Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 17
A Pooled Analysis of Mortality in Patients with COPD Receiving Dual Bronchodilation with and without Additional Inhaled Corticosteroid
Authors Miravitlles M , Verhamme K , Calverley PMA , Dreher M, Bayer V, Gardev A, de la Hoz A, Wedzicha J, Price D
Received 25 November 2021
Accepted for publication 21 February 2022
Published 11 March 2022 Volume 2022:17 Pages 545—558
DOI https://doi.org/10.2147/COPD.S350167
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Richard Russell
Marc Miravitlles,1 Katia Verhamme,2 Peter MA Calverley,3 Michael Dreher,4 Valentina Bayer,5 Asparuh Gardev,6 Alberto de la Hoz,6 Jadwiga Wedzicha,7 David Price8,9
1Pneumology Department, Hospital Universitari Vall d’Hebron, Vall d’Hebron Research Institute (VHIR), Vall d’Hebron Barcelona Hospital Campus, CIBER de Enfermedades Respiratorias (CIBERES), Barcelona, Spain; 2Department of Medical Informatics, Erasmus MC, Rotterdam, the Netherlands; 3Clinical Science Centre, Institute of Ageing and Chronic Disease, University of Liverpool, Liverpool, UK; 4Department of Pneumology and Intensive Care Medicine, University Hospital Aachen, Aachen, Germany; 5Boehringer Ingelheim Pharmaceuticals, Inc., Ridgefield, CT, USA; 6Boehringer Ingelheim International GmbH, Ingelheim am Rhein, Germany; 7Head Respiratory Division, National Heart and Lung Institute, Imperial College London, London, UK; 8Observational and Pragmatic Research Institute, Singapore; 9Centre of Academic Primary Care, Division of Applied Health Sciences, University of Aberdeen, Aberdeen, UK
Correspondence: Marc Miravitlles, Pneumology Department, Hospital Universitari Vall d’Hebron, Vall d’Hebron Research Institute (VHIR), Vall d’Hebron Barcelona Hospital Campus, CIBER de Enfermedades Respiratorias (CIBERES), P° Vall d’Hebron 119-129, Barcelona, 08035, Spain, Email [email protected]
Background: Recent studies report a lower mortality rate during treatment with long-acting muscarinic antagonist (LAMA)/long-acting β2-agonist (LABA)/inhaled corticosteroid (ICS) versus LAMA/LABA in patients with symptomatic chronic obstructive pulmonary disease (COPD) and a history of exacerbations.
Objective: We compared time to all-cause mortality with LAMA/LABA versus LAMA/LABA/ICS in patients with mild-to-very-severe COPD and a predominantly low exacerbation risk.
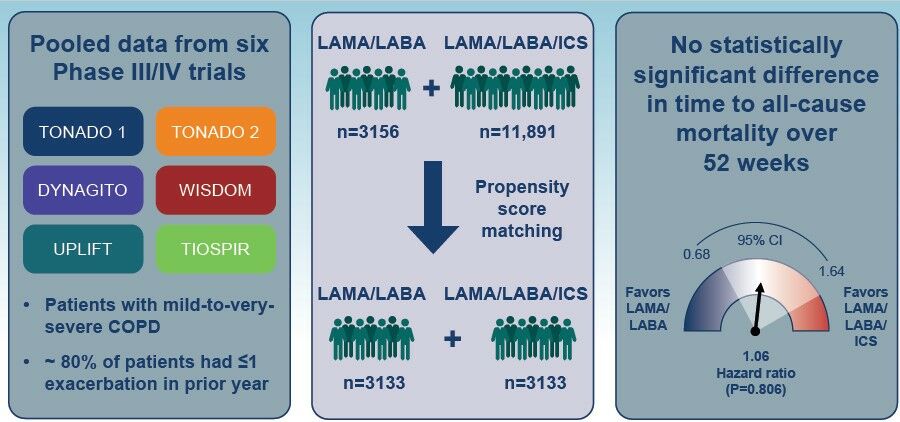
Methods: Data were pooled from six randomized controlled trials (TONADO 1/2, DYNAGITO, WISDOM, UPLIFT and TIOSPIR; LAMA/LABA: n = 3156, LAMA/LABA/ICS: n = 11,891). Analysis was on-treatment and data were censored at 52 weeks. Patients on LAMA/LABA/ICS received ICS prior to study entry, which was not withdrawn at randomization. Patients on LAMA/LABA/ICS were propensity score (PS)-matched to patients on LAMA/LABA who had not previously received ICS; covariates included age, sex, geographical region, smoking status, post-bronchodilator forced expiratory volume in 1 second percent predicted, exacerbation history in previous year, body mass index and time since diagnosis. Time to all-cause mortality was assessed using Cox proportional hazard regression models.
Results: After PS matching, 3133 patients on LAMA/LABA and 3133 patients on LAMA/LABA/ICS were analyzed. Fewer than 20% of patients reported ≥ 2 exacerbations in the prior year (LAMA/LABA: 19.1%; LAMA/LABA/ICS: 19.0%). There were 41 (1.3%) deaths on LAMA/LABA and 45 (1.4%) deaths on LAMA/LABA/ICS. No statistically significant difference in time to death was observed between treatment arms (hazard ratio for LAMA/LABA 1.06; 95% confidence intervals 0.68, 1.64; P = 0.806). Sensitivity analyses conducted using different covariates or in an intent-to-treat population showed similar results.
