Back to Journals » Lung Cancer: Targets and Therapy » Volume 8
A pilot study of zoledronic acid in the treatment of patients with advanced malignant pleural mesothelioma
Authors Jamil MO, Jerome MS, Miley D, Selander KS, Robert F
Received 9 March 2017
Accepted for publication 21 April 2017
Published 12 June 2017 Volume 2017:8 Pages 39—44
DOI https://doi.org/10.2147/LCTT.S135802
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Pan-Chyr Yang
Muhammad Omer Jamil, Mary S Jerome, Deborah Miley, Katri S Selander, Francisco Robert
Division of Hematology and Oncology, Department of Medicine, Comprehensive Cancer Center, University of Alabama at Birmingham, Birmingham, AL, USA
Purpose: Malignant pleural mesothelioma (MPM) is a rare malignancy with a dismal median survival of <12 months with current therapy. Single and combination chemotherapy regimens have shown only modest clinical benefit. In preclinical studies, nitrogen-containing bisphosphonates (zoledronic acid) inhibit growth of mesothelioma cells by different mechanisms: inhibition of mevalonate pathway, inhibition of angiogenesis, activation of apoptosis through caspase activation, and alteration in activity of matrix metalloproteinases, thereby affecting invasiveness of cancer cells.
Patients and methods: We investigated the role of zoledronic acid in a pilot, single-arm trial of MPM patients with Eastern Cooperative Oncology Group (ECOG) performance status (PS) 0–2 who had progressed on prior treatments or had not received systemic therapy due to poor PS. Primary end point was composite response rate by modified response evaluation criteria in solid tumors and/or metabolic response by 2-deoxy-2-[fluorine-18]fluoro-d-glucose (18F-FDG) positron emission tomography criteria. Secondary end points were progression-free survival (PFS) and overall survival (OS). Exploratory end points include the effect of zoledronic acid therapy on vascular endothelial growth factor (VEGF), basic fibroblast growth factor, interleukin 8, transforming growth factor beta, mesothelin, and osteopontin levels.
Results: Eight male patients (median age of 62 years) with the following clinical characteristics were treated; ECOG PS was 0–2, 75% with epithelioid type, and 62% had prior chemotherapy. Overall composite response rate was 12.5% and the clinical benefit rate (response + stable disease) was 37.5%. Median PFS was 2 months (0.5–21 months) and median OS was 7 months (0.8–28 months). No treatment-related toxicities were observed. Lower VEGF levels were predictive of favorable response and mesothelin levels correlated with disease course.
Conclusion: Zoledronic acid shows modest clinical activity without significant toxicity in patients with advanced MPM.
Keywords: mesothelioma, treatment, bisphosphonates
Introduction
Malignant pleural mesothelioma (MPM) is an aggressive tumor of serosal surfaces. Its incidence is increasing worldwide due to asbestos use.1 MPM occurs mainly in men, aged 60–80 years, and the majority of them die due to local extension and/or respiratory failure.1,2 Median survival of patients ranges from 12 to 19 months with combination chemotherapy. Pemetrexed plus cisplatin, the current standard chemotherapy regimen, yield a median overall survival (OS) of 12.1 months and a median time to progression of 5.7 months.3 Poor prognostic markers include: poor performance status (PS), high leukocyte count, high platelet count (>400,000/µL), nonepithelial tumor type, and male gender.4 Despite new chemotherapy combinations, MPM patient outcome is modest,5–11 thus a great need for improvement exists.
