Back to Journals » Journal of Inflammation Research » Volume 16
A Pilot Study on Tocilizumab in Very-Late-Onset Myasthenia Gravis
Authors Yang TT , Wang ZY, Fan ZX, Yuan BY, Ma L, Lu JF, Liu PJ, He Y, Liu GZ
Received 26 May 2023
Accepted for publication 7 September 2023
Published 8 December 2023 Volume 2023:16 Pages 5835—5843
DOI https://doi.org/10.2147/JIR.S423098
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Ting-Ting Yang,1,* Ze-Yi Wang,1,* Ze-Xin Fan,1 Bo-Yi Yuan,1 Lin Ma,1 Jian-Feng Lu,1 Pen-Ju Liu,1 Yang He,2 Guang-Zhi Liu1
1Department of Neurology, Beijing Anzhen Hospital, Capital Medical University, Beijing, 100029, People’s Republic of China; 2Department of Neurology, Peking University People’s Hospital, Beijing, 100044, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Guang-Zhi Liu, Department of Neurology, Beijing Anzhen Hospital, Capital Medical University, Beijing, 100029, People’s Republic of China, Tel +86-10-64456149, Fax +86-10-64456119, Email [email protected]
Objective: This study aimed to initially investigate the efficacy and safety of low-dose tocilizumab combined with glucocorticoid for the treatment of very-late-onset myasthenia gravis (VLOMG).
Methods: We conducted a retrospective study in VLOMG patients who were administered intravenous methylprednisolone therapy and subsequently received low-dose oral corticosteroid, in combination with intravenous injection of tocilizumab given once every month for three months.
Results: Five patients (mean age 75.0 ± 4.5 years) were included, and all of them were new-onset, and anti-acetylcholine receptor (AChR) antibody-positive generalized MG. The Quantitative Myasthenia Gravis Scale (QMGS) and Myasthenia Gravis Activities of Daily Living (MG-ADL) scores before treatment were 15.4 ± 4.3 and 9.6 ± 2.3, respectively, and they exhibited a continuously decreasing trend after the first, second, and third injection of tocilizumab until 6 months after treatment. At 6 months post-treatment, the QMGS and MG-ADL scores were 5.0 ± 2.9 and 2.0 ± 1.2, respectively, and the difference between scores at baseline and 6-month follow-up was significant (P = 0.005 and P < 0.0001, respectively). No serious adverse drug reactions were reported in any patient during the study period.
Discussions and Conclusion: The therapeutic efficacy of tocilizumab in VLOMG remains uncertain. The results from our study support the efficacy and safety of this combination treatment option for VLOMG, and strongly suggests the therapeutic potential of tocilizumab in VLOMG. However, considering the limitation of retrospective nature and small sample size in this study, prospective randomized controlled studies including a larger sample size of selected patients are needed to validate our results.
