Back to Journals » Journal of Pain Research » Volume 16
A Pragmatic Randomized Controlled Trial on the Effectiveness and Safety of Pharmacopuncture for Chronic Lower Back Pain
Authors Park KS, Kim C, Kim JW, Kim S, Lee JY , Lee YJ , Lee J, Kim MJ, Choi YE, Yang C , Han CH , Ha IH
Received 14 April 2023
Accepted for publication 21 July 2023
Published 3 August 2023 Volume 2023:16 Pages 2697—2712
DOI https://doi.org/10.2147/JPR.S413512
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Houman Danesh
Kyoung Sun Park,1 Changnyun Kim,2 Joo Won Kim,3 Sang‐Don Kim,4 Jee Young Lee,5 Yoon Jae Lee,5 Jinho Lee,1 Min Ji Kim,6 Young Eun Choi,6 Changsop Yang,7 Chang-Hyun Han,7,8,* In-Hyuk Ha5,*
1Jaseng Hospital of Korean Medicine, Seoul, Republic of Korea; 2Daejeon Jaseng Hospital of Korean Medicine, Daejeon, Republic of Korea; 3Bucheon Jaseng Hospital of Korean Medicine, Bucheon, Republic of Korea; 4Haeundae Jaseng Hospital of Korean Medicine, Busan, Republic of Korea; 5Jaseng Spine and Joint Research Institute, Jaseng Medical Foundation, Seoul, Republic of Korea; 6Clinical Research Coordinating Team, Korea Institute of Oriental Medicine, Daejeon, Republic of Korea; 7Clinical Medicine Division, Korea Institute of Oriental Medicine, Daejeon, Republic of Korea; 8Korean Convergence Medical Science, Korea Institute of Oriental Medicine School, University of Science & Technology, Daejeon, Republic of Korea
*These authors contributed equally to this work
Correspondence: Chang-Hyun Han, Clinical Medicine Division, Korea Institute of Oriental Medicine, Daejeon, Republic of Korea, Tel +82 42 868 9498, Email [email protected] In-Hyuk Ha, Jaseng Spine and Joint Research Institute, Jaseng Medical Foundation, Seoul, Republic of Korea, Tel +82 2 2222 2740, Email [email protected]
Purpose: Chronic lower back pain (LBP) is a major global health concern. Pharmacopuncture has been widely used to treat LBP in Korea; however, randomized clinical trials (RCT) or active control have not been conducted to evaluate its effectiveness. Therefore, this RCT aimed to compare the effectiveness of pharmacopuncture and physical therapy (PT) for the treatment of chronic LBP.
Patients and Methods: A two-arm, parallel, and multicenter RCT was conducted at four hospitals of Korean medicine. Participants with chronic LBP were randomly assigned at a 1:1 ratio using block randomization to undergo 10 sessions of pharmacopuncture or PT over 5 weeks and followed up for 25 weeks. The numerical rating scale (NRS) and visual analog scale scores of LBP and radiating leg pain and the Oswestry disability index (ODI), 5-level EuroQol-5 dimension (EQ-5D-5L), and the patient global impression of change were recorded at baseline and at 6, 13, and 25 weeks. An intention-to-treat analysis was conducted as the primary analysis using a linear mixed model.
Results: One-hundred patients (mean age, 49.27 years; 58 women) were recruited. At 6 weeks after randomization, pharmacopuncture showed statistically superior results compared with PT in LBP (difference in NRS, 1.54; 95% CI, 0.94– 2.13), function (difference in ODI, 4.52%; 95% CI, 0.93– 8.11%), and quality of life (difference in EQ-5D-5L) scores (− 0.05; 95% CI, − 0.08 to − 0.01). This effect persisted for 25 weeks. In the survival analysis for participants with at least a 50% reduction in the NRS scores of LBP during the 182-day follow-up, the pharmacopuncture group showed significantly faster recovery than the PT group (P< 0.001, Log rank test).
Conclusion: Pharmacopuncture significantly reduced pain and improved functional outcomes and quality of life in patients with low back pain compared with physical therapy. Based on the findings of this study, pharmacopuncture could be recommended as a treatment for patients with chronic low back pain.
Keywords: low back pain, pharmacopuncture, physiotherapy, Oswestry disability index, Roland–Morris disability questionnaire, pragmatic randomized controlled trial
Plain Language Summary
Pharmacopuncture was more effective than physical therapy at reducing pain and improving lower back function and quality of life in patients with chronic lower back pain.