Bisphosphonates are osteoporosis drugs that have been in clinical use for decades.12 In addition to their antiresorptive effects in osteoporosis and cancer-induced osteolytic bone disease, nitrogen-containing bisphosphonates (such as zoledronic acid and risedronic acid) inhibit tumor proliferation.12–14 Zoledronic acid effectively inhibits proliferation of mesothelioma cell lines in vitro and in vivo.15 This result is likely an outcome of mevalonate pathway inhibition,12,16 leading to depletion of prenyl groups needed in posttranslational modification and activation of small GTPases,14 ultimately inhibiting cell growth. In addition, zoledronic acid reduces experimental malignant pleural effusions.17 It demonstrates antiangiogenic effects18–20 and suppresses vascular endothelial growth factor (VEGF) blood levels.21 VEGF levels have been associated with MPM progression and poor survival22,23 in relation to high tumor microvessel density.22,24
Computed tomography (CT) response evaluation criteria in solid tumors (RECIST) is difficult to use in MPM due to circumferential and axial growth. Thus, MPM response to treatment is usually measured with imaging using modified RECIST assessment criteria.25 MPM is 2-deoxy-2-[fluorine-18]fluoro-d-glucose (18F-FDG) positron emission tomography (PET) avid,26 and 18F-FDG PET scans can be used to document early responses27,28 and have been useful in the assessment of prognosis and staging.29,30 Mesothelin and osteopontin are glycoproteins that are overexpressed in mesothelioma and are associated with tumor progression. Serum markers such as mesothelin and osteopontin are of interest for prognosis, tumor response, and tumor progression.31–33 On the basis of these findings, we conducted a pilot, proof-of-concept feasibility study of zoledronic acid in the treatment of advanced MPM. We measured the levels of antiangiogenic factors (VEGF, bFGF, IL-8, and TGF-β), mesothelin, and osteopontin before and during treatment.
Patients and methods
Study design and patients
This single-arm, prospective study was conducted at the University of Alabama at Birmingham Comprehensive Cancer Center. The primary objective of this study was to evaluate the antitumor activity of zoledronic acid in subjects with unresectable, advanced MPM assessed by the modified RECIST criteria,25 and/or metabolic response 18F-FDG PET assessment.34 The secondary objectives were to assess duration of response, progression-free survival (PFS), OS, and safety and tolerability of zoledronic acid in MPM patients. Blood levels of VEGF, bFGF, IL-8, TGF-β, mesothelin, and osteopontin were measured to investigate the influence of zoledronic acid.
Inclusion criteria included: adult patients (age>18) with unresectable MPM who had progressed after one or more prior systemic therapies, had not received prior systemic therapy due to poor PS, and/or were unwilling to receive systemic chemotherapy. Other eligibility criteria included life expectancy of at least 2 months, measurable disease by CT or 18F-FDG PET, willing to consent to contraception (if applicable), and ability to provide consent. Exclusion criteria were brain metastasis, second cancer diagnosis, heart disease (class 3 or 4 heart failure by New York Heart failure classification), acute coronary syndrome (within 6 months), known human immunodeficiency virus or hepatitis, clinically significant arrhythmias, serious acute systemic disease, dental disease, and history of osteonecrosis of the jaw.
This study was approved by the Institutional Review Board at the University of Alabama at Birmingham Comprehensive Cancer Center. Written informed consent was obtained from all study participants.
The study was registered with the National Clinical Trials Network, number NCT01204203.
Treatment and assessments
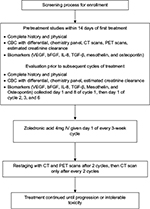
Study schema is presented in Figure 1. Patients were treated with intravenous (IV) infusion of zoledronic acid (Novartis Pharmaceuticals, East Hanover, NJ, USA) 4 mg IV over 15 minutes on day 1 of a 3-week cycle. Patients were evaluated for response every 2 cycles with CT scans and 18F-FDG PET scans only on cycle 2. Subjects with either stable disease or response continued treatment until disease progression and/or intolerable toxicity at which patients were taken off study. Dose adjustment was allowed per standard guidelines for zoledronic acid for decreased creatinine clearance. Subjects were monitored for toxicity using National Cancer Institute Common Terminology Criteria for Adverse Events Version 3.0 criteria. Patients who completed at least one treatment cycle were included in data analysis.