Keywords: very late-onset myasthenia gravis, immunotherapy, tocilizumab, glucocorticoid
Introduction
Myasthenia gravis (MG) is an autoimmune disease caused by autoantibodies that act against the acetylcholine receptor (AChR), muscle-specific kinase (MuSK), or other AChR-related proteins on the postsynaptic membrane of the neuromuscular junction, leading to muscle weakness and fatigability.1,2 Based on the age at onset, with 50 and 65 years set as the limits, MG patients can be categorized into three different age-based subgroups: early-onset MG (< 50 years), late-onset MG (≥ 50 and < 65 years), and very-late-onset MG (VLOMG, ≥ 65 years).3,4 Compared with patients in the former two MG subgroups, those with VLOMG are more likely to have a larger number of comorbidities (ie, hypertension, diabetes mellitus, and tumor development), greater disease severity at onset, higher risk of exacerbation, poor tolerance to medication side effects, and aging-related changes in immune function.5–7 To date, the results of a variety of retrospective studies have supported the clinical benefit of immunotherapy (eg, glucocorticoid, azathioprine, and tacrolimus) in treating patients with VLOMG.5,8,9
The pro-inflammatory cytokine, interleukin (IL)-6, is an important mediator of the inflammatory process in MG and may contribute to the pathogenesis of VLOMG. IL-6 has a variety of biological properties, such as the stimulation of B cell differentiation, antibody production, activation of T cells, and induction of naïve T cells to differentiate into T helper type 17 (Th17) and follicular T cells. In accordance with this finding, it was recently reported that patients with anti-AchR antibody-positive MG exhibited elevated serum IL-6 levels, which were also associated with MG disease activity and which decreased following immunosuppressant therapy, suggesting that IL-6 signaling is involved in the pathogenesis of anti-AChR antibody-positive MG and could be a potential therapeutic target in patients with MG.10,11 Furthermore, the administration of anti-IL-6 antibodies caused the downregulation of Th17-related genes (IL-17, IL-17R, IL-23R, and IL-21) in the lymph nodes, indicating that IL-6 plays a major role in regulating the autoimmune response associated with MG.12 In Torpedo californica AChR-induced experimental autoimmune MG (EAMG) mice, only 25% of IL-6(-/-) mice developed MG-like symptoms compared to 83% of wild-type C57BL/6 mice.13 Intraperitoneal injection of anti-IL-6 antibodies administered in EAMG rats remarkably ameliorated their clinical symptoms when treatment was started during either the acute or chronic phase of the disease, and when compared with a control treatment, this treatment led to a decreased overall anti-AChR antibody titer and reduced number of B cells.
As a recombinant humanized monoclonal antibody of the immunoglobulin (Ig) G1 subclass, tocilizumab inhibits the binding of IL-6 to its receptors and thus downregulates pro-inflammatory activity by competing with both the soluble and membrane-bound forms.14 Tocilizumab is currently approved for the treatment of rheumatoid arthritis, juvenile idiopathic arthritis, and giant cell arteritis and can prevent cardiovascular events caused by chimeric antigen receptor-modified T-cell (CAR-T) therapy.15–17 In addition, a recent randomized controlled study reported that compared with azathioprine, tocilizumab (8 mg/kg, once every 4 weeks) significantly reduced the relapse rate in patients with neuromyelitis optica spectrum disorders (NMOSDs) and produced mild ADRs (ie, elevated alanine aminotransferase levels and upper respiratory tract and urinary tract infections), which is indicative of its therapeutic potential against NMOSDs.18 Notably, satralizumab, a product similar to tocilizumab, has recently been approved for the treatment of NMOSDs. More importantly, it has been reported that in two patients with anti-AChR antibody-seropositive MG that was refractory to rituximab, subsequent tocilizumab therapy (8 mg/kg, once a month) remarkably ameliorated myasthenic symptoms over several months without obvious adverse events,19 indicating the beneficial effects of tocilizumab in carefully selected MG cases (eg, treatment-refractory MG). Hence, this study aimed to further investigate the efficacy and safety of combination therapy with low-dose tocilizumab and glucocorticoid in treating VLOMG.
Materials and Methods
The single-center, retrospective case series study was performed in patients with VLOMG at outpatient and inpatient clinics of the Department of Neurology, Beijing Anzhen Hospital between September 10, 2018 and March 09, 2022. The study was approved by the local ethics committee of Beijing Anzhen Hospital (no. 2022138X) and carried out in compliance with the Declaration of Helsinki. The patient’s personal data was kept confidential and informed consent was waived due to the retrospective observational nature of this study.