Conclusion: This pooled analysis of over 6000 patients with mild-to-very-severe COPD and predominantly low exacerbation risk showed no differences in mortality with LAMA/LABA versus LAMA/LABA/ICS, suggesting that the survival benefit of triple therapy seen in some recent studies may be specific to a high-risk population. This supports current Global Initiative for Chronic Obstructive Lung Disease recommendations that triple therapy should be reserved for the subpopulations of patients who need it the most (eg, those with an eosinophilic phenotype and a high risk of exacerbations) to avoid ICS overuse.
Keywords: COPD, exacerbation history, inhaled corticosteroid, long-acting β2-agonist, long-acting muscarinic antagonist, mortality
Graphical Abstract:
Plain Language Summary
For patients with chronic obstructive pulmonary disease (COPD), inhaled bronchodilator therapy can help to improve airflow in the lungs and provide symptom control. Several studies have looked at the rate of death in patients with COPD who were treated with a combination of two bronchodilators (dual therapy) either with or without an additional anti-inflammatory drug ─ namely an inhaled corticosteroid (ICS). These studies suggest that patients who received triple therapy (dual therapy plus ICS) had a lower risk of death; however, they only included patients with a history of exacerbations (episodes of symptom worsening). Since the majority of patients with COPD do not have frequent exacerbations, our study investigated the risk of death in patients who mostly had a low exacerbation risk. This had not been studied in this patient population before. We used data from over 6000 patients from six clinical trials and analyzed the time to death. We found that there was no difference in the risk of death for patients with COPD who were treated with either dual therapy or triple therapy. These findings suggest that the survival benefit of triple therapy seen in some recent studies may be specific to a high-risk population. This supports current COPD guideline recommendations that triple therapy should be reserved for patients who need it the most (eg, those with a high risk of exacerbations).
Introduction
Chronic obstructive pulmonary disease (COPD) is associated with a substantial healthcare and economic burden,1 and in 2019 was the third leading cause of mortality worldwide.2
There are multiple therapies that can be used as long-term treatment options for COPD, including long-acting bronchodilators (long-acting muscarinic antagonists [LAMAs] and/or long-acting β2-agonists [LABAs]) and inhaled corticosteroids (ICS). The Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2021 strategy report recommends a stepwise approach to pharmacologic treatment, starting with a LAMA or LABA for most patients with COPD, and escalating to dual bronchodilation as the next step.1 For patients who are highly symptomatic (eg, COPD Assessment Test score >20) and have a history of exacerbations (≥2 moderate exacerbations or ≥1 leading to hospitalization), LAMA/LABA dual therapy is recommended as initial treatment.1 The GOLD 2021 strategy report recommends triple therapy with LAMA/LABA/ICS as initial treatment for patients who are at an increased risk of exacerbations and have blood eosinophil levels ≥300 cells/µL and as follow-up for patients with increased exacerbation risk despite treatment with LABA/ICS or LAMA/LABA and with blood eosinophil levels ≥100 cells/µL.1
Several randomized controlled trials have assessed the effect of different inhaled COPD therapies on mortality. In both the TORCH and SUMMIT trials, no statistically significant difference in mortality was observed with LABA/ICS, LABA alone or ICS alone compared with placebo.3,4 In comparison, the reduction in all-cause mortality with the LAMA tiotropium versus placebo did reach statistical significance at the end of the protocol-defined treatment period in the UPLIFT trial, although not when patients were followed up for 30 days thereafter.5 Previous studies have also shown that there is no difference in mortality amongst patients treated with LAMA/LABA dual therapy versus either LAMA or LABA monotherapy or LABA/ICS combinations.6,7 The relative benefits on mortality of triple therapy versus dual LAMA/LABA therapy for patients with COPD are widely debated.8–16 Recent studies report a possible mortality benefit of treatment with LAMA/LABA/ICS versus LAMA/LABA combinations in patients with highly symptomatic COPD and a history of exacerbations.11–13 However, neither of these trials were designed or powered for mortality; furthermore, they only enrolled patients with highly symptomatic COPD and a high risk of future exacerbations and, as such, they are not representative of the majority of patients, who have moderate COPD and are infrequent exacerbators.17,18
Data on mortality in patients receiving LAMA/LABA versus LAMA/LABA/ICS therapy who have COPD with a lower exacerbation risk are currently lacking. Given that the majority of patients who either have established COPD or who are initiating COPD treatment are not at high risk of exacerbations, this is a key patient population to consider. This pooled analysis of data from six Phase III/IV randomized controlled trials therefore aimed to compare the time to all-cause mortality in a population of patients with mild-to-very-severe COPD and a predominantly low exacerbation risk who received treatment with either LAMA/LABA or LAMA/LABA/ICS.