Sample collection and analysis for correlative objectives
Blood samples for VEGF, bFGF, IL-8, TGF-β, mesothelin, and osteopontin were collected prior to treatment on days 1 and 8 of cycle 1 and then prior to treatment on day 1 of cycles 2, 3, and 6. Plasma samples were aliquoted and stored at −70°C. Blood levels of these biomarkers were analyzed using ELISA (enzyme-linked immunosorbent assay) kits from R&D systems (R&D Systems, Inc., Minneapolis, MN, USA) Quantikine assays, according to manufacturer’s instructions.
Statistical analysis
Responses were assessed as composite response rate which include modified RECIST objective responses and metabolic response 18F-FDG PET assessment. Data are presented as descriptive statistics. OS and PFS are presented as median, whereas VEGF, bFGF, IL-8, TGF-β, mesothelin, and osteopontin are presented as mean values.
Results
Demographics
From November 2010 to January 2015, 9 patients consented, but only 8 patients were treated. All patients were male (7 Caucasian and 1 African-American), median age of 62 years (age range 49–77), and Eastern Cooperative Oncology Group ( ECOG) PS 0–2. Three patients were never-smokers. One patient had stage 2 disease, and 7 patients had stage 4 disease. The majority of the patients (n=6) had epithelioid mesothelioma. All except 2 patients reported a history of asbestos exposure. All patients included in this study had symptomatic, active disease with measurable parameters by CT and PET scans; previously treated patients had progressive symptomatic disease after the last line of treatment. Three patients were treatment naïve, whereas others had received one or more lines of chemotherapy agents, including pemetrexed/cisplatin or carboplatin, single-agent carboplatin, pemetrexed, and cediranib.
Treatment outcomes
Median duration of follow-up was 1.3 months (0.3–21 months), median number of treatment cycles was 2 (1–28 cycles). Baseline tumor parameters and response assessment after cycle 2 of treatment are summarized in Table 1. No response was seen when patients were evaluated by modified RECIST criteria; 2 patients had the best response of stable disease, 1 response lasted 21 months. When assessed with 18F-FDG PET, 1 patient with stable disease by modified RECIST had partial response by 18F-FDG PET criteria, whereas another patient thought to have progressive disease by modified RECIST had stable disease by 18F-FDG PET criteria. Overall composite response rate was 12.5%. Clinical benefit rate (response + stable disease) was 37.5%. Median PFS was 2 months (0.5–21 months). Median OS was 7 months (0.8–28 months). The histopathological characteristics of the MPM responders include 1 epithelioid and 1 mixed histology; all 6 MPM nonresponders had epithelioid histology. No treatment-related toxicities were observed.
Correlative studies result
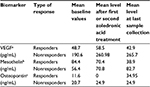
Higher baseline levels of VEGF and osteopontin were observed in nonresponders (Table 2). Decreasing levels of mesothelin and osteopontin were seen in patients with response or stable disease. Zoledronic acid initially led to increase in VEGF levels in all patients with subsequent decrease. Increasing VEGF levels was seen in patients with progressive disease. No association with disease burden or progression was seen with IL-8, bFGF, or TGF-β.
Discussion
This pilot feasibility study assessed whether bisphosphonates could have a role in the treatment of mesothelioma due to their antiangiogenic properties. Our findings suggest that zoledronic acid has single-agent activity in MPM treatment. Angiogenesis is a complex process, difficult to target due to multiple VEGF-dependent and VEGF-independent pathways.35,36 Multiple antiangiogenic agents have been previously used for the treatment of MPM; however, responses have been modest with bevacizumab having the most evidence.37–47 A recent publication reporting the combination of bevacizumab with pemetrexed and cisplatin showed increased PFS and OS.41 Zoledronic acid can mitigate angiogenesis at subcellular level by mevalonate pathway inhibition and is beneficial in antitumor therapy.14,15,48–50 In our study, clinical benefit in at least 2 patients was observed without toxicity, which makes this drug an attractive agent for combination with chemotherapy or other antiangiogenic agents for future trials. Lower VEGF levels were associated with favorable responses similar to previous results.41,42,51 However, an interesting finding was the initial increase and then decrease in VEGF levels with zoledronic therapy in responder patients (Table 2). It has been shown that maximal tolerated chemotherapy increases the expression of VEGF and other proangiogenic growth factors in a rebound response to treatment. It is possible that similar initial rebound effect can be seen with zolendronic acid in responders; and more profound effect in nonresponders, indicative of an adaptive (evasive) mechanism of resistance.52
IL-8 and bFGF, which have been shown to enhance neovascularization and promote tumor growth,53 did not correlate with zoledronic acid therapy. Serum mesothelin levels are elevated in MPM, but is not used as a biomarker for diagnosis due to low sensitivity.31,54,55 It has been shown to correlate with prognosis56–58 and has been a target for MPM immunotherapy.59,60 Our study reports that mesothelin and osteopontin levels decline in patients who have favorable therapy response and potentially may represent promising biomarkers of this disease.