Introduction
Lower back pain (LBP) is a growing major global health concern. According to the 2016 Global Burden of Disease Study, LBP is a leading cause of disability worldwide.1 Acute LBP has a favourable prognosis, with pain alleviation being observed within six weeks in most cases; however, the rate of recovery declines beyond this period. Although the severity of pain and disability may not be high, progression to chronic LBP occurs in approximately 40% of cases.2,3 Moreover, chronic LBP is associated with a high recurrence rate, with a 1-year recurrence rate of 20–44% and a lifetime recurrence rate of 85%.4
Pharmacological treatment is only recommended for patients with chronic back pain if nonpharmacological therapy is ineffective, while nonpharmacological therapies, such as exercise, multidisciplinary rehabilitation, and acupuncture, are strongly recommended in most cases.5 Patients with chronic LBP have concerns, such as frequent recurrence and chronic productivity loss,4,6 which increases the demand for more effective treatment options. A growing interest exists involving complementary and alternative medical treatments.7
Pharmacopuncture is a pharmacological therapy that combines acupuncture and herbal medicine, including the administration of herbal medicine extracts, with acupoints.8 Acupuncture alters the abnormal default mode network of the brain in patients with chronic LBP, which is correlated with pain.9 It also exerts anti-inflammatory and analgesic effects via neuroendocrine-immune regulation.10 Pharmacopuncture has the advantage of simultaneously stimulating the acupuncture points and extending the therapeutic window for absorption of the pharmacopuncture solution over acupuncture.8 Pharmacopuncture is widely used in Korea to treat spinal diseases, including back pain. The frequency and specific details of pharmacopuncture usage in 12 Korean medicine (KM) hospitals and clinics were investigated in 2016. The study found that 33,145 patients received inpatient care and 373,755 patients received outpatient care at KM hospitals and clinics over a 4-year period. Among these patients, 32,947 inpatients (98.6%) and 289,860 outpatients (77.6%) received pharmacopuncture.11 In addition, a 2015 survey of KM doctors at KM institutions who specialized in musculoskeletal disorders reported that 95.9% of KM doctors used pharmacopuncture in patients with lumbar herniated intervertebral disc (LHID).12
Randomized controlled trials (RCTs) comparing bee venom pharmacopuncture have been conducted for chronic back pain; however, comparisons were made with sham in these studies.13,14 Pragmatic RCTs must be conducted to confirm whether pharmacopuncture can be a new treatment option for chronic back pain compared with active control. Therefore, this study aimed to compare the effects of pharmacopuncture, including various clinically used solutions, with those of physical therapy (PT). Consequently, the purpose of this pragmatic RCT was to compare the effectiveness of pharmacopuncture with that of PT in the treatment of chronic LBP. We hypothesized that pharmacopuncture is superior to PT for the treatment of LBP.
Materials and Methods
Study Design
This two-armed, parallel, multicenter RCT competitively recruited 100 patients from four Jaseng Hospitals of KM (Seoul, Daejeon, Bucheon, and Haeundae). Prior to participant recruitment, the Institutional Review Board (IRB) authorized the study protocol (JASENG 2021-02-012, JASENG 2021-02-013, JASENG 2021-02-014, and JASENG 2021-02-032). Clinicaltrials.gov (NCT04833309) and Clinical Research Information Service (CRIS) were notified of the study protocol (KCT0006088).
Participant Schedule
The participants filled out an informed consent form (ICF) during the first visit after being informed about the purpose of the study by the investigator. Subsequently, screening was conducted by the investigator, and participants were enrolled based on the inclusion/exclusion criteria. The participants were scheduled to attend fifteen sessions, including ten treatment sessions (twice per week for 5 weeks). Follow-up sessions were conducted at 6, 13, and 25 weeks post-baseline in person or via telephone. The participants received pharmacopuncture or PT starting from the second visit, depending on their random assignment. The time schedule for participant enrolment, intervention, and assessment is presented in Supplementary Table 1 and Supplementary Figure 1.
Inclusion and Exclusion Criteria
Inclusion Criteria
- Presence of LBP for at least 6 months.
- Numerical Rating Scale (NRS) score of ≥ 5 for LBP.
- Age of 19–70 years.
- Consent for participation with a signed ICF.
Exclusion Criteria
- Diagnosis of a severe specific disorder that may cause LBP (eg, tumor metastasis to the spine, acute fracture, or spinal dislocation).
- Presence of progressive neurological deficit or serious neurological symptoms.
- Pain attributable to a soft tissue disorder, but not spine disorders (eg, tumour, fibromyalgia, rheumatoid arthritis, or gout).
- Chronic disorders that may interfere with the therapeutic effect or interpretation of results (eg, stroke, myocardial infarction, kidney disease, diabetic neuropathy, dementia, or epilepsy).
- Ongoing intake of medications, such as steroids, immunosuppressants, psychiatric drugs, or other drugs, that may influence the research results.
- Conditions in which pharmacopuncture may not be appropriate or safe, including bleeding disorders, ongoing anticoagulant therapy, and severe diabetes that may lead to an increased risk of infection.
- History of intake of medications, such as non-steroidal anti-inflammatory drugs or pharmacopuncture within the past week that may affect the pain levels.
- Pregnancy, planning for pregnancy, or breastfeeding.
- History of lumbar spine surgery within the past 3 months.
- Completion of participation in another clinical trial within the past month or planned participation in another clinical trial within 6 months of enrolment in the present clinical trial or during the follow-up period.
- Difficulty in completing the ICF.
- Other reasons, as determined by the investigator.
Interventions
Experimental Group: Pharmacopuncture
Pharmacopuncture was performed twice a week for 5 weeks (10 sessions) in this group. Based on a previous study that analysed the use of pharmacopuncture through electronic medical records (EMR) from 12 KM institutions11, pharmacopuncture was performed according to the clinical judgment of the KM doctor. The physician selected the pharmacopuncture solutions, acupoints, and amount depending on patient’s symptoms, severity, and area of pain. All procedure-related details were documented in the EMR. The pharmacopuncture methods used were reviewed retrospectively and recorded in a case report form (CRF) for analysis.
Control Group: PT
According to a review that used the Korean Health Insurance Review and Assessment Service (HIRA) National Patient Sample (NPS) dataset, various combinations of deep heat therapy, superficial heat therapy, transcutaneous electrical nerve stimulation, and intermittent pelvic traction therapy are used to manage LBP in clinical practice in Korea.15 These findings indicate that the physician selected the PT technique, treatment site, and treatment length based on patient symptoms, radiological findings, and level of improvement. PT was performed twice per week for 5 weeks (10 sessions). Details regarding the type of PT used and its length and site were recorded in the EMR. The PT methods were analysed retrospectively and recorded in the CRF.
Criteria for Discontinuation and Dropout
Participants were withdrawn from the study in the following cases:
- The participant was diagnosed with a disease that had not been detected during the examinations conducted prior to the clinical trial, which might affect the determination of the research results.
- The participant or their authorized representative requested cessation of participation, or the participant revoked his/her consent.
- The participant was confirmed to be pregnant during the study.