Diagnosis of MG was based on clinical symptoms indicative of fluctuating skeletal muscle weakness and results of auxiliary tests for serological antibody detection or of a neurophysiological examination according to the guidelines of the Association of British Neurologists on MG management;20 The following inclusion criteria were applied: (1) age ≥ 65 years; (2) newly diagnosed MG; (3) confirmed diagnosis of MG in accordance with the Association of British Neurologists; (4) measurable severity of weakness (Myasthenia Gravis Foundation of America [MGFA] classification of ≥ II); (4) positivity for serum anti-AChR antibody, as detected by radioimmunoassay (DIAsource Immunoassay, Belgium). The exclusion criteria were as follows: (1) patients with thymoma or thymic hyperplasia; (2) patients who received immunotherapy within 6 months prior to tocilizumab treatment; (3) patients who had been taking other immunosuppressants during the study period.
All clinical data were collected from the electronic medical record system at the time of first hospital admission, including demographics, past medical history, electromyography, chest CT and laboratory examinations, drug use, and treatment.
A combination treatment of glucocorticoid and tocilizumab were performed in selected patients with VLOMG. The glucocorticoid treatment regimen was tailored to the individual patient according to the appropriate guidelines,20,21 clinician experience, and patient preferences. The participants were administered intravenous methylprednisolone at different doses (minimum dose of 120 mg once daily to a maximum dose of 500 mg once daily) for 3 consecutive days after admission. Subsequently, oral prednisone (40 mg/day) or an equivalent dose of methylprednisolone was administered. After 8 weeks of maintenance, the glucocorticoid dose was gradually tapered to the lowest effective dose (prednisone, 15 mg/day). Tocilizumab was administered intravenously at a fixed dose (4 mg/kg body weight, once every 4 weeks) dissolved in 100 mL of 0.9% NaCl one day after the initiation of glucocorticoid, and the patients received this treatment for at least 3 consecutive times. In addition, the dosage of pyridostigmine (180 mg/d in three divided doses) was stable during the study period.
To evaluate the efficacy of the therapeutic regimen and its influence on quality of life, the Quantitative Myasthenia Gravis Scale (QMGS) and MG Activities of Daily Living (MG-ADL) questionnaire were collected from the electronic medical record system before the start of the combination treatment (baseline), as well as at the time points of tocilizumab administration and 6 months after the first dose.
All data were obtained retrospectively from medical records. Two experienced neurologists assessed the adverse drug reactions (ADRs) in the hospital, following which they continued the assessments via outpatient clinic and phone calls. Serious adverse event (SAE) is defined as an event that meets one of the following criteria: (1) becomes fatal or life threatening; (2) results in persistent or significant disability or incapacity; (3) constitutes a congenital anomaly or birth defect; (4) becomes clinically meaningful (ie defined as an event that jeopardizes the participant or requires potential medical or surgical intervention to prevent one of the outcomes listed above) or requires inpatient hospitalization or prolongation of existing hospitalization. Non-serious adverse reactions are adverse reactions that do not meet the definition of a serious adverse reaction. The main safety outcome measure was the frequency of infection.
The kinetic alteration in the blood lymphocyte subsets was collected in the five patients at the following time points: baseline (before the start of treatment); time at which the first, second, and third doses of tocilizumab were administered; and 6 months after treatment. Peripheral blood mononuclear cells were isolated from heparinized blood specimens and were stained with monoclonal antibodies against CD4-APC, CD8-PE, CD19-APC, and CD16+56-PE (BD Biosciences, CA, USA). The cells were washed twice with staining buffer and analyzed with the FACScan system using CellQuest software (BD Biosciences, San Jose, CA, USA).
Statistical analyses were performed using SPSS (version 22.0; SPSS, Chicago, IL, USA). Continuous variables were presented as mean ± standard deviation or median [interquartile range (IQR)]. Categorical variables were expressed as percentages. Normally distributed data were analyzed with a one-way analysis of variance (ANOVA) by Student-Newman-Keul’s post-hoc test. Categorical variables were analyzed using the chi-square test. Statistical significance was set at P < 0.05.