In our patient population, as well as in the SEER Cancer Statistics, the incidence of mesothelioma is much more common in men than in women.61 This prevalence is reflected in the demographic data of our study. Previous studies have shown women diagnosed with mesothelioma typically have more favorable outcomes and a statistically better chance of survival than males.61
Conclusion
Our pilot study suggests modest activity of zoledronic acid as a single agent in the treatment of mesothelioma and warrants further investigation in combination with other agents.
Acknowledgments
This work was supported by the UAB Comprehensive Cancer Center Core Grant-CA13148 and Novartis. The funding sources provided financial resources and had no involvement in the study design, collection/analysis/interpretation of data, report writing, or in the decision to submit the article for publication. The authors greatly appreciate the participation of the study patients. The authors would like to thank the Clinic Research team and Tasha Renee Smith PhD, MPH who assisted with manuscript preparation.
Disclosure
Dr Robert reports grants from UAB Comprehensive Cancer Center Core Grant-CA13148 and Novartis during the conduct of the study. He reports grants from TRACON, Stemcentrx, Inc., Pfizer, AstraZeneca, Boehringer Ingelheim, Sanofi, EMD Serono, Clovis Oncology, Threshold Pharmaceuticals, Mirati Therapeutics, and Novartis and personal fees from Merck and Boehringer Ingelheim outside the submitted work. The other authors report no conflicts of interest in this work.
References
Grondin SC, Sugarbaker DJ. Malignant mesothelioma of the pleural space. Oncology (Williston Park). 1999;13(7):919–926; discussion 926, 931–932. | ||
Yang H, Testa JR, Carbone M. Mesothelioma epidemiology, carcinogenesis, and pathogenesis. Curr Treat Options Oncol. 2008;9(2–3):147–157. | ||
Vogelzang NJ, Rusthoven JJ, Symanowski J, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol. 2003;21(14):2636–2644. | ||
Curran D, Sahmoud T, Therasse P, van Meerbeeck J, Postmus PE, Giaccone. Prognostic factors in patients with pleural mesothelioma: the European Organization for Research and Treatment of Cancer experience. J Clin Oncol. 1998;16(1):145–152. | ||
Ceresoli GL, Zucali PA, Favaretto AG, et al. Phase II study of pemetrexed plus carboplatin in malignant pleural mesothelioma. J Clin Oncol. 2006;24(9):1443–1448. | ||
van Meerbeeck JP, Gaafar R, Manegold C, et al. Randomized phase III study of cisplatin with or without raltitrexed in patients with malignant pleural mesothelioma: an intergroup study of the European Organisation for Research and Treatment of Cancer Lung Cancer Group and the National Cancer Institute of Canada. J Clin Oncol. 2005;23(28):6881–6889. | ||
Byrne MJ, Davidson JA, Musk AW, et al. Cisplatin and gemcitabine treatment for malignant mesothelioma: a phase II study. J Clin Oncol. 1999;17(1):25–30. | ||
Nowak AK, Byrne MJ, Williamson R, et al. A multicentre phase II study of cisplatin and gemcitabine for malignant mesothelioma. Br J Cancer. 2002;87(5):491–496. | ||
Castagneto B, Zai S, Dongiovanni D, et al. Cisplatin and gemcitabine in malignant pleural mesothelioma: a phase II study. Am J Clin Oncol. 2005;28(3):223–226. | ||