- Performing medical or KM procedures for LBP posed a problem for the participant.
- Other reasons, as determined by the investigator.
Concomitant Treatment
In the event of severe pain during intervention or follow-up, the use of medications and treatment at alternative medical facilities was permitted. The content and frequency of such treatments were documented in the CRF and analysed.
Outcomes
Primary Outcome (NRS Score of LBP)
NRS was utilized to evaluate the severity of LBP over the past week. The participants rated their level of pain on a scale ranging from 0 to 10 (0 = no pain; 10 = worst pain imaginable).
Secondary Outcomes
NRS Score of Radiating Leg Pain
The intensity of radiating leg pain in the previous week was assessed using NRS, with the participants indicating a number from 0 to 10 that best described their pain level (0 = no pain; 10 = worst pain imaginable).
Visual Analog Scale of LBP and Radiating Leg Pain
The visual analog scale (VAS), a method used to assess pain levels among patients, consists of a 100-mm line anchored at each end with “no pain” or “worst pain imaginable”. The participants marked a point on the line to indicate the LBP and radiating leg pain levels they experienced in the past week.
Oswestry Disability Index
The functional status of the participants was assessed using the Oswestry disability index (ODI) questionnaire, which is a 10-item questionnaire developed to assess the level of disability caused by LBP. Each item was rated on a 6-point scale (0–5 points), with higher scores indicating a more severe disability. The official Korean version of the ODI questionnaire was used in the present study, which has been shown to be reliable and valid.16
Korean Version of the Roland–Morris Disability Questionnaire
The Korean version of the Roland–Morris disability questionnaire (RMDQ) is a tool often used to assess the functional status associated with LBP. It comprises 24 items that help identify the limitations in normal activities caused by LBP. The official Korean version of RMDQ was used in the present study, which has been shown to be reliable and valid.17
Patient Global Impression of Change
The Patient Global Impression of Change (PGIC) is a 7-point scale used to subjectively assess the level of improvement perceived by the patient with each score indicating the following: 1, significant improvement; 2, marked improvement; 3, minimal improvement; 4, no change; 5, minimal worsening; 6, marked worsening; and 7, significant worsening.18
Short-Form-12 Health Survey Version 2
The short-form-12 health survey (SF-12) is a questionnaire designed to assess health-related QoL based on 12 items in eight domains: physical functioning, role-physical, bodily pain, general health, vitality, social functioning, role-emotional, and mental health. It typically requires less than 5 minutes to complete, with higher scores indicating better health-related QoL. This study utilized the Korean version of SF-12, whose reliability and validity have been demonstrated.19
Five-Level EuroQol-5 Dimension
The 5-level EuroQol-5 dimension (EQ-5D-5L) is one of the most widely used methods to indirectly assess certain health states related to the QoL using a pre-assigned preference score for each functional level after a multidimensional investigation of these health states. The EQ-5D-5L comprises five questions related to mobility, self-care, usual activities, pain, and anxiety/depression. Weights are assigned to each item, and these weights and constant numbers are used to calculate the preference score.20 The Korean-adapted version was used in the present study, which has been shown to be reliable and valid.20
Credibility and Expectancy Scale (CES)
The treatment expectancy of the participants was assessed using a 9-point Likert scale. During the enrolment visit, the participants were instructed to choose a score corresponding to the question, “to what extent do you think pharmacopuncture and PT can reduce your symptoms?” (1 = not at all, 5 = somewhat, and 9 = very much).
Drug Consumption
The types and doses of rescue medications consumed for LBP during the study period were investigated using a questionnaire survey during each visit. For PT or injection therapies, the frequency of such treatments was recorded, in addition to the drugs consumed.
AEs
For safety assessment, the following clinical pathology tests were performed at the start and end of the treatment period: complete blood count, liver function tests (aspartate transaminase, alanine transaminase, and alkaline phosphatase), blood urea nitrogen, creatinine, erythrocyte sedimentation rate, and C-reactive protein.
AEs were recorded based on the symptoms reported by the patients and observed by the investigators. The frequency of suspected AEs, abnormal laboratory values, and serious AEs associated with the intervention were analysed. The causality of AEs related to the intervention was assessed on a 6-point scale based on the WHO-Uppsala Monitoring Centre causality assessment system (1 = definitely related, 2 = probably related, 3 = possibly related, 4 = probably not related, 5 = definitely not related, and 6 = unknown). Spilker’s classification categorizes the severity of all adverse events into three levels: mild (1): No treatment is required, and the AE does not significantly impair the participant’s normal life (function); moderate (2): AE significantly impairs the participant’s normal life (function), may necessitate treatment, and can recover after treatment; and severe (3): AE necessitates advanced treatment and may have aftereffects.
Sample Size Calculation
A pilot study comparing the effectiveness of pharmacopuncture and PT in patients with chronic LBP was conducted prior to the present study. The pilot study revealed that at the primary endpoint (immediately after 5 weeks of intervention), the mean difference in the NRS score of LBP between the PTT and PT groups was 1.37±1.94 points, and Cohen’s d was 0.71. The correlation between baseline and endpoint was 0.53 21, and based on the assumption of a 95% significance level and 90% power, the minimum sample size required was 64. Assuming a dropout rate of 30% and considering that participants were being recruited from four institutions, a total of 100 participants were recruited.
Recruitment
The participants in this clinical trial were recruited through press releases about the trial, promotional posters posted inside the participating research centres, and recruitment announcements posted on the internet.
Randomization and Allocation Concealment
The randomization table generated by a statistician using R studio 1.1.463 (© 2009–2018 RStudio, Inc.) was used to allocate the same number of participants to the two groups (n=50 each). A random sequence was generated using block randomization, with the size of each block set at random between 2 and 4. A third party unrelated to the study sealed the generated randomization results in an opaque envelope and placed them in a double-locked locker. The person in charge at each participating centre opened the randomization envelope to assign each participant to the group.