Methods
Patient Population
Data were pooled from patients with COPD who participated in six Phase III/IV randomized controlled trials (TONADO® 1 [NCT01431274]/TONADO® 2 [NCT01431287],19 DYNAGITO [NCT02296138],20 WISDOM [NCT00975195],21 UPLIFT [NCT00144339]22 and TIOSPIR [NCT01126437]23,24) and received treatment with either LAMA/LABA (n=3156) or LAMA/LABA/ICS (n=11,891). The individual trials have been described in detail previously.19–24 Severity of COPD was assessed according to the following GOLD spirometric groups: GOLD 1 (mild, forced expiratory volume in 1 second [FEV1] ≥80% predicted); GOLD 2 (moderate, FEV1 50–<80% predicted); GOLD 3 (severe, FEV1 30–<50% predicted); GOLD 4 (very severe, FEV1 <30% predicted).1
Details on the treatment arms included from these trials are outlined in Table 1. The LAMA/LABA arm (n=3156) included patients: (1) not receiving ICS at trial entry and randomized to tiotropium/olodaterol (TONADO 1/TONADO 2/DYNAGITO; 53.6%); and (2) those already receiving LABA only who were randomized to add-on tiotropium (UPLIFT/TIOSPIR; 46.4%). The LAMA/LABA/ICS arm (n=11,891) included patients: (1) already receiving ICS (either alone or in combination with a LAMA and/or LABA) at trial entry, who were randomized to receive tiotropium/olodaterol (TONADO 1/TONADO 2/DYNAGITO; 27.6%); (2) already receiving LABA/ICS, who were randomized to receive tiotropium in addition (UPLIFT/TIOSPIR; 62.0%); and (3) those randomized to receive tiotropium/salmeterol/fluticasone propionate (WISDOM; 10.5%). Patients receiving ICS before enrolment continued their ICS treatment (or the ICS component alone if taken as a fixed combination with bronchodilator) at the same equivalent dose and regimen during the study.
 |
Table 1 Summary of Patients Included in the Pooled Analysis |
There was no withdrawal of prior treatment at randomization in either arm, therefore the ICS withdrawal arm of the WISDOM study was not included in the analysis. The main analysis was on-treatment (censored at treatment discontinuation) and all data were censored at 52 weeks, since some trials (ie, TIOSPIR and UPLIFT) were longer than 52 weeks; the other four trials were 52 weeks long. Additional intention-to-treat (ITT) analyses were also conducted including on- and off-treatment deaths, and Week 52 vital status data are reported.
Propensity Score Matching
To address any imbalance in characteristics between treatment arms, analyses were performed in a propensity score-matched cohort, with age, sex, geographical region, smoking status, post-bronchodilator FEV1% predicted, exacerbation history in the previous year, body mass index (BMI) and time since diagnosis as selected variables. Patients in the LAMA/LABA/ICS treatment arm were propensity score-matched to those in the LAMA/LABA treatment arm, using caliper matching (caliper width, 0.1). Baseline characteristics are reported for patients before and after matching; variables with a standardized difference of less than 0.1 were considered balanced between treatment arms.25
All-Cause Mortality
The primary outcome of this analysis was to compare the time to all-cause mortality, defined as time to on-treatment death from any cause, in the LAMA/LABA arm versus LAMA/LABA/ICS arm.
Time to all-cause mortality was analyzed by fitting a Cox proportional hazard regression model with treatment, study, geographical region, smoking status, FEV1% predicted (post bronchodilator) and number of prior COPD exacerbations as covariates (main analysis). Additional sensitivity analyses were conducted by fitting a Cox proportional hazard regression model with: treatment, study, age and sex as covariates (sensitivity analysis 1); treatment, study, age, sex and number of prior COPD exacerbations as covariates (sensitivity analysis 2); and with treatment, study, age, sex, geographical region, smoking status, FEV1% predicted (post bronchodilator), number of prior COPD exacerbations, BMI and duration of COPD diagnosis as covariates (sensitivity analysis 3). Sensitivity analysis 4 used the same model and covariates as in the main analysis but was an ITT analysis, including deaths on and off treatment.
Compliance with Ethics Guidelines
The studies included in this pooled analysis were all performed in accordance with the Declaration of Helsinki, International Conference on Harmonization Harmonized Tripartite Guideline for Good Clinical Practice and local regulations. The protocols were approved by the authorities and the ethics committees of the respective institutions, and signed informed consent was obtained from all patients, as described in the earlier publications.19–24
Results
Patient Population and Baseline Characteristics
Baseline patient characteristics for the total population and for the population following propensity score matching are shown in Table 2. Prior to propensity score matching, most demographic characteristics in the total population were similar, including mean age and gender distribution. However, there were differences in smoking history: a greater proportion of patients in the LAMA/LABA arm were current smokers compared with those in the LAMA/LABA/ICS arm (40.8% vs 31.8%; standardized difference = 0.19). The LAMA/LABA arm had a higher mean FEV1% predicted (post bronchodilator), a lower proportion of patients with a prior history of frequent exacerbations and a higher proportion of patients with mild-to-moderate COPD compared with the LAMA/LABA/ICS arm.
 |  |  |
Table 2 Baseline Patient Characteristics in Patients with COPD Receiving LAMA/LABA versus LAMA/LABA/ICS |
After propensity score matching, there were 3133 patients in each of the LAMA/LABA and LAMA/LABA/ICS treatment arms. Baseline characteristics were well balanced, as demonstrated by low values of standardized difference (Table 2). Both arms were composed mostly of infrequent exacerbators (patients with 0–1 exacerbation in prior year: 80.8% on LAMA/LABA vs 81.0% on LAMA/LABA/ICS) and patients with mild-to-very-severe COPD (GOLD 1: 0.2% vs 0.5%; GOLD 2: 49.5% vs 47.5%; GOLD 3: 40.5% vs 41.9%; and GOLD 4: 9.4% vs 9.5%). Although comorbidities were not matched for, they were found to be well balanced across the two cohorts (Table 2). There was, however, a slightly higher proportion of patients with diabetes in the LAMA/LABA versus LAMA/LABA/ICS arm (13.5% vs 9.4%, respectively).
