Back to Journals » Drug Design, Development and Therapy » Volume 11
A novel transdermal nanoethosomal gel of betahistine dihydrochloride for weight gain control: in-vitro and in-vivo characterization
Authors El-Menshawe SF, Ali AA, Halawa AA, Srag El-Din AS
Received 22 June 2017
Accepted for publication 18 October 2017
Published 28 November 2017 Volume 2017:11 Pages 3377—3388
DOI https://doi.org/10.2147/DDDT.S144652
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Sukesh Voruganti
Shahira F El-Menshawe,1 Adel Ahmed Ali,1 Abdelkhalk Ali Halawa,2 Ahmed SG Srag El-Din2
1Department of Pharmaceutics and Industrial Pharmacy, Beni-Suef University, Beni-Suef, 2Department of Pharmaceutics and Clinical Pharmacy, Nahda University, Beni-Suef, Egypt
Background: Betahistine dihydrochloride (BDH) is a histamine analog used to control weight gain, with short elimination half-life and gastric irritation as side effects.
Objective: The aim of the current investigation is to formulate and optimize a topical BDH ethosomal gel for weight gain control.
Materials and methods: Box–Behnken design was applied to study the effect of independent variables: phosphatidylcholine (PC), propylene glycol (PG), and ethanol on vesicle size; entrapment efficiency; % drug release; and flux. The morphology and zeta potential of the optimized formulation were evaluated. The % drug release, flux, and pharmacodynamics of the optimized formulation gel were studied.
Results: The size and entrapment efficiency percent had a direct positive relationship with the concentration of PC and negative relationship with ethanol and PG. The % drug release and flux decreased with increasing PC and PG, while ethanol enhanced both responses. Regression modeling indicated a good correlation between dependent and independent variables, where F16 was chosen as the optimized formulation. F16 showed well-defined spherical vesicles and zeta potential of −24 mV, and % release from the gel exceeded 99.5% over 16 h with the flux of 0.28 mg/cm2/h. Food intake and weight gain of rats were significantly decreased after transdermal application of the BDH ethosomal gel when compared with control, placebo, and BDH gel. The histopathological findings proved the absence of inflammation and decrease in adipose tissue.
Conclusion: Results obtained showed a significant, sustained transdermal absorption of BDH ethosomal gel and, consequently, a decrease in food intake and weight gain.
Keywords: Box–Behnken design, regression modeling, neural histamine, pharmacodynamics, obesity
Introduction
Obesity is a common metabolic disorder attributed to increased food intake and physical inactivity; it is considered as a serious risk factor for various diseases such as type II diabetes, coronary artery disease, atherosclerosis, hypertension, and hyperlipidemia.1 Although the pathophysiology behind obesity is poorly understood, overeating is likely one of the most important factors. Thus, decreasing food intake might be effective in the treatment or prevention of obesity. Brain histamine has been shown to have a significant effect on weight gain and food intake. It has been suggested that H1 receptor activation and H3 inhibition decrease food intake.2 Masaki et al have demonstrated that central infusion of histamine decreased fat accumulation and leptin, and enhanced insulin sensitivity in a leptin-resistant obese mouse.3 Kasaoka reported that the histamine neurons system suppresses fat accumulation by regulating mRNA expression of uncoupling protein, which expends energy consumption in rats.4 Yoshimatsu et al reported that histamine neurons downregulate ob gene expression in rat white adipose tissue.5 These data suggest that drugs with H1 agonist and H3 antagonist properties would inhibit food intake.
