevent it. Curr Pain Headache Rep. 2007;11(1):5–13. | ||
Perkins FM, Kehlet H. Chronic pain as an outcome of surgery. A review of predictive factors. Anesthesiology. 2000;93(4):1123–1133. | ||
Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet. 2006;367(9522):1618–1625. | ||
Alam A, Gomes T, Zheng H, Mamdani MM, Juurlink DN, Bell CM. Long-term analgesic use after low-risk surgery: a retrospective cohort study. Arch Intern Med. 2012;172(5):425–430. | ||
Practice guidelines for acute pain management in the perioperative setting: an updated report by the American Society of Anesthesiologists Task Force on Acute Pain Management. Anesthesiology. 2012;116(2):248–273. | ||
Fletcher D, Fermanian C, Mardaye A, Aegerter P. Pain and Regional Anesthesia Committee of the French Anesthesia Intensive Care Society (SFAR). A patient-based national survey on postoperative pain management in France reveals significant achievements and persistent challenges. Pain. 2008;137(2):441–451. | ||
Marret E, Kurdi O, Zufferey P, Bonnet F. Effects of nonsteroidal antiinflammatory drugs on patient-controlled analgesia morphine side effects: meta-analysis of randomized controlled trials. Anesthesiology. 2005;102(6):1249–1260. | ||
Somogyi AA, Barratt DT, Coller JK. Pharmacogenetics of opioids. Clin Pharmacol Ther. 2007;81(3):429–444. | ||
Overholser BR, Foster DR. Opioid pharmacokinetic drug-drug interactions. Am J Manag Care. 2011;17(Suppl 11):S276–S287. | ||
Sadhasivam S, Chidambaran V. Pharmacogenomics of opioids and perioperative pain management. Pharmacogenomics. 2012;13(15):1719–1740. | ||
Meissner K, Kharasch ED. Pain and anesthesia. In: Altman R, Altman RB, Flockhart D, Goldstein DB, editors. Principles of Pharmacogenetics and Pharmacogenomics. Cambridge, UK: Cambridge University Press; 2012. | ||
Bohn LM, Dykstra LA, Lefkowitz RJ, Caron MG, Barak LS. Relative opioid efficacy is determined by the complements of the G protein-coupled receptor desensitization machinery. Mol Pharmacol. 2004;66(1):106–112. | ||
DeWire SM, Ahn S, Lefkowitz RJ, Shenoy SK. Beta-arrestins and cell signaling. Annu Rev Physiol. 2007;69:483–510. | ||
Raehal KM, Bohn LM. Mu opioid receptor regulation and opiate responsiveness. AAPS J. 2005;7(3):E587–E591. | ||
Bohn LM, Lefkowitz RJ, Gainetdinov RR, Peppel K, Caron MG, Lin FT. Enhanced morphine analgesia in mice lacking beta-arrestin 2. Science. 1999;286(5449):2495–2498. | ||
Raehal KM, Walker JK, Bohn LM. Morphine side effects in beta-arrestin 2 knockout mice. J Pharmacol Exp Ther. 2005;314(3):1195–1201. | ||
Violin JD, Crombie AL, Soergel DG, Lark MW. Biased ligands at G-protein-coupled receptors: promise and progress. Trends Pharmacol Sci. 2014;35(7):308–316. | ||
Smith HS. Opioid metabolism. Mayo Clin Proc. 2009;84(7):613–624. | ||
Hughes MM, Atayee RS, Best BM, Pesce AJ. Observations on the metabolism of morphine to hydromorphone in pain patients. J Anal Toxicol. 2012;36(4):250–256. | ||
Klimas R, Mikus G. Morphine-6-glucuronide is responsible for the analgesic effect after morphine administration: a quantitative review of morphine, morphine-6-glucuronide, and morphine-3-glucuronide. Br J Anaesth. 2014;113(6):935–944. | ||
Chen XT, Pitis P, Liu G, et al. Structure-activity relationships and discovery of a G protein biased mu opioid receptor ligand, [(3-methoxythiophen-2-yl)methyl]({2-[(9R)-9-(pyridin-2-yl)-6-oxaspiro-[4.5]decan- 9-yl]ethyl})amine (TRV130), for the treatment of acute severe pain. J Med Chem. 2013;56(20):8019–8031. | ||
DeWire SM, Yamashita DS, Rominger DH, et al. A G protein-biased ligand at the mu-opioid receptor is potently analgesic with reduced gastrointestinal and respiratory dysfunction compared with morphine. J Pharmacol Exp Ther. 2013;344(3):708–717. | ||