Overall, the propensity score distribution was similar among patients treated with LAMA/LABA compared with LAMA/LABA/ICS and largely overlapped, indicating balance between the two treatment arms (e-Figure 1).
At Week 52, vital status data were available for 99.4% of patients in the LAMA/LABA treatment arm and 99.6% in the LAMA/LABA/ICS arm (Table 3). A similar proportion of patients discontinued trial medication in both the LAMA/LABA (12.1%) and LAMA/LABA/ICS (13.1%) treatment arms (e-Table 1).
 |
Table 3 Vital Status at 52 Weeksa |
All-Cause Mortality
In the propensity score-matched population, there were 41 (1.3%) on-treatment deaths in the LAMA/LABA arm and 45 (1.4%) on-treatment deaths in the LAMA/LABA/ICS arm prior to 52 weeks (Table 3). No statistically significant difference in the hazard of death was observed between treatment arms (hazard ratio 1.06; 95% confidence interval [CI] 0.68, 1.64; P=0.806; Figure 1). Sensitivity analyses using three additional models with different covariates showed similar results (Figure 1).
In the ITT analysis including both on-treatment and off-treatment deaths, there were 74 (2.4%) deaths in the LAMA/LABA arm and 66 (2.1%) deaths in the LAMA/LABA/ICS arm prior to 52 weeks (Table 3). Again, no statistically significant difference in the time to death was observed between treatment arms (hazard ratio 1.19; 95% CI 0.84, 1.68; P=0.338; Figure 1, sensitivity analysis 4).
Plots of estimated probability of all-cause mortality with LAMA/LABA versus LAMA/LABA/ICS are shown in Figure 2 (Log rank test P=0.6390) for the on-treatment analysis and e-Figure 2 for the ITT analysis (Log rank test P=0.4938).
Cause of Death
Adjudicated causes of death for patients who died in the on-treatment (as well as on- and off-treatment) analysis are summarized in e-Table 2. No meaningful difference in cause of death was observed, including for cardiac, respiratory, and any other cause. The proportion of respiratory deaths based on the adjudicated cause of death were 9/41 (22% of all deaths) in the LAMA/LABA arm and 11/45 (24% of all deaths) in the LAMA/LABA/ICS arm.
Discussion
This pooled analysis of over 6000 propensity score-matched patients from six Phase III/IV randomized controlled trials showed no difference in mortality between treatment with LAMA/LABA and LAMA/LABA/ICS therapy in patients with COPD and a predominantly low risk of exacerbations. This finding remained when sensitivity analyses were conducted using three additional models with different combinations of covariates and when the same analyses were conducted in the ITT population, which included on- and off-treatment deaths.
Importantly, our study includes a population of patients at a predominantly low risk of exacerbations; only 19% of patients had experienced >1 exacerbation in the year prior to study entry. Our findings suggest that triple therapy does not carry a survival benefit versus LAMA/LABA therapy in patients with a low exacerbation risk, who are reflective of the majority of patients with COPD.18
In contrast to our study, the IMPACT and ETHOS randomized controlled trials report that, in patients with a higher risk of exacerbations (at least 1 moderate or severe exacerbation in the previous year), there is a survival benefit of treatment with LAMA/LABA/ICS versus LAMA/LABA combinations.11–13,26 In IMPACT, the hazard ratio for on-treatment all-cause mortality was 0.58 (95% CI 0.38, 0.88; P=0.011) for the comparison of triple therapy (umeclidinium/vilanterol/fluticasone furoate) with LAMA/LABA (umeclidinium/vilanterol).11 Similarly, in a recent analysis of data from ETHOS, the hazard ratio for reduction in on-treatment all-cause mortality was 0.50 (95% CI 0.30, 0.81; unadjusted P=0.0056) with glycopyrrolate/formoterol/budesonide relative to glycopyrrolate/formoterol.26 It is worth noting that, in both the IMPACT and ETHOS trials,11–13 treatment with ICS was discontinued in the LAMA/LABA arm at the time of randomization and this could have confounded the benefit of triple therapy. In fact, 72% and 81% of patients in the LAMA/LABA arm of IMPACT and ETHOS, respectively, were receiving ICS at screening; in both trials, 40% of patients entering the LAMA/LABA arm were on triple therapy at screening.12,13 A potential difference in mortality benefit has been seen when stratifying by follow-up time after treatment initiation.27 Moreover, the small subgroup of patients who were not on ICS at screening was the only group that did not show a trend towards reduced mortality with triple therapy versus LAMA/LABA in either trial.11,26 However, the authors of the most recent ETHOS publication suggest that the overall results for mortality cannot be explained solely by acute treatment withdrawal, although it cannot be ruled out that discontinuation of ICS may have contributed to some of the early death events.26 In contrast to the IMPACT and ETHOS trials, there was no withdrawal of prior ICS treatment at randomization in patients included in our analysis. Note that, in the future, use of an “adaptive selection” clinical trial design would avoid these issues concerning treatment withdrawal effects.28
For inclusion in the ETHOS trial, patients were required to have a post-bronchodilator FEV1 of less than 65% of the predicted normal value,13 which would have skewed the inclusion of patients with moderate GOLD 2 COPD toward the more severe end of this range. Compared with both ETHOS and IMPACT, the mean FEV1% predicted (post bronchodilator) was higher in our study (48.4─48.6% versus 43.4% [ETHOS] and 45.5% [IMPACT]).11,26 In both IMPACT and ETHOS, the inclusion criteria required patients to have a history of exacerbations (≥1 moderate or severe exacerbation in the prior year if FEV1% predicted <50%; or ≥2 moderate/≥1 severe exacerbation if FEV1% predicted ≥50%).12,13 As a result, these patients had a substantially higher risk of exacerbations than in our study, with 54% of patients from IMPACT and 57% from ETHOS experiencing ≥2 moderate or severe exacerbations in the previous year, compared with only 19% in our study.12,13 In our study, over 35% of patients had no history of exacerbations in the prior year. The ETHOS study also enriched for patients with high eosinophil counts (>150 cells/μL),13 who are more likely to benefit from addition of ICS. Unfortunately, we do not have eosinophil data available and therefore cannot investigate the influence of blood eosinophils on outcomes. Another possible explanation for the different results observed in our analysis is that IMPACT and ETHOS included patients with a previous history of asthma, whereas patients with a history of asthma were excluded in the studies that we used for our analysis.