Betahistine dihydrochloride (BDH), a centrally acting H3 antagonist and H1 agonist, is used for the management of Ménière’s disease due to its vasodilatation action in the cerebral vasculature region and reduction in food intake by increasing brain histamine.6–8 Tighilet et al reported that BDH administration for 1 or 3 weeks induced an upregulation of histidine decarboxylase mRNA expression suggesting that histamine synthesis and release are increased under BDH treatment.9 Barak et al reported that BDH, at doses ranging from 16 to 48 mg/day, did not have a significant effect on weight loss compared with a placebo over a 12 weeks interval in 281 adults. However, a subgroup post hoc analysis suggested that weight loss occurred in non-Hispanic women <50 years old, which was consistent with a potential sexual dimorphism.10 The acute effect of BDH on food intake and appetite has been studied in obese women for 24 h, where the highest dose in that study was 1.3 mg/kg of body weight. Results showed no difference to the given BDH dose. However, longer period of administration or higher dose may show the effect of BDH on food intake.11 In animal models, there is some evidence for sexual dimorphism in the histaminergic control of feeding. Female Wistar King rats exposed to dietary histidine decreased their food intake more than do male rats or ovariectomized female rats.12 Two animal studies carried out by Rossi et al and Szelag et al have proved the effectiveness of BDH in reducing food intake.13,14 Unfortunately, BDH has a short plasma half-life (3–4 h), which necessitates its frequent dosing, and consequently, increases the risk of peptic ulcer and gastric irritation when taken orally.15 Several studies have been carried out to control BDH release and explore a new route for its administration like the transdermal route and mucoadhesive tablets for buccal delivery.8,16,17
Ethosomes are specially tailored vesicular carriers characterized by their ability to entrap both hydrophilic and lipophilic drugs. In addition, ethosomes could be applied topically to produce systemic action with high skin tolerability and safety.18–21
Furthermore, application of various ethosomal systems containing acyclovir and salicylic acids and clindamycin or prostaglandin E1 to human skin in three clinical studies have not shown adverse skin reactions.22 Paolino et al studied skin tolerability of ethosomes on a healthy human by reflectance spectrophotometry after topical application. The results showed that the ethosomal system did not induce skin erythema whereas the application of hydroethanolic solution with an equal water/ethanol ratio to that of ethosomes resulted in significant skin erythema, which indicate the safety of ethosomes.23
The aim of the current study was to optimize ethosomal gel containing BDH and investigate its effect on decreasing food intake and weight gain. Several ethosomal formulations were prepared and characterized to select an optimum composition. The optimized formulation was subjected to both in-vitro and in-vivo characterizations.
Materials and methods
Materials
BDH was obtained as a gift sample from Pharco Pharmaceuticals (Amriya, Alexandria, Egypt), Soya beans phosphatidylcholine (PC) was purchased from Across Organics (Geel, Belgium), and propylene glycol (PG) was obtained as a gift from Arabcomed Company (Cairo, Egypt). Carbopol 934 (CP) was obtained from SERVA Electrophoresis GmbH (Heidelberg, Germany). Poloxamer 407 was obtained from Sigma-Aldrich (St Louis, MO, USA). The other ingredients used were of analytical grade.
Methods
Experimental design and data analysis
A three-factors, three-level Box–Behnken design was constructed using Design Expert® (Version 7.0.0, Stat-Ease Inc. Minneapolis, MN, USA), and a design matrix comprising 15 experimental runs (Table 1) was used in ethosomes formulations. The concentration range and ingredient of independent variables were chosen based on preliminary research experiments and data collected from the literature review. The nonlinear computer-generated quadratic model was given as
|
where Y is the measured response associated with each factor level combination; b0 is the intercept; b1 to b33 are the regression coefficients computed from the observed experimental values of Y; X1, X2, and X3 are the coded levels of independent variables. The terms X1X2 and 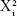 (i=1, 2, or 3) represent the interaction and quadratic terms, respectively.
(i=1, 2, or 3) represent the interaction and quadratic terms, respectively.
Ethosomes preparation
BDH ethosomes were prepared by the method described by Touitou et al with a slight modification.19 Briefly, the ethosomal suspension was prepared by dissolving PC and BDH in absolute alcohol using covered vessels under vigorous stirring at room temperature. PG was added, followed by water under constant mixing at 700 rpm. Mixing was continued for an extra 30 min. The system was maintained at 30°C during the preparation. The preparation was cooled at room temperature before sonication at 4°C for three cycles, 5 min each, and a 5 min rest between cycles.
Characterization of BDH vesicles
Determination of vesicle size
The vesicle size was determined by dynamic light scattering.24 One-milliliter ethosomal suspension was diluted to 10 mL with distilled water and measured using Malvern Zeta Sizer (Zetasizer Ver. 7.11; Malvern Instruments, Malvern, UK) at 25°C. Three replicates of each sample were taken.