Utkan G, Buyukcelik A, Yalcin B, et al. Divided dose of cisplatin combined with gemcitabine in malignant mesothelioma. Lung Cancer. 2006;53(3):367–374. | ||
van Haarst JM, Baas P, Manegold C, et al. Multicentre phase II study of gemcitabine and cisplatin in malignant pleural mesothelioma. Br J Cancer. 2002;86(3):342–345. | ||
Luckman SP, Hughes DE, Coxon FP, Graham R, Russell G, Rogers MJ. Nitrogen-containing bisphosphonates inhibit the mevalonate pathway and prevent post-translational prenylation of GTP-binding proteins, including Ras. J Bone Miner Res. 1998;13(4):581–589. | ||
Conte P, Coleman R. Bisphosphonates in the treatment of skeletal metastases. Semin Oncol. 2004;31(5 Suppl 10):59–63. | ||
Rogers MJ. New insights into the molecular mechanisms of action of bisphosphonates. Curr Pharm Des. 2003;9(32):2643–2658. | ||
Wakchoure S, Merrell MA, Aldrich W, et al. Bisphosphonates inhibit the growth of mesothelioma cells in vitro and in vivo. Clin Cancer Res. 2006;12(9):2862–2868. | ||
Oades GM, Senaratne SG, Clarke IA, et al. Nitrogen containing bisphosphonates induce apoptosis and inhibit the mevalonate pathway, impairing Ras membrane localization in prostate cancer cells. J Urol. 2003;170(1):246–252. | ||
Stathopoulos GT, Moschos C, Loutrari H, et al. Zoledronic acid is effective against experimental malignant pleural effusion. Am J Respir Crit Care Med. 2008;178(1):50–59. | ||
Vincenzi B, Santini D, Dicuonzo G, et al. Zoledronic acid-related angiogenesis modifications and survival in advanced breast cancer patients. J Interferon Cytokine Res. 2005;25(3):144–151. | ||
Wood J, Bonjean K, Ruetz S, et al. Novel antiangiogenic effects of the bisphosphonate compound zoledronic acid. J Pharmacol Exp Ther. 2002;302(3):1055–1061. | ||
Ferretti G, Fabi A, Carlini P, et al. Zoledronic-acid-induced circulating level modifications of angiogenic factors, metalloproteinases and proinflammatory cytokines in metastatic breast cancer patients. Oncology. 2005;69(1):35–43. | ||
Santini D, Vincenzi B, Dicuonzo G, et al. Zoledronic acid induces significant and long-lasting modifications of circulating angiogenic factors in cancer patients. Clin Cancer Res. 2003;9(8):2893–2897. | ||
Strizzi L, Catalano A, Vianale G, et al. Vascular endothelial growth factor is an autocrine growth factor in human malignant mesothelioma. J Pathol. 2001;193(4):468–475. | ||
Karrison T, Kindler HL, Gandara DR, et al. Final analysis of a multi-center, double-blind, placebo-controlled, randomized phase II trial of gemcitabine/cisplatin (GC) plus bevacizumab (B) or placebo (P) in patients (pts) with malignant mesothelioma (MM). J Clin Oncol. 2007;25(18S):7526. | ||
Ohta Y, Shridhar V, Bright RK, et al. VEGF and VEGF type C play an important role in angiogenesis and lymphangiogenesis in human malignant mesothelioma tumours. Br J Cancer. 1999;81(1):54–61. | ||
Byrne MJ, Nowak AK. Modified RECIST criteria for assessment of response in malignant pleural mesothelioma. Ann Oncol. 2004;15(2):257–260. | ||
Bénard F, Sterman D, Smith RJ, Kaiser LR, Albelda SM, Alavi A. Metabolic imaging of malignant pleural mesothelioma with fluorodeoxyglucose positron emission tomography. Chest. 1998;114(3):713–722. | ||