All-cause mortality (on-treatment) was slightly lower in the LAMA/LABA arm in our analysis (proportion of patients with deaths: 1.3%) compared with that reported in the IMPACT (1.9%) and ETHOS (2.1%) trials, but was similar for the triple therapy arm (our analysis: 1.4% compared with 1.2% in IMPACT and 1.2%/1.7% for 320/160 µg budesonide, respectively, in ETHOS).11,26 We may hypothesize that the higher mortality risk in patients on LAMA/LABA from IMPACT and ETHOS may be explained by the proportion of patients with frequent exacerbations and high blood eosinophil counts who were randomized to LAMA/LABA, a significant number of whom were previously treated with ICS that was removed as a consequence of randomization.26 Our findings in a lower risk population showed no significant reduction in the risk of mortality with the use of triple therapy compared with LAMA/LABA in all patients with COPD. This suggests that ICS are not needed in patients at a low risk of exacerbations, likely irrespective of baseline eosinophil count. This is consistent with current guidelines that strongly suggest prescribing (and not discontinuing) ICS only in patients with exacerbations and high blood eosinophil counts.1,29 Unfortunately, blood eosinophil data are not available for all studies in our pooled analysis, which limits our interpretation.
Overall, our findings support the recommendations from the GOLD 2021 strategy report that triple therapy should be reserved for those patients who have an increased risk of exacerbations despite treatment with LABA/ICS or despite treatment with LAMA/LABA and who have eosinophil levels ≥100 cells/µL.1 Given that there are a number of safety concerns associated with ICS use, including an increased risk of pneumonia, osteoporosis and poor diabetes control, this further emphasizes the importance of only using ICS-containing regimens in those patients for whom they are recommended.30–32 In addition, triple therapy has been associated with higher costs compared with LAMA/LABA combinations.33,34
The fact that this was not a new user cohort is an important limitation of the study and introduces the possibility of on-treatment bias, since confounding cannot be controlled for pre-treatment variables that were not captured at baseline, such as prior treatment duration.35–37 Nevertheless, it is worth noting that, similar to our study, a relevant proportion of patients included in the triple arms of the IMPACT (40%) and ETHOS (39.1─39.5%) randomized clinical trials were not new users either.11,13 There is also an inherent limitation of any such pooled analysis, which, unlike a randomized controlled trial, involved pooling data from different trials. Note that tiotropium was the only LAMA evaluated in this analysis, which may impact on the generalizability of the results. The duration of 52 weeks may also be a limitation as longer follow-up could potentially have revealed a delayed survival benefit in patients with less severe COPD.
A key strength of our study is that it provides mortality data for LAMA/LABA versus LAMA/LABA/ICS treatment in a representative population of patients with a lower risk of exacerbations and with less severe COPD than has previously been reported.11–13 Another strength is the large number of patients (N>6000) in the propensity score-matched population, and that both on-treatment and off-treatment mortality were assessed, showing similar results. In addition, vital status was available for ≥99.4% of patients. In the total population, there were some differences in baseline characteristics between treatment arms (eg, exacerbation history); however, propensity score matching provided balance between the cohorts. Nevertheless, this was only possible for measured covariates, and therefore an impact of residual confounding by unmeasured confounders cannot be ruled out. Of note, there was an imbalance in prior ICS use between the treatment groups. No patients in the LAMA/LABA group had previously used ICS, whereas those in the LAMA/LABA/ICS group had received ICS prior to study entry. Thus, since there was no obligatory withdrawal of prior treatment at randomization in either treatment arm, discontinuation of ICS at study entry could not have confounded the results.