Blinding
Due to the evident differences between the interventions performed in the two groups in the present study, double-blinding was impossible, and only the assessor was blinded. Prior to the intervention, an assessment was conducted in a distinct area by a professional resident who was blinded to group assignment.
Data Collection and Management
The present study used an electronic CRF (e-CRF) operated by the Korea Disease Control and Prevention Agency. The investigator of the lead research centre provided the standard operating procedure (SOP) for completing the CRF and data entry to train the assessors and investigators at each institution. Queries were generated for a range check for data values, while data entered into the e-CRF were locked after cleaning. Data access was restricted to all investigators except the data manager.
Statistical Methods
ITT and per-protocol (PP) analyses were conducted in this study, with ITT analysis serving as the primary analysis. The participants who received at least seven treatment sessions during the 5-week intervention period were analysed separately for the PP analysis.
The current study’s effectiveness endpoint was the difference between the two groups in the change in continuous outcomes (NRS, VAS, NDI, NPQ, EQ-5D-5L, and SF-12 scores) at multiple points in time relative to the baseline. For the primary analysis, a linear mixed model was implemented with the baseline value of each variable and covariant factors, with statistically significant baseline differences between the treatment groups set as covariant factors. The assigned group was fixed as a factor. The analysis of covariance (ANCOVA) was then conducted on the multiple imputation (MI) and the last observation carried forward (LOCF) data sets.
To compare the total difference in each outcome within the period (the intervention period and the entire study period) for both groups, the area under the curve (AUC) was calculated for each time point since randomization, and Student’s t-test was performed for comparison. In addition, a comparative analysis was performed for participants with at least a 50% reduction in pain indicators (NRS and VAS scores) at each time point relative to the baseline. Kaplan–Meier survival analysis was used to compare the time required to achieve recovery from LBP, which was indicated by at least a 50% reduction in pain indicators since randomization, and the curves were compared using the Log rank test. The Cox proportional hazards model was used to compare hazard ratios (HRs).
Missing data for the linear mixed model were handled using a mixed model for repeated measures (MMRM). For AUC and ANCOVA, missing data were processed using MI. The outcomes of the survival analysis were analysed after incomplete values were censored. During the sensitivity analysis, missing values were replaced with the LOCF, and the normality of the data distribution was examined subsequently. The Wilcoxon rank sum test was utilized for the primary analysis of nonparametric distributions.
For each group, sociodemographic characteristics and treatment expectations of the participants were evaluated. Depending on the distribution, continuous variables are expressed as mean and standard deviation (SD) or median and quartile, and differences between the groups are analysed using Student’s t-test or Wilcoxon rank sum test. The chi-square test or Fisher’s exact test was utilized to examine categorical variables expressed as frequency and percentage (%).
All statistical analyses were performed using SAS (version 9.4; SAS Institute, Inc., Cary, NC, USA) or R studio 1.1.463 (2009–2018 RStudio, Inc.). Statistical significance was set at P<0.05.
Data Monitoring
The clinical trial monitor supervised the clinical trial process and regularly reviewed and verified that the trial was conducted and recorded in accordance with the protocol, SOP, Good Clinical Practice, and relevant guidelines. The clinical trial was monitored through internal monitoring by the research centre and the supporting institutions through regular visits by the investigators or via telephone. During the visits, the monitor checked the original records of the participants and data storage. In addition, the monitor closely observed the clinical trial process and discussed any problems with investigators.
Results
Participant Flow
A total of 245 patients were screened between April and September 2021, among whom 100 candidates were enrolled and randomly assigned to the pharmacopuncture and PT groups (n=50 each). One participant in the PT group withdrew consent during the intervention period. Thus, 99 patients were followed up for 25 weeks, except for one patient who withdrew consent. ITT analysis was performed using data from 50 participants each in the pharmacopuncture and PT groups, whereas PP analysis was performed using data from 50 participants in the pharmacopuncture group and 48 participants in the PT group who had received at least seven sessions of treatment during the 5-week intervention period (Figure 1).
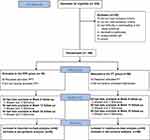 |
Figure 1 Flow chart of the study. Abbreviations: PPT, pharmacopuncture therapy; PT, physical therapy. |
Baseline Characteristics
No significant differences were observed in the baseline characteristics, except for age, between the pharmacopuncture and PT groups (n=50 each). The mean participant age in the pharmacopuncture and PT groups was 51.60 ± 10.92 and 46.94 ± 12.44 years, respectively (P = 0.049; Table 1). Thirty (60.0%) and 28 (56.0%) participants were females in the pharmacopuncture and PT groups, respectively. The participants had chronic LBP for 29.62 ±43.8 months since the onset of LBP in the pharmacopuncture group and 43.42±75.81 months in the PT group (P=0.269). No significant difference was observed in the NRS score of LBP at baseline (6.42±0.93 in the pharmacopuncture group and 6.30±0.84 in the PT group).
 |
Table 1 Baseline Characteristics of the Patients |
Treatment
Forty-four patients (88%) received all 10 treatment sessions in the pharmacopuncture group, whereas 37 (74%) received all 10 treatment sessions in the PT group. Fifty (100%) patients in the pharmacopuncture group and 46 (92%) patients in the PT group received more than eight treatment sessions (Supplementary Table 2).
The most frequently used treatments in the pharmacopuncture group, in decreasing order, were Shinbaro 2, hwangryunhaedok-tang, Shinbaro 1, harpagophytum procumbens, hominis placenta, jungsongouhyul, and muscle relaxation, with an average of 1.92±0.75 types of pharmacopuncture used during each visit. The total dose of pharmacopuncture, depending on the type of pharmacopuncture, ranged from 1.00–1.71 mL (Supplementary Table 3). Pharmacopuncture was performed at the acupoints of the lower back such as BL23, GV40, BL52, BL25, EX-B2, BL26, GV3, and CV2. However, it was also performed on the acupoints of the hip, such as GB30, lower extremities, such as BL58, and upper extremities, such as PC6 and TE5 (Supplementary Table 4).