Francis RJ, Byrne MJ, van der Schaaf AA, et al. Early prediction of response to chemotherapy and survival in malignant pleural mesothelioma using a novel semiautomated 3-dimensional volume-based analysis of serial 18F-FDG PET scans. J Nucl Med. 2007;48(9):1449–1458. | ||
Ceresoli GL, Chiti A, Zucali PA, et al. Early response evaluation in malignant pleural mesothelioma by positron emission tomography with [18F] fluorodeoxyglucose. J Clin Oncol. 2006;24:4587–4593. | ||
Bénard F, Sterman D, Smith RJ, Kaiser LR, Albelda SM, Alavi A. Prognostic value of FDG PET imaging in malignant pleural mesothelioma. J Nucl Med. 1999;40(8):1241–1245. | ||
Flores RM, Akhurst T, Gonen M, Larson SM, Rusch VW. Positron emission tomography defines metastatic disease but not locoregional disease in patients with malignant pleural mesothelioma. J Thorac Cardiovasc Surg. 2003;126(1):11–16. | ||
Robinson BW, Creaney J, Lake R, et al. Mesothelin-family proteins and diagnosis of mesothelioma. Lancet. 2003;362(9396):1612–1616. | ||
Sandhu H, Dehnen W, Roller M, Abel J, Unfried K. mRNA expression patterns in different stages of asbestos-induced carcinogenesis in rats. Carcinogenesis. 2000;21(5):1023–1029. | ||
Wai PY, Kuo PC. The role of Osteopontin in tumor metastasis. J Surg Res. 2004;121(2):228–241. | ||
Young H, Baum R, Cremerius U, et al. Measurement of clinical and subclinical tumour response using [18F]-fluorodeoxyglucose and positron emission tomography: review and 1999 EORTC recommendations. European Organization for Research and Treatment of Cancer (EORTC) PET Study Group. Eur J Cancer. 1999;35:1773–1782. | ||
Gaur P, Bose D, Samuel S, Ellis LM. Targeting tumor angiogenesis. Semin Oncol. 2009;36(2 Suppl 1): S12–19. | ||
Abdullah SE, Perez-Soler R. Mechanisms of resistance to vascular endothelial growth factor blockade. Cancer. 2012;118(14):3455–3467. | ||
Ceresoli GL, Zucali PA. Anti-angiogenic therapies for malignant pleural mesothelioma. Expert Opin Investig Drugs. 2012;21(6):833–844. | ||
Toyooka S, Kishimoto T, Date H. Advances in the molecular biology of malignant mesothelioma. Acta Med Okayama. 2008;62(1):1–7. | ||
Marquez-Medina D, Popat S. Closing faucets: the role of anti-angiogenic therapies in malignant pleural diseases. Clin Transl Oncol. 2015;18(8):760–768. | ||
Buikhuisen WA, Scharpfenecker M, Griffioen AW, Korse CM, van Tinteren H, Baas P. A randomized phase II study adding axitinib to pemetrexed-cisplatin in patients with malignant pleural mesothelioma: a single-center trial combining clinical and translational outcomes. J Thorac Oncol. 2016;11(5):758–768. | ||
Zalcman G, Mazieres J, Margery J, et al. Bevacizumab for newly diagnosed pleural mesothelioma in the Mesothelioma Avastin Cisplatin Pemetrexed Study (MAPS): a randomised, controlled, open-label, phase 3 trial. Lancet. 2015;387(10026):1405–1414. | ||
Kindler HL, Karrison TG, Gandara DR, et al. Multicenter, double-blind, placebo-controlled, randomized phase II trial of gemcitabine/cisplatin plus bevacizumab or placebo in patients with malignant mesothelioma. J Clin Oncol. 2012;30(20):2509–2515. | ||
Tsao AS, Harun N, Lee JJ, et al. Phase I trial of cisplatin, pemetrexed, and imatinib mesylate in chemonaive patients with unresectable malignant pleural mesothelioma. Clin Lung Cancer. 2014;15(3):197–201. | ||