Results
Five VLOMG patients (one man and four women) were identified (Table 1). The mean age at disease onset was 75.0 ± 4.5 years, and the median (Range) disease course was 14 (0.5–36) months. At the time of enrollment, the mean QMGS and MG-ADL scores were 15.4 ± 4.3 and 9.6 ± 2.3, respectively. All the patients were new-onset, and anti-AChR antibody-positive generalized MG, and were hospitalized for the disease thereafter. The MGFA classifications were as follows: IIb (2/5, 40%) and IIIb (3/5, 60%). All patients in this study had no thymoma or thymic hyperplasia, and none of them received thymectomy. The comorbidities were as follows: hypertension (n = 4), diabetes mellitus (n = 4), coronary heart disease (n = 1), and breast cancer (postoperative) (n = 1). The study was approved by the local ethics committee of Beijing Anzhen Hospital.
 |
Table 1 Low-Dose Tocilizumab Combined with Glucocorticoid Therapy in the Five Cases of Very-Late-Onset Myasthenia Gravis (VLOMG) |
The baseline and follow-up clinical assessment results are presented in Figure 1. Before the start of the combination treatment, the mean MG-ADL and QMGS scores of the VLOMG patients were 9.6 ± 2.3 and 15.4 ± 4.3, respectively; these scores exhibited a continuously decreased trend after 1, 2, 3, and 6 months of treatment. The difference in MG-ADL scores reached a significant level between baseline and 1, 2, 3 or 6 months after treatment initiation (4.6 ± 2.9 vs 9.6 ± 2.3, P = 0.003; 3.2 ± 1.5 vs 9.6 ± 2.3, P = 0.0002; 1.6 ± 1.3 vs 9.6 ± 2.3, P < 0.0001; 2.0 ± 1.2 vs 9.6 ± 2.3, P < 0.0001) (Figure 1A). Similarly, the QMGS score in the patients with VLOMG were significantly lower at 2, 3 or 6 months than at baseline (15.4 ± 4.3 vs 6.6 ± 4.6, P = 0.011; 15.4 ± 4.3 vs 4.8 ± 2.7, P = 0.002; 15.4 ± 4.3 vs 5.0 ± 2.9, P = 0.005) (Figure 1B). There were no cases of mortality during the study period. Additionally, none of the patients had COVID-19 infection or received the COVID-19 vaccinations. However, the 6-month MG-ADL and QMGS assessments were not performed at outpatient clinic in one patient, mainly due to the COVID-19 pandemic. Anti-AChR antibodies were evaluated in two patients, and showed a decreasing trend.
Skin infection occurred in one patient among those with VLOMG (20%, 1/5) (Supplementary Table S1), but clinical signs were mild and disappeared after short-term antibiotic treatment. None of the recipients of the therapeutic regimen experienced any serious adverse events. In addition, blood pressure, heart rate, and routine laboratory test results before and after treatment revealed no remarkable changes. These findings suggest that the use of tocilizumab does not generate safety concern in the short-term (six months).
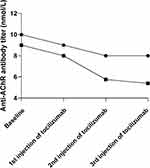
There were slight or irregular changes in the number of blood lymphocytes and CD4+ T, CD8+ T, CD19+ B, and CD56+ NK cells before treatment and at the time of the doses, as well as 6 months after treatment with tocilizumab (Supplementary Figure S1). However, two patients underwent measurement of anti-AChR antibody follow-up and exhibited a decreasing trend in serum anti-AChR antibody titers at the time of the first, second and third injections of tocilizumab (Figure 2).