The most frequently used treatments in the PT group, in decreasing order, were interferential current therapy, deep heat therapy, superficial heat therapy, laser therapy, transcutaneous electrical nerve stimulation, and extracorporeal shock wave therapy, which is consistent with the frequently used PT described by the 2014 Korean HIRA NPS data.15 In the PT group, an average of 2.20±0.51 types of PT were used during each visit (Supplementary Table 2). PT was mainly performed on the lower back and hip, and it was performed for 5–15 minutes depending on the type of PT (Supplementary Table 2).
Primary and Secondary Outcomes
The analyses at different time points using a linear mixed model by MMRM revealed that pharmacopuncture had statistically significantly superior effectiveness at the primary endpoint (week 6) as measured by the NRS score of LBP, NRS score of radiating leg pain, VAS score of LBP, VAS score of radiating leg pain, ODI, RMDQ, EQ-5D, and PGIC scores (difference in LBP NRS score, 1.54; 95% CI, 0.94–2.13; RP NRS score, 1.01; 95% CI, 0.38–1.63; LBP VAS score, 16.96; 95% CI, 10.96–2.96; RP VAS score, 11.37; 95% CI, 5.23–17.50; ODI, 4.52; 95% CI, 0.93–8.11, RMDQ, 1.51; 95% CI, 0.42–2.60, EQ-5D, −0.05; 95% CI −0.08 to −0.01) and PGIC, 0.97; 95% CI 0.60–1.34).
At the 13-week follow-up, there were statistically significant differences in the NRS score of LBP, NRS score of radiating leg pain, VAS score of LBP, VAS score of radiating leg pain, ODI, and PGIC scores between the two groups (difference in LBP NRS score, 1.35; 95% CI, 0.76–1.94; RP NRS score, 0.74; 95% CI, 0.11–1.37; LBP VAS score, 15.03; 95% CI, 8.78–21.29; RP VAS score, 8.47; 95% CI, 2.13–14.81; ODI, 4.36; 95% CI, 0.77–7.95, and PGIC, 0.65; 95% CI 0.28–1.02).
Moreover, the pharmacopuncture and PT groups also showed statistically significant differences in the NRS score of LBP, NRS score of radiating leg pain, VAS score of LBP, ODI, RMDQ, EQ-5D, and PGIC scores at the 25-week follow-up (difference in LBP NRS score, 1.63; 95% CI, 1.04–2.23; RP NRS score, 0.96; 95% CI, 0.33–1.59; LBP VAS score, 16.97; 95% CI, 10.24–23.71; ODI, 5.71; 95% CI, 2.12–9.30, EQ-5D, −0.04; 95% CI −0.08 to −0.01 and PGIC, 0.55; 95% CI 0.18–0.92) (Table 2, Figure 2). In addition, the pharmacopuncture group also showed more favorable outcomes in the ANCOVA analysis set with missing values substituted by MI and LOCF (Supplementary Tables 5 and 6, respectively) and the PP analysis set for patients who received more than seven treatment sessions over 5 weeks (Supplementary Table 7). In particular, the NRS and VAS scores of LBP showed significant differences in the effectiveness maintained at 25 weeks between both groups, regardless of the analysis method.
 |
Table 2 Intention-to-Treat Analysis Using Mixed Model Repeated Measures |
On analyzing the 25-week cumulative values of each outcome using AUC, the pharmacopuncture group showed statistically significantly superior effectiveness as measured by the NRS score of LBP, NRS score of radiating leg pain, VAS score of LBP, VAS score of radiating leg pain, ODI, and EQ-5D scores compared with those of the PT group (AUC difference in LBP NRS, −31.29; 95% CI, −45.20 to −17.39; RP NRS score, −20.19; 95% CI, −34.81 to −5.57; LBP VAS score, −358.45; 95% CI, −500.08 to −216.82; RP VAS score, −222.75; 95% CI, −368.70 to −76.81; ODI, −85.80; 95% CI, −145.54 to −26.06, and EQ5D, 0.66; 95% CI 0.06–1.27) (Table 3).
 |
Table 3 AUCs of Outcomes According to the Treatment |
Survival Analysis
In the survival analysis of participants with at least a 50% reduction in the NRS score of LBP during the 182 days of observation, the median time to recovery was 28 (95% CI: 22–38) and 171 (95% CI: 87–NA) days from randomization in the pharmacopuncture and PT groups, respectively. The Log rank test results showed significantly faster recovery in the pharmacopuncture group than that in the PT group (P<0.001). The HR for participants with at least a 50% reduction in the NRS score of LBP during the 182 days of observation was 2.62 (95% CI: 1.56–4.40), which was more favorable than that in the pharmacopuncture group (P<0.001; Figure 3).
Subgroup Analysis
In the subgroup analysis on the effects of sex, age, body mass index (BMI), severity of symptoms, baseline NRS score of LBP, baseline NRS score of radiating leg pain, ODI, RMDQ, EQ-5D-5L, SF-12 (physical component score), SF-12 (mental component score), and current work status on the improvement of NRS score of LBP, the participants with a baseline NRS score of ≥4 for radiating leg pain showed greater improvement in the NRS score of LBP than their counterparts. However, other factors had no significant effects on pain reduction (Supplementary Figure 1).
AEs
A case of intervention-related AE was reported in the pharmacopuncture group. One participant in the pharmacopuncture group experienced localized itching at the treatment site. The participant was prescribed one tablet (Cetirizine) per day for 6 days until the symptoms disappeared. The severity of this AE was determined to be moderate as only the administration of medications was required to relieve the symptoms. There was no reported case of intervention-related severe adverse events (SAE).