Nowak AK, Millward MJ, Creaney J, et al. A phase II study of intermittent sunitinib malate as second-line therapy in progressive malignant pleural mesothelioma. J Thorac Oncol. 2012;7(9):1449–1456. | ||
Papa S, Popat S, Shah R, et al. Phase 2 study of sorafenib in malignant mesothelioma previously treated with platinum-containing chemotherapy. J Thorac Oncol. 2013;8(6):783–787. | ||
Dubey S, Jänne PA, Krug L, et al. A phase II study of sorafenib in malignant mesothelioma: results of cancer and leukemia group B 30307. J Thorac Oncol. 2010;5(10):1655–1661. | ||
Campbell NP, Kunnavakkam R, Leighl N, et al. Cediranib in patients with malignant mesothelioma: a phase II trial of the University of Chicago Phase II Consortium. Lung Cancer. 2012;78(1):76–80. | ||
Ishtiaq S, Edwards S, Sankaralingam A, et al. The effect of nitrogen containing bisphosphonates, zoledronate and alendronate, on the production of pro-angiogenic factors by osteoblastic cells. Cytokine. 2015;71(2):154–160. | ||
Barghash RF, Abdou WM. Pathophysiology of metastatic bone disease and the role of the second generation of bisphosphonates: from basic science to medicine. Curr Pharm Des. 2016;22(11):1546–1557. | ||
Zekri J, Mansour M, Karim SM. The anti-tumour effects of zoledronic acid. J Bone Oncol. 2014;3(1):25–35. | ||
Duysinx BC, Corhay JL, Hubin L, Nguyen D, Henket M, Louis R. Diagnostic value of interleukine-6, transforming growth factor-beta 1 and vascular endothelial growth factor in malignant pleural effusions. Respir Med. 2008;102(12):1708–1714. | ||
Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer. 2008;8(8):592–603. | ||
Judge S, Thomas P, Govindarajan V, Sharma P, Loggie B. Malignant peritoneal mesothelioma: characterization of the inflammatory response in the tumor microenvironment. Ann Surg Oncol. 2015;23(5):1496–1500. | ||
Creaney J, Dick IM, Robinson BW. Comparison of mesothelin and fibulin-3 in pleural fluid and serum as markers in malignant mesothelioma. Curr Opin Pulm Med. 2015;21(4):352–356. | ||
Franceschini MC, Ferro P, Canessa PA, et al. Mesothelin in serum and pleural effusion in the diagnosis of malignant pleural mesothelioma with non-positive cytology. Anticancer Res. 2014;34(12):7425–7429. | ||
Pass HI, Goparaju C, Espin-Garcia O, et al. Plasma biomarker enrichment of clinical prognostic indices in malignant pleural mesothelioma. J Thorac Oncol. 2016;11(6):900–909. | ||
Vigneri P, Martorana F, Manzella L, Stella S. Biomarkers and prognostic factors for malignant pleural mesothelioma. Future Oncol. 2015; 11(24 Suppl):29–33. | ||
Linch M, Gennatas S, Kazikin S, et al. A serum mesothelin level is a prognostic indicator for patients with malignant mesothelioma in routine clinical practice. BMC Cancer. 2014;14:674. | ||
Morello A, Sadelain M, Adusumilli PS. Mesothelin-targeted CARs: driving T cells to solid tumors. Cancer Discov. 2016;6(2):133–146. | ||
Mazor R, Zhang J, Xiang L, et al. Recombinant immunotoxin with T-cell epitope mutations that greatly reduce immunogenicity for treatment of mesothelin-expressing tumors. Mol Cancer Ther. 2015;14(12):2789–2796. | ||
Howlander N, Noone AM, Krapcho M, et al, editors. SEER Cancer Statistics Review 1975–2010. National Cancer Institute [updated April 2013]. Bethesda, MD. Available from: http://seer.cancer.gov/csr/1975_2010/. Accessed April 2013. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.