Conclusion
This pooled analysis of over 6000 propensity score-matched patients showed no differences in mortality between LAMA/LABA and LAMA/LABA/ICS therapy in patients with COPD and a predominantly low risk of exacerbations. Our findings therefore suggest that triple therapy does not carry a survival benefit versus LAMA/LABA therapy in this population, and that the survival benefit of triple therapy seen in some recent studies may be specific to a high-risk population. This supports current GOLD recommendations that triple therapy should be reserved for the subpopulations of patients who need it the most (eg, those with an eosinophilic phenotype and a high risk of exacerbations) to avoid ICS overuse.
Abbreviations
BMI, body mass index; CI, confidence interval; COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 second; GOLD, Global Initiative for Chronic Obstructive Lung Disease; ICS, inhaled corticosteroids; ITT, intention to treat; LABA, long-acting β2-agonist; LAMA, long-acting muscarinic antagonist.
Data Sharing Statement
To ensure independent interpretation of clinical study results and enable authors to fulfill their role and obligations under the ICMJE criteria, Boehringer Ingelheim grants all external authors access to relevant clinical study data. In adherence with the Boehringer Ingelheim Policy on Transparency and Publication of Clinical Study Data, scientific and medical researchers can request access to clinical study data after publication of the primary manuscript in a peer-reviewed journal, regulatory activities are complete and other criteria are met. Researchers should use the https://vivli.org/ link to request access to study data and visit https://www.mystudywindow.com/msw/datasharing for further information.
Ethics Approval and Informed Consent
The studies included in this pooled analysis were all performed in accordance with the Declaration of Helsinki, International Conference on Harmonisation Harmonised Tripartite Guideline for Good Clinical Practice and local regulations. The protocols were approved by the authorities and the ethics committees of the respective institutions, and signed informed consent was obtained from all patients, as described in the earlier publications.
Acknowledgments
Support for this project was provided by Boehringer Ingelheim International GmbH (BI). The authors did not receive payment related to the development of the manuscript. Medical writing assistance, in the form of the preparation and revision of the manuscript, was supported financially by BI and provided by Vicki Cronin of MediTech Media, under the authors’ conceptual direction and based on feedback from the authors. BI was given the opportunity to review the manuscript for medical and scientific accuracy as well as intellectual property considerations. The authors would like to thank Wenqiong Xue for her contributions to the statistical analysis.
This manuscript includes some content that was previously presented at the 2021 American Thoracic Society International Conference as a poster presentation. The poster’s abstract was published in “Meeting Abstracts” in Am J Respir Crit: https://www.atsjournals.org/doi/10.1164/ajrccm-conference.2021.203.1_MeetingAbstracts.A2251.
Author Contributions
The authors meet criteria for authorship as recommended by the International Committee of Medical Journal Editors (ICMJE). All authors made a significant contribution to the work reported, whether that was in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
Support for this project was provided by Boehringer Ingelheim International GmbH.
Disclosure
Marc Miravitlles has received speaker fees from AstraZeneca, Bial, Boehringer Ingelheim, Chiesi, Cipla, CSL Behring, GlaxoSmithKline, Grifols, Menarini, Novartis, Rovi, Sandoz and Zambon; consulting fees from AstraZeneca, Atriva Therapeutics, Bial, Boehringer Ingelheim, Chiesi, CSL Behring, Gebro Pharma, Ferrer, GlaxoSmithKline, Grifols, Kamada, Laboratorios Esteve, Mereo Biopharma, Novartis, ONO Pharma, pH Pharma, Sanofi, Palobiofarma SL, Spin Therapeutics, Takeda, TEVA and Verona Pharma; and research grants from Grifols. Peter M.A. Calverley has received grants from GlaxoSmithKline; advisory board fees from Boehringer Ingelheim; and speaker and advisory board fees from Phillips Respironics, Recipharm and Zambon. Katia Verhamme has received consultancy fees from Boehringer Ingelheim; payment for a lecture to the Department of Medical Informatics from AstraZeneca; and received unconditional research grants from Yamanouchi, Pfizer/Boehringer Ingelheim, Novartis, GlaxoSmithKline, Amgen, UCB and Chiesi. Michael Dreher has received speaker or advisory fees from Actelion, AstraZeneca, Bayer, Berlin-Chemie, Boehringer Ingelheim, Chiesi, Hamilton, Heinen und Löwenstein, Insmed, InterMune, Linde, Novartis, Pfizer, Philips Respironics, ResMed, Roche and Weinmann. Valentina Bayer, Asparuh Gardev and Alberto de la Hoz are employees of Boehringer Ingelheim. Jadwiga Wedzicha has received grants from AstraZeneca, Boehringer Ingelheim, Chiesi, Genentech, GlaxoSmithKline, Johnson and Johnson, and Novartis, and meeting expenses from AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline and Novartis. David Price has board membership with AstraZeneca, Boehringer Ingelheim, Chiesi, Mylan, Novartis, Regeneron Pharmaceuticals, Sanofi Genzyme and Thermo Fisher; consultancy agreements with Airway Vista Secretariat, AstraZeneca, Boehringer Ingelheim, Chiesi, EPG Communication Holdings Ltd, FIECON Ltd, Fieldwork International, GlaxoSmithKline, Mylan, Mundipharma, Novartis, OM Pharma SA, PeerVoice, Phadia AB, Spirosure Inc, Strategic North Limited, Synapse Research Management Partners S.L., Talos Health Solutions, Theravance and WebMD Global LLC; grants and unrestricted funding for investigator-initiated studies (conducted through Observational and Pragmatic Research Institute Pte Ltd) from AstraZeneca, Boehringer Ingelheim, Chiesi, Mylan, Novartis, Regeneron Pharmaceuticals, Respiratory Effectiveness Group, Sanofi Genzyme, Theravance and the UK National Health Service; payment for lectures/speaking engagements from AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, GlaxoSmithKline, Kyorin, Mylan, Mundipharma, Novartis, Regeneron Pharmaceuticals and Sanofi Genzyme; payment for travel/accommodation/meeting expenses from AstraZeneca, Boehringer Ingelheim, Mundipharma, Mylan, Novartis and Thermo Fisher; stock/stock options from AKL Research and Development Ltd, which produces phytopharmaceuticals; owns 74% of the social enterprise Optimum Patient Care Ltd (Australia and UK) and 92.61% of Observational and Pragmatic Research Institute Pte Ltd (Singapore); 5% shareholding in Timestamp which develops adherence monitoring technology; is peer reviewer for grant committees of the UK Efficacy and Mechanism Evaluation programme and Health Technology Assessment; and was an expert witness for GlaxoSmithKline. The authors report no other conflicts of interest in this work.