Discussion
An RCT conducted on patients with LBP revealed that acupuncture treatment combined with Calculus Bovis, Fel Ursi, and Moschus pharmacopuncture as add-ons was significantly more effective than acupuncture alone.22 In addition, another RCT on the efficacy of bee venom pharmacopuncture for LBP reported that bee venom pharmacopuncture was significantly superior in reducing pain and improving function than sham bee venom pharmacopuncture.13,14 Moreover, a three-arm RCT on the effectiveness of pharmacopuncture for LHID revealed that jungsongouhyul pharmacopuncture had superior therapeutic efficacy compared with those of acupuncture or bee venom pharmacopuncture in the early stage of the treatment; however, bee venom pharmacopuncture demonstrated the most superior efficacy over time.23 Furthermore, the group that received integrative KM treatment with soyem pharmacopuncture as an add-on for LHID showed significantly lower VAS scores of LBP than the controls24, whereas an RCT on integrative KM treatment with add-on Shinbaro pharmacopuncture also showed that combination therapy had greater efficacy than control treatment.17 Previous RCTs have used placebo or add-on treatments; however, no pragmatic RCTs with a wide range of interventions reflecting actual clinical settings have been reported.
In the present study, compared with PT, pharmacopuncture significantly improved pain indicators, functional outcomes, and QoL after 5 weeks of treatment, and this effect persisted for up to 25 weeks. In the survival analysis of the participants with at least a 50% reduction in the NRS score of LBP during the 182 days of observation, the pharmacopuncture group showed significantly faster recovery than the PT group. On analysing the 25-week cumulative values of each outcome using AUC, the pharmacopuncture group showed significantly superior effectiveness as indicated by the NRS score of LBP, NRS score of radiating leg pain, VAS score of LBP, VAS score of radiating leg pain, ODI, and EQ-5D scores compared with those of the PT group. Thus, pharmacopuncture enabled faster recovery from LBP than standard treatment, and its therapeutic effect was sustained for up to 25 weeks.
The minimal clinically important difference (MCID) in the NRS score of LBP has been reported to be 1.0–2.0 25. In the present study, the mean difference in the pain NRS score at the primary endpoint (6 weeks) was approximately 1.54, indicating an improvement exceeding the MCID. This finding suggests that pharmacopuncture has statistically significant effects on LBP and induces clinically significant differences in pain NRS compared with standard treatment. However, the MCID of RMDQ is 4–5, and that of ODI is 8–17,26 and this study did not show an effect beyond this MCID.
The primary mechanism of action of pharmacopuncture may involve the interaction between the mechanical characteristics of acupoint stimulation and the chemical stimulation of the injected solution.8 Acupuncture exerts anti-inflammatory and analytical actions via a neuroendocrine-immune regulating mechanism, thereby improving an abnormal brain default mode network correlated with pain in patients with chronic low back pain.9,10 Pharmacopuncture involves the simultaneous application of physical (eg, irrigation and hydrodissection) and chemical stimulation of the pharmacological action of the pharmacopuncture solution.8 In decreasing order, the most frequently used types of pharmacopuncture in this study included Shinbaro 2, hwangryunhaedok-tang, Shinbaro 1, harpagophytum procumbens, hominis placenta, jungsongouhyul, and muscle relaxation. This finding is consistent with the results of a questionnaire survey conducted by clinicians in Korea who treated lumbar spinal stenosis27 and cervical disc herniation28 in spine-specialty hospitals. Shinbaro 2, which comprises nine herbal medicinal ingredients is used to relieve pain or treat inflammation in musculoskeletal disorders.29 Shinbaro 1 (GCSB-5) comprises six herbal medicinal ingredients, of which five ingredients are also included in Shinbaro 2. GCSB-5 is frequently used to treat neuropathy and inflammation, including arthritis.30 In addition, harpagophytum procumbens has been reported to be effective in alleviating major clinical symptoms caused by osteoarthritis31 and acute LBP32. Harpagophytum procumbens functions by inducing the migration of interleukins and leukocytes to the site of pain and inflamed joints. Interestingly, it has been found to be effective for the treatment of inflammatory diseases, due to its anti-inflammatory and peripheral analgesic properties,33–35 and spinal stenosis, a severe spinal disorder.36
A retrospective review on the safety of pharmacopuncture showed that the incidence of adverse events (AEs) associated with pharmacopuncture among 80,523 patients who received pharmacopuncture was low, and most AEs were not severe.37 In this study, one case of AE was reported in the pharmacopuncture group. The participant experienced localized itching at the site of treatment. However, the symptoms disappeared after the administration of oral medication. There were no reported cases of intervention-related SAE.
The limitations of this study are as follows. Blinding of both investigators and participants was impossible owing to the obvious differences in the interventions used in the two groups. To overcome this limitation, an investigator who did not participate in the intervention and was blinded to group allocation assessed the outcomes to minimize bias. Moreover, as the effectiveness of pharmacopuncture was maintained for up to 25 weeks in this study, further follow-up was not performed; thus, the long-term therapeutic effects could not be verified. The participating centers in this study did not include any clinics, which might have prevented sufficient reflection of the actual clinical settings. This aspect might have affected the pragmatic design of the study.
Since the type of pharmacopuncture solution was not determined in advance for the current clinical study, a variety of pharmacopuncture solutions were used. The physical effects of pharmacopuncture were similar regardless of the type of pharmacopuncture solution; however, the chemical effects may have varied. In the future, it will be more significant if clinical studies confirm which pharmacopuncture solution is most effective for chronic LBP. In addition, since patients with chronic back pain frequently experience recurrence of symptoms, it was necessary to confirm the effect on long-term recurrence. However, although follow-up observation was performed for 25 weeks in this study, the time-point until which the long-term effect was maintained could not be verified. A longer-term follow-up is required in future studies.