Discussion
To date, the current management guidelines for the treatment of MG recommend the administration of pyridostigmine as part I initial treatment regimen in most patients with generalized MG. In cases in which symptoms persist despite the administration of pyridostigmine, immunotherapy with corticosteroids or immunosuppressive (IS) agents is administered in all MG patients, and corticosteroids are usually used as the first-line IS therapy. Moreover, a nonsteroidal IS agent, such as tacrolimus, azathioprine, mycophenolate mofetil, and cyclosporine, should be introduced initially in combination with corticosteroids when the risk of corticosteroid-related side effects is high due to medical comorbidities (ie, diabetes mellitus, osteoporosis, or ischemic heart disease) or when the steroid dose cannot be reduced because of symptom relapse.21,22 However, it is also necessary to monitor patients for potential ADRs and complications associated with these IS drugs.21 In the present study, all the patients were elderly and had generalized MG, which in most cases was concomitant with hypertension, diabetes, osteoporosis, and coronary heart disease. Hence, different doses of corticosteroids were used to avoid ADRs as much as possible, and low-dose tocilizumab was used as a replacement for the abovementioned IS drugs. Consequently, the clinical symptoms in the patients with anti-AChR antibody-positive VLOMG significantly improved without the occurrence of serious ADRs in the short term, thus substantiating the potential efficacy and safety of combination therapy regimens. On the other hands, our results should be interpreted with caution, since patients with anti-AChR antibody-positive MG have different response to immunomodulatory regimens compared to those with anti-MuSK antibody-positive MG.23 Future studies should be conducted to validate our viewpoint, and determine which subtype of MG may benefit from the therapy.
Under some circumstances, MG patients require hospitalization due to potentially fatal exacerbation, which may be triggered by a multitude of stressors such as infections, certain medicines, surgery, and vaccines.24 The recent COVID-19 pandemic has disproportionately affected people with chronic diseases, including MG.25 Various studies have reported that an increased risk of hospitalization and death due to respiratory sequelae among MG patients infected with COVID-19 as compared with matched controls,26–29 as well as safety and effectiveness of vaccination for this population.29,30 In our study, all unvaccinated patient received both corticosteroids and tocilizumab treatment, but none of them had COVID-19 infection. This finding may, at least partially, support the safety of tocilizumab in MG patients with COVID-19 in clinical practice. More research is warranted to address the issue.
Consistent with the findings reported by Jonsson et al,19 our study reconfirmed the feasibility and safety of tocilizumab for the treatment of MG; furthermore, our findings suggest that low-dose tocilizumab could be used as an alternative to conventional IS drugs in elderly patients with MG, particularly in those with concomitant cardiovascular or metabolic diseases. Interestingly, we also observed an 81-year-old male MG patient who exhibited a marked improvement in MG symptoms within 6 months after receiving three consecutive monthly intravenous injections of low-dose tocilizumab (4 mg/kg body weight) in the absence of corticosteroids and other IS therapy (Supplementary Table S2). Moreover, parallel to the clinical improvement in MG symptoms, a decreasing trend in anti-AChR antibody titers was found following therapy in our serial studies of two patients with VLOMG. Nevertheless, additional evidence is required to confirm the findings and elucidate the exact therapeutic mechanisms of this agent.
Conclusion
In conclusion, our preliminary study showed that the use of low-dose tocilizumab combined with glucocorticoid in the treatment of VLOMG significantly relieved MG symptoms without causing serious adverse events at least in the short term, thereby validating the feasibility and safety of this treatment option in managing VLOMG; furthermore, our results strongly suggests the therapeutic potential of tocilizumab against VLOMG, by improving clinical outcomes and possibly, decreasing autoantibody production. These findings might pave the way for the development of a new immunotherapy strategy for the treatment of MG. However, this study has several limitations. First, considering the small number of participants with MG, an obvious limitation is the lack of statistical power. Second, it was difficult to fully assess the effectiveness of tocilizumab, mainly owing to its combination glucocorticoid; therefore, one cannot conclude tocilizumab is effective for this group of MG patients, based on data from this study. Finally, this is a retrospective study, and the follow-up period was relatively short; therefore, prospective randomized controlled studies including a larger sample size of selected patients and longer follow-up periods are needed to validate our results.
Data Sharing Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethical Approval and Consent to Participate
The study was approved by the local ethics committee of Beijing Anzhen Hospital (no. 2022138X) and carried out in compliance with the Declaration of Helsinki. The patient’s personal data was kept confidential and informed consent was waived due to the retrospective observational nature of this study.