References
1. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease (2021 report); 2020 [Updated November 25, 2020]. Available from: https://goldcopd.org/wp-content/uploads/2020/11/GOLD-REPORT-2021-v1.1-25Nov20_WMV.pdf.
2. World Health Organization. The top 10 causes of death; 2020. Available from: https://www.who.int/en/news-room/fact-sheets/detail/the-top-10-causes-of-death.
3. Calverley PM, Anderson JA, Celli B, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356(8):775–789. doi:10.1056/NEJMoa063070
4. Vestbo J, Anderson JA, Brook RD, et al. Fluticasone furoate and vilanterol and survival in chronic obstructive pulmonary disease with heightened cardiovascular risk (SUMMIT): a double-blind randomised controlled trial. Lancet. 2016;387(10030):1817–1826. doi:10.1016/S0140-6736(16)30069-1
5. Celli B, Decramer M, Kesten S, et al. Mortality in the 4-year trial of tiotropium (UPLIFT) in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2009;180(10):948–955. doi:10.1164/rccm.200906-0876OC
6. Rogliani P, Matera MG, Ora J, Cazzola M, Calzetta L. The impact of dual bronchodilation on cardiovascular serious adverse events and mortality in COPD: a quantitative synthesis. Int J Chron Obstruct Pulmon Dis. 2017;12:3469–3485. doi:10.2147/COPD.S146338
7. Oba Y, Keeney E, Ghatehorde N, Dias S. Dual combination therapy versus long-acting bronchodilators alone for chronic obstructive pulmonary disease (COPD): a systematic review and network meta-analysis. Cochrane Database Syst Rev. 2018;12:CD012620. doi:10.1002/14651858.CD012620.pub2
8. Suissa S, Ariel A. Triple therapy trials in COPD: a precision medicine opportunity. Eur Respir J. 2018;52(6):1801848. doi:10.1183/13993003.01848-2018
9. Calzetta L, Ritondo BL, de Marco P, Cazzola M, Rogliani P. Evaluating triple ICS/LABA/LAMA therapies for COPD patients: a network meta-analysis of ETHOS, KRONOS, IMPACT, and TRILOGY studies. Expert Rev Respir Med. 2021;15(1):143–152. doi:10.1080/17476348.2020.1816830
10. Han MK, Lipson DA, Singh D, Martinez FJ. Reply to: ‘evaluating triple ICS/LABA/LAMA therapies for COPD patients: a network meta-analysis of ETHOS, KRONOS, IMPACT, and TRILOGY studies’. Expert Rev Respir Med. 2021;15(4):577–578. doi:10.1080/17476348.2021.1865813
11. Lipson DA, Crim C, Criner GJ, et al. Reduction in all-cause mortality with fluticasone furoate/umeclidinium/vilanterol in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2020;201(12):1508–1516. doi:10.1164/rccm.201911-2207OC
12. Lipson DA, Barnhart F, Brealey N, et al. Once-daily single-inhaler triple versus dual therapy in patients with COPD. N Engl J Med. 2018;378(18):1671–1680. doi:10.1056/NEJMoa1713901
13. Rabe KF, Martinez FJ, Ferguson GT, et al. Triple inhaled therapy at two glucocorticoid doses in moderate-to-very-severe COPD. N Engl J Med. 2020;383(1):35–48. doi:10.1056/NEJMoa1916046
14. Calzetta L, Ludovica Ritondo B, de Marco P, Cazzola M, Rogliani P. Reply to Han et al.: impact on mortality of triple ICS/LABA/LAMA therapy in a population of COPD patients including also subjects with asthma-like profile. Expert Rev Respir Med. 2021;15(4):579–581. doi:10.1080/17476348.2021.1866835
15. Calverley PMA, Magnussen H, Miravitlles M, Wedzicha JA. Triple therapy in COPD: what we know and what we don’t. COPD. 2017;14(6):648–662. doi:10.1080/15412555.2017.1389875
16. López-Campos JL, Carrasco-Hernández L, Román Rodríguez L, Quintana-Gallego E, Carmona Bernal C, Alcázar Navarrete B. Implicaciones clínicas del uso de la triple terapia en combinación de dosis fija en EPOC: del ensayo al paciente. Arch Bronconeumol. 2020;56(4):242–248. doi:10.1016/j.arbres.2019.11.011
17. Halpin DM, Kerkhof M, Soriano JB, Mikkelsen H, Price DB. Eligibility of real-life patients with COPD for inclusion in trials of inhaled long-acting bronchodilator therapy. Respir Res. 2016;17(1):120. doi:10.1186/s12931-016-0433-5
18. Han MK, Quibrera PM, Carretta EE, et al. Frequency of exacerbations in patients with chronic obstructive pulmonary disease: an analysis of the SPIROMICS cohort. Lancet Respir Med. 2017;5(8):619–626. doi:10.1016/S2213-2600(17)30207-2