The present study is significant in that it is the first pragmatic RCT on the effectiveness of pharmacopuncture for LBP. In the present study, a broad range of patients with chronic LBP were recruited, regardless of radiological findings. The physicians were allowed to administer the treatment by freely choosing the type of pharmacopuncture or PT according to their clinical judgment based on the symptoms and radiological findings identified in actual outpatient practice without being provided specific details about the treatment. These aspects make the present study more pragmatic than the previous RCTs on pharmacopuncture. The present study is significant in that it presented the possibility of offering patients with chronic back pain a new treatment option.
Conclusion
The present study compared the effectiveness of pharmacopuncture for LBP with that of standard treatments widely used in Korean medicine clinical practice. In patients with LBP, pharmacopuncture administered for 5 weeks significantly reduced pain and improved functional outcomes and quality of life compared with PT administered for up to 25 weeks. Based on the findings of this study, pharmacopuncture could be recommended as a suitable treatment for patients with chronic LBP.
Data Sharing Statement
The data presented in this study are available upon request from the corresponding author.
Acknowledgments
We would like to thank all individuals who participated in the study, including the patients and clinical research coordinators.
Author Contributions
All authors made a substantial contribution to the work reported, including the conception, study design, execution, acquisition of data, analysis, and/or interpretation; participated in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; agreed on the journal to which the article was submitted; and accept responsibility for all aspects of the work.
Funding
This research was funded by the Korea Institute of Oriental Medicine, Republic of Korea (grant number: KSN1823211). This research project was an investigator-initiated trial in which the investigator played a leading role in the study design, performance of the trial, analyses, data interpretation, and report writing.
Disclosure
The authors declare that they have no competing interests.
References
1. Vos T, Abajobir AA, Abate KH, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390(10100):1211–1259.
2. Costa L, Maher CG, Hancock MJ, McAuley JH, Herbert RD, Costa LO. The prognosis of acute and persistent low-back pain: a meta-analysis. CMAJ. 2012;184(11):E613–E624. doi:10.1503/cmaj.111271
3. Costa L, Maher CG, McAuley JH, et al. Prognosis for patients with chronic low back pain: inception cohort study. BMJ. 2009;339:b3829.
4. Krismer M, Van Tulder M. Low back pain (non-specific). Best Pract Res. 2007;21(1):77–91. doi:10.1016/j.berh.2006.08.004
5. Qaseem A, Wilt TJ, McLean RM, Forciea MA. Physicians* CGCotACo. Noninvasive treatments for acute, subacute, and chronic low back pain: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2017;166(7):514–530. doi:10.7326/M16-2367
6. Ekman M, Jönhagen S, Hunsche E, Jönsson L. Burden of illness of chronic low back pain in Sweden: a Cross-Sectional, Retrospective Study in primary care setting. Spine. 2005;30(15):1777–1785. doi:10.1097/01.brs.0000171911.99348.90
7. Sherman KJ, Cherkin DC, Connelly MT, et al. Complementary and alternative medical therapies for chronic low back pain: what treatments are patients willing to try? BMC Complement Altern Med. 2004;4:9. doi:10.1186/1472-6882-4-9
8. Kim M-R, Lee SM, Lee YJ, Ha I-H. Clinical research on pharmacopuncture in Korea: a scoping review. Perspect Integr Med. 2023;2(1):8–23. doi:10.56986/pim.2023.02.003
9. Li J, Zhang JH, Yi T, Tang WJ, Wang SW, Dong JC. Acupuncture treatment of chronic low back pain reverses an abnormal brain default mode network in correlation with clinical pain relief. Acupunct Med. 2014;32(2):102–108. doi:10.1136/acupmed-2013-010423
10. Xu Z-F, Hong S-H, Wang S-J, et al. Neuroendocrine-immune regulating mechanisms for the anti-inflammatory and analgesic actions of acupuncture. World J Trad Chin Med. 2020;6(4):384–392. doi:10.4103/wjtcm.wjtcm_41_20
11. Lee YJ, Shin J-S, Lee J, et al. Usage report of pharmacopuncture in musculoskeletal patients visiting Korean medicine hospitals and clinics in Korea. BMC Complement Altern Med. 2016;16(1):292. doi:10.1186/s12906-016-1288-5
12. Shin Y-S, Shin J-S, Lee J, et al. A survey among Korea Medicine doctors (KMDs) in Korea on patterns of integrative Korean Medicine practice for lumbar intervertebral disc displacement: preliminary research for clinical practice guidelines. BMC Complement Altern Med. 2015;15(1):432. doi:10.1186/s12906-015-0956-1
13. Shin B-C, Kong JC, Park T-Y, Yang C-Y, Kang K-W, Choi S-M. Bee venom acupuncture for chronic low back pain: a randomised, sham-controlled, triple-blind clinical trial. Eur J Integr Med. 2012;4(3):e271–e280. doi:10.1016/j.eujim.2012.02.005
14. Seo B-K, Han K, Kwon O, Jo D-J, Lee J-H. Efficacy of bee venom acupuncture for chronic low back pain: a randomized, double-blinded, sham-controlled trial. J Toxins. 2017;9(11):361. doi:10.3390/toxins9110361
15. Ahn Y-J, Shin J-S, Lee J, et al. Evaluation of use and cost of medical care of common lumbar disorders in Korea: cross-sectional study of Korean health insurance review and assessment service national patient sample data. BMJ Open. 2016;6(9):e012432. doi:10.1136/bmjopen-2016-012432
16. Kim D-Y, Lee S-H, Lee H-Y, et al. Validation of the Korean version of the Oswestry disability index. Spine. 2005;30(5):E123–E127. doi:10.1097/01.brs.0000157172.00635.3a
17. Lee JS, Lee DH, Suh KT, Kim JI, Lim JM, Goh TS. Validation of the Korean version of the Roland–Morris disability questionnaire. Eur Spine J. 2011;20(12):2115–2119. doi:10.1007/s00586-011-1788-4
18. Farrar JT, Young JP, LaMoreaux L, Werth JL, Poole RM. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. 2001;94(2):149–158. doi:10.1016/S0304-3959(01)00349-9
19. Kim S-H, Jo M-W, Ahn J, Ock M, Shin S, Park J. Assessment of psychometric properties of the Korean SF-12 v2 in the general population. BMC Public Health. 2014;14(1):1086. doi:10.1186/1471-2458-14-1086
20. Kim S-H, Ahn J, Ock M, et al. The EQ-5D-5L valuation study in Korea. Qual Life Res. 2016;25(7):1845–1852. doi:10.1007/s11136-015-1205-2
21. Borm GF, Fransen J, Lemmens WA. A simple sample size formula for analysis of covariance in randomized clinical trials. J Clin Epidemiol. 2007;60(12):1234–1238. doi:10.1016/j.jclinepi.2007.02.006
22. Jeong S-Y, Park Z-W, Shin J-M, Kim J-Y, Youn I-Y. The comparative study of effectiveness between acupuncture and its cotreatment with Calculus Bovis. Fel Ursi. Moschus pharmacopuncture on the treatment of acute low back pain. J Acupunct Res. 2011;28(4):105–110.