Acknowledgments
We thank Ms. Xiao-Ran Shen, Ms. Dan Lu, and Ms. Ling-Ye Qian for their technical assistance in the flow cytometry and radioimmunoassay tests performed in this study. We also thank Dr. Ming Ren for the careful discussion during the study period. Ms. Xiao-Ran Shen, Ms. Dan Lu, Ms. Ling-Ye Qian and Dr. Ming Ren acknowledged with their permission.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
No funding was received for this study or publication of this article.
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
1. Meriggioli MN, Sanders DB. Autoimmune myasthenia gravis: emerging clinical and biological heterogeneity. Lancet Neurol. 2009;8(5):475–490. doi:10.1016/S1474-4422(09)70063-8
2. Lascano AM, Lalive PH. Update in immunosuppressive therapy of myasthenia gravis. Autoimmun Rev. 2021;20(1):102712. doi:10.1016/j.autrev.2020.102712
3. Gilhus NE. Myasthenia Gravis. N Engl J Med. 2016;375(26):2570–2581. doi:10.1056/NEJMra1602678
4. Barnett C, Bril V. New insights into very-late-onset myasthenia gravis. Nat Rev Neuro. 2020;16(6):299–300. doi:10.1038/s41582-020-0345-3
5. Cortés-Vicente E, Álvarez-velasco R, Segovia S, et al. Clinical and therapeutic features of myasthenia gravis in adults based on age at onset. Neurology. 2020;94(11):e1171–e1180. doi:10.1212/WNL.0000000000008903
6. Alkhawajah NM, Oger J. Treatment of myasthenia gravis in the aged. Drugs Aging. 2015;32(9):689–697. doi:10.1007/s40266-015-0297-2
7. Vijayan J, Menon D, Barnett C, Katzberg H, Lovblom LE, Bril V. Clinical profile and impact of comorbidities in patients with very-late-onset myasthenia gravis. Muscle Nerve. 2021;64(4):462–466. doi:10.1002/mus.27369
8. Casetta I, Groppo E, De Gennaro R, et al. Myasthenia gravis: a changing pattern of incidence. J Neurol. 2010;257(12):2015–2019. doi:10.1007/s00415-010-5651-z
9. Zheng Y, Yuan X, Zhang C, et al. Efficacy and safety of Tacrolimus Therapy for a single Chinese Cohort with Very-Late-Onset Myasthenia Gravis. Front Neurol. 2022;13:843523. doi:10.3389/fneur.2022.843523
10. Uzawa A, Akamine H, Kojima Y, et al. High levels of serum interleukin-6 are associated with disease activity in myasthenia gravis. J Neuroimmunol. 2021;358:577634. doi:10.1016/j.jneuroim.2021.577634
11. Uzawa A, Kuwabara S, Suzuki S, et al. Roles of cytokines and T cells in the pathogenesis of myasthenia gravis. Clin Exp Immunol. 2021;203(3):366–374. doi:10.1111/cei.13546
12. Aricha R, Mizrachi K, Fuchs S, Souroujon MC. Blocking of IL-6 suppresses experimental autoimmune myasthenia gravis. J Autoimmun. 2011;36(2):135–141. doi:10.1016/j.jaut.2010.12.001
13. Deng C, Goluszko E, Tüzün E, Yang H, Christadoss P. Resistance to experimental autoimmune myasthenia gravis in IL-6-deficient mice is associated with reduced germinal center formation and C3 production. J Immunol. 2002;169(2):1077–1083. doi:10.4049/jimmunol.169.2.1077
14. Sheppard M, Laskou F, Stapleton PP, Hadavi S, Dasgupta B. Tocilizumab (Actemra). Hum Vaccin Immunother. 2017;13(9):1972–1988. doi:10.1080/21645515.2017.1316909
15. Venkiteshwaran A. Tocilizumab. MAbs. 2009;1(5):432–438. doi:10.4161/mabs.1.5.9497