Soergel DG, Subach RA, Burnham N, et al. Biased agonism of the mu-opioid receptor by TRV130 increases analgesia and reduces on-target adverse effects versus morphine: a randomized, double-blind, placebo-controlled, crossover study in healthy volunteers. Pain. 2014;155(9):1829–1835. | ||
Viscusi ER, Webster L, Kuss M, et al. A randomized, phase 2 study investigating TRV130, a biased ligand of the mu-opioid receptor, for the intravenous treatment of acute pain. Pain. 2016;157(1):264–272. | ||
International Conference of Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. ICH Harmonised Tripartite Guideline: Guideline for Good Clinical Practice E6(R1), Vol. 2015. Geneva, Switzerland: International Conference on Harmonisation, 1996. | ||
World Medical Association General Assembly. World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects, Vol. 2015. Ferney-Voltaire, France: World Medical Association, Inc.; 2008. | ||
Pergolizzi J, Böger RH, Budd K, et al. Opioids and the management of chronic severe pain in the elderly: consensus statement of an International Expert Panel with focus on the six clinically most often used World Health Organization Step III opioids (buprenorphine, fentanyl, hydromorphone, methadone, morphine, oxycodone). Pain Pract. 2008;8(4):287–313. | ||
Minkowitz HS, Gruschkus SK, Shah M, Raju A. Adverse drug events among patients receiving postsurgical opioids in a large health system: risk factors and outcomes. Am J Health Syst Pharm. 2014;71(18):1556–1565. | ||
Boccara G, Mann C, Pouzeratte Y, Bellavoir A, Rouvier A, Colson P. Improved postoperative analgesia with isoflurane than with propofol anaesthesia. Can J Anaesth. 1998;45(9):839–842. | ||
Mentz HA, Ruiz-Razura A, Newall G, Patronella CK. Use of a regional infusion pump to control postoperative pain after an abdominoplasty. Aesthetic Plast Surg. 2005;29(5):415–421; discussion 422. | ||
Sun T, Sacan O, White PF, Coleman J, Rohrich RJ, Kenkel JM. Perioperative versus postoperative celecoxib on patient outcomes after major plastic surgery procedures. Anesth Analg. 2008;106(3):950–958. | ||
Edwards MC, Sorokin E, Brzezienski M, et al. Impact of liposome bupivacaine on the adequacy of pain management and patient experiences following aesthetic surgery: Results from an observational study. Plast Surg (Oakv). 2015;23(1):15–20. |
Supplementary materials
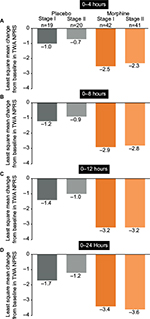
In stage I, 19 and 42 patients were treated with placebo and the morphine regimen, respectively. In stage II, 20 and 41 patients were treated with placebo and the morphine regimen, respectively. Analyses were performed to test whether there were statistically significant differences in efficacy (time-weighted average change in numeric pain rating scale over 24 hours from baseline) between stage I and stage II in each treatment group.
In comparisons over 0–4, 0–8, 0–12, and 0–24 hours, no statistically significant differences were seen in the placebo treatment groups between stage I and II (Figure S2A–D). Similarly, over 0–4, 0–8, 0–12, and 0–24 hours, the difference in the morphine treatment groups between stage I and II did not reach statistical significance (Figure S2A–D). Because of the similarity in efficacy demonstrated in each treatment group between stage I and II, the respective groups were combined for the primary efficacy and safety analyses to provide greater sample size and more robust evaluation versus oliceridine.
  | Figure S1 Patient disposition. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.