23. Sung-hwan L, Min-wan K, Hyun L, So-yol L. Effectiveness of Bee-venom acupuncture and ouhyul herbal acupuncture in herniation of nucleus pulposus-comparison with acupuncture therapy only. J Korean Acupunct Moxibust Soc. 2007;24:197–205.
24. Song H-G, Choe J-Y, Kang J-H, Lee H. The effect of the acupuncture therapy in combination with soyeom pharmacopuncture therapy on the improvement of the symptoms of the patients with herniated intervertebral disk of L-spine in his initial stage of hospitalization. J Pharmacopunct. 2009;12(4):111–118. doi:10.3831/KPI.2009.12.4.111
25. Ostelo RW, Deyo RA, Stratford P, et al. Interpreting change scores for pain and functional status in low back pain: towards international consensus regarding minimal important change. Spine. 2008;33(1):90–94. doi:10.1097/BRS.0b013e31815e3a10
26. Maughan EF, Lewis JS. Outcome measures in chronic low back pain. Eur Spine J. 2010;19:1484–1494. doi:10.1007/s00586-010-1353-6
27. Lee YJ, Shin J-S, Lee J, et al. Survey of integrative lumbar spinal stenosis treatment in Korean medicine doctors: preliminary data for clinical practice guidelines. BMC Complement Altern Med. 2017;17:1–12. doi:10.1186/s12906-017-1942-6
28. Choi HS, Lee YJ, Kim M-R, et al. A survey of integrative treatment practices of Korean medicine doctors for cervical disc herniation: preliminary data for clinical practice guidelines. Evid Based Complement Altern Med. 2019;2019:1–14. doi:10.1155/2019/2345640
29. Park SH, Hong J-Y, Kim WK, et al. Effects of SHINBARO2 on rat models of lumbar spinal stenosis. Mediators Inflamm. 2019;2019:1–11. doi:10.1155/2019/7651470
30. Kim WK, Chung H-J, Pyee Y, et al. Effects of intra-articular SHINBARO treatment on monosodium iodoacetate-induced osteoarthritis in rats. Chin Med. 2016;11:1–10. doi:10.1186/s13020-016-0089-6
31. Brien S, Lewith GT, McGregor G. Devil’s Claw (Harpagophytum procumbens) as a treatment for osteoarthritis: a review of efficacy and safety. J Altern Complement Med. 2006;12(10):981–993. doi:10.1089/acm.2006.12.981
32. Chrubasik S, Zimpfer C, Schütt U, Ziegler R. Effectiveness of Harpagophytum procumbens in treatment of acute low back pain. Phytomedicine. 1996;3(1):1–10. doi:10.1016/S0944-7113(96)80003-1
33. Mahomed IM, Ojewole JA. Analgesic, antiinflammatory and antidiabetic properties of Harpagophytum procumbens DC (Pedaliaceae) secondary root aqueous extract. Phytother Res. 2004;18(12):982–989. doi:10.1002/ptr.1593
34. Andersen ML, Santos EH, Maria de Lourdes VS, da Silva AA, Tufik S. Evaluation of acute and chronic treatments with Harpagophytum procumbens on Freund’s adjuvant-induced arthritis in rats. J Ethnopharmacol. 2004;91(2–3):325–330. doi:10.1016/j.jep.2004.01.003
35. McGregor G, Fiebich B, Wartenberg A, Brien S, Lewith G, Wegener T. Devil’s claw (Harpagophytum procumbens): an anti-inflammatory herb with therapeutic potential. Phytochem Rev. 2005;4(1):47–53. doi:10.1007/s11101-004-2374-8
36. Hong JY, Kim H, Lee J, Jeon W-J, Lee YJ, Ha I-H. Harpagophytum procumbens inhibits iron overload-induced oxidative stress through activation of Nrf2 signaling in a rat model of lumbar spinal stenosis. Oxid Med Cell Longev. 2022;2022:1–18. doi:10.1155/2022/3472443
37. Kim M-R, Shin J-S, Lee J, et al. Safety of acupuncture and pharmacopuncture in 80,523 musculoskeletal disorder patients: a retrospective review of internal safety inspection and electronic medical records. Medicine. 2016;95(18). doi:10.1097/MD.0000000000003635
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