16. Alvi RM, Frigault MJ, Fradley MG, et al. Cardiovascular events among adults treated with Chimeric Antigen Receptor T-Cells (CAR-T). J Am Coll Cardiol. 2019;74(25):3099–3108. doi:10.1016/j.jacc.2019.10.038
17. Tanaka T, Narazaki M, Kishimoto T. Interleukin (IL-6) Immunotherapy. Cold Spring Harb Perspect Biol. 2018;10(8):a028456. doi:10.1101/cshperspect.a028456
18. Zhang C, Zhang M, Qiu W, et al.; TANGO Study Investigators. Safety and efficacy of tocilizumab versus azathioprine in highly relapsing neuromyelitis optica spectrum disorder (TANGO): an open-label, multicentre, randomised, Phase 2 trial. Lancet Neurol. 2020;19(5):391–401. doi:10.1016/S1474-4422(20)30070-3
19. Jonsson DI, Pirskanen R, Piehl F. Beneficial effect of tocilizumab in myasthenia gravis refractory to rituximab. Neuromuscul Disord. 2017;27(6):565–568. doi:10.1016/j.nmd.2017.03.007
20. Sussman J, Farrugia ME, Maddison P, Hill M, Leite MI, Hilton-Jones D. Myasthenia gravis: association of British Neurologists’ management guidelines. Pract Neurol. 2015;15(3):199–206. doi:10.1136/practneurol-2015-001126
21. Sanders DB, Wolfe GI, Benatar M, et al. International consensus guidance for management of myasthenia gravis: executive summary. Neurology. 2016;87(4):419–425. doi:10.1212/WNL.0000000000002790
22. Alhaidar MK, Abumurad S, Soliven B, Rezania K. Current treatment of Myasthenia gravis. J Clin Med. 2022;11(6):1597. doi:10.3390/jcm11061597
23. Di Stefano V, Lupica A, Rispoli MG, Di Muzio A, Brighina F, Rodolico C. Rituximab in AChR subtype of myasthenia gravis: systematic review. J Neurol Neurosurg Psychiatry. 2020;91(4):392–395. doi:10.1136/jnnp-2019-322606
24. Gummi RR, Kukulka NA, Deroche CB, Govindarajan R. Factors associated with acute exacerbations of myasthenia gravis. Muscle Nerve. 2019;60(6):693–699. doi:10.1002/mus.26689
25. Kassardjian CD, Widdifield J, Paterson JM, et al. Serious infections in patients with myasthenia gravis: population-based cohort study. Eur J Neurol. 2020;27(4):702–708. doi:10.1111/ene.14153
26. Muppidi S, Guptill JT, Jacob S, et al. COVID-19–associated risks and effects in myasthenia gravis (CARE-MG). Lancet Neurol. 2020;19(12):970–971. doi:10.1016/S1474-4422(20)30413-0
27. Jakubíková M, Týblová M, Tesař A, et al. Predictive factors for a severe course of COVID-19 infection in myasthenia gravis patients with an overall impact on myasthenic outcome status and survival. Eur J Neurol. 2021;28(10):3418–3425. doi:10.1111/ene.14951
28. Roy B, Kovvuru S, Nalleballe K, Onteddu SR, Nowak RJ. Electronic health record–derived impact of COVID-19 on myasthenia gravis. J Neurol Sci. 2021;423:117362. doi:10.1016/j.jns.2021.117362
29. Lupica A, Di Stefano V, Iacono S, et al. Impact of COVID-19 in AChR Myasthenia Gravis and the Safety of Vaccines: data from an Italian Cohort. Neurol Int. 2022;14(2):406–416. doi:10.3390/neurolint14020033
30. Alcantara M, Koh M, Park AL, Bril V, Barnett C. Outcomes of COVID-19 infection and vaccination among individuals with Myasthenia Gravis. JAMA Netw Open. 2023;6(4):e239834. doi:10.1001/jamanetworkopen.2023.9834
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.