19. Buhl R, Maltais F, Abrahams R, et al. Tiotropium and olodaterol fixed-dose combination versus mono-components in COPD (GOLD 2–4). Eur Respir J. 2015;45(4):969–979. doi:10.1183/09031936.00136014
20. Calverley PMA, Anzueto AR, Carter K, et al. Tiotropium and olodaterol in the prevention of chronic obstructive pulmonary disease exacerbations (DYNAGITO): a double-blind, randomised, parallel-group, active-controlled trial. Lancet Respir Med. 2018;6(5):337–344. doi:10.1016/S2213-2600(18)30102-4
21. Magnussen H, Disse B, Rodriguez-Roisin R, et al. Withdrawal of inhaled glucocorticoids and exacerbations of COPD. N Engl J Med. 2014;371(14):1285–1294. doi:10.1056/NEJMoa1407154
22. Tashkin DP, Celli B, Senn S, et al. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med. 2008;359(15):1543–1554. doi:10.1056/NEJMoa0805800
23. Wise RA, Anzueto A, Cotton D, et al. Tiotropium Respimat inhaler and the risk of death in COPD. N Engl J Med. 2013;369(16):1491–1501. doi:10.1056/NEJMoa1303342
24. Wise RA, Anzueto A, Calverley P, et al. The Tiotropium Safety and Performance in Respimat Trial (TIOSPIR), a large scale, randomized, controlled, parallel-group trial-design and rationale. Respir Res. 2013;14(1):40. doi:10.1186/1465-9921-14-40
25. Austin PC. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun Stat Simul Comput. 2009;38(6):1228–1234. doi:10.1080/03610910902859574
26. Martinez FJ, Rabe KF, Ferguson GT, et al. Reduced all-cause mortality in the ETHOS trial of budesonide/glycopyrrolate/formoterol for chronic obstructive pulmonary disease: a randomized, double-blind, multicenter, parallel-group study. Am J Respir Crit Care Med. 2021;203(5):553–564. doi:10.1164/rccm.202006-2618OC
27. Suissa S. Perplexing mortality data from triple therapy trials in COPD. Lancet Respir Med. 2021;9(7):684–685. doi:10.1016/S2213-2600(21)00238-1
28. Suissa S. Triple therapy in COPD: time for adaptive selection trials. COPD. 2021:1–5. doi:10.1080/15412555.2021.1982886
29. Chalmers JD, Laska IF, Franssen FME, et al. Withdrawal of inhaled corticosteroids in COPD: a European Respiratory Society guideline. Eur Respir J. 2020;55(6):2000351. doi:10.1183/13993003.00351-2020
30. Yawn BP, Suissa S, Rossi A. Appropriate use of inhaled corticosteroids in COPD: the candidates for safe withdrawal. NPJ Prim Care Respir Med. 2016;26:16068. doi:10.1038/npjpcrm.2016.68
31. Avdeev S, Aisanov Z, Arkhipov V, et al. Withdrawal of inhaled corticosteroids in COPD patients: rationale and algorithms. Int J Chron Obstruct Pulmon Dis. 2019;14:1267–1280. doi:10.2147/COPD.S207775
32. Miravitlles M, Auladell-Rispau A, Monteagudo M, et al. Systematic review on long-term adverse effects of inhaled corticosteroids in the treatment of COPD. Eur Respir Rev. 2021;30(160):160. doi:10.1183/16000617.0075-2021
33. Palli SR, Frazer M, DuCharme M, Buikema AR, Anderson AJ, Franchino-Elder J. Differences in real-world health and economic outcomes among patients with COPD treated with combination tiotropium/olodaterol versus triple therapy. J Manag Care Spec Pharm. 2020;26(10):1363–1374. doi:10.18553/jmcp.2020.20159
34. Nici L, Mammen MJ, Charbek E, et al. Pharmacologic management of chronic obstructive pulmonary disease: an official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med. 2020;201(9):e56–e69. doi:10.1164/rccm.202003-0625ST
35. Yoshida K, Solomon DH, Kim SC. Active-comparator design and new-user design in observational studies. Nat Rev Rheumatol. 2015;11(7):437–441. doi:10.1038/nrrheum.2015.30
36. Suissa S, Moodie EE, Dell’Aniello S. Prevalent new-user cohort designs for comparative drug effect studies by time-conditional propensity scores. Pharmacoepidemiol Drug Saf. 2017;26(4):459–468. doi:10.1002/pds.4107
37. Johnson ES, Bartman BA, Briesacher BA, et al. The incident user design in comparative effectiveness research. Pharmacoepidemiol Drug Saf. 2013;22(1):1–6. doi:10.1002/pds.3334
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