Entrapment efficiency percent
Ultracentrifugation method was used for the determination of entrapment efficiency percent (EE%). Vesicular preparations were centrifuged using a cooling centrifuge (Sigma Laborzentrifugen D-37520, Osterode, Germany) at 4°C and 15,000 rpm for 3 h with 15 min pauses after every 30 min. The supernatant was diluted with ethanol and measured with a UV spectrophotometer (Shimadzu UV-1800, Tokyo, Japan) at 261 nm. The mean of the three EE% measurements was then calculated based on the following equation:
|
|
where DT is the theoretical amount of BDH and DS is the detected amount.
In-vitro release studies
The drug released from the prepared vesicles was determined using the dialysis method. A glass cylinder open at both ends was attached to the shaft of the United States Pharmacopoeia (USP) dissolution apparatus (Erweka DT-720, Erweka GmbH, Heusenstamm, Germany), and dialysis membrane was tied at one end of the glass cylinder.25 A volume of ethosomes formulations equivalent to 10 mg of the BDH was accurately pipetted into the cylinder. The cylinder was then suspended in 500 mL of phosphate buffer (pH 7.4) and the dissolution medium was maintained at 37°C±0.5°C. The speed of the device shaft was set at 50 rpm. The time for test run was 6 h and aliquots (2 mL) were withdrawn at intervals of 1, 2, 3, 4, 5, and 6 h and analyzed by UV spectrophotometer at 261 nm.26–28 The cumulative amount of BDH released after 6 h (Q6 h) was calculated for each formulation and represented as mean ± SD.
Ex-vivo permeation studies
Permeation studies were performed using the dialysis method described above under the same experimental conditions for in-vitro release studies except for the use of freshly acquired abdominal skin from female albino rats instead of the dialysis membrane.29 The whole abdominal region was carefully shaved and excised immediately before the start of experiment. The skin was later mounted in a receptor compartment with the stratum corneum side facing toward the donor compartment. The cumulative amount of drug permeated per unit area of skin during 6 h (Q6 in μg/cm2) is calculated as follows:
|
|
where Jss is the BDH flux (μg/cm2/h), time is 6 h, and effective diffusion surface area is 4.6 cm2.30,31 All experiments were done in triplicate and mean values ± SD were determined.
Optimization and evaluation of the selected formulation
Experimental model evaluation
The coefficient of determination (R2), adjusted R2, predicted R2, and CV% values were used to evaluate the fitness of the model to experimental data. Furthermore, F test was conducted for the analysis of variance (ANOVA) to assess the statistical significance of the quadratic models. The percentage prediction error was calculated for the optimized formulation to verify the above findings.
Formulation and characterization of the optimized BDH ethosomal formulation
The optimized formulation was selected based on minimum particle size and minimum % released as well as maximum EE% and flux. The selected formula was characterized by transmission electron microscopy, zeta potential, in-vitro release study, and ex-vivo permeation study, and eventually incorporated into gel base.
Transmission electron microscopy
The morphology of optimized BDH ethosomal formulation (F16) was visualized by transmission electron microscopy. A droplet of ethosomes suspension after appropriate dilution was placed on carbon-coated copper grid till it gets dry. The formed film was then negatively stained with 1% phosphotungstic acid (PTA); the excess liquid was removed by filter paper and finally allowed to dry again. The air-dried sample was examined by a transmission electron microscopy (TEM) analyzer (FEI Tecnai G2 Spirit Twin, Brno, Czech Republic) at an accelerating voltage of 120 KV, conducted by a VELETA camera.18,32
Zeta potential
Zeta potential was measured for the optimized formulation (F16) using Malvern Zeta Sizer (Zetasizer Ver. 7.11, Malvern Instruments, Malvern, UK) and the mean of three replicates was taken.
Preparation of BDH ethosomal gel
BDH ethosomal gel of the optimized formulation was prepared using the cold method.33 Briefly, carbopol was allowed to be hydrated overnight, and then poloxamer was slowly added to the solution with continuous agitation using a magnetic stirrer. The polymers were stored in a refrigerator overnight, and then the optimized BDH ethosomes (F16) were added to the mixture and probably stirred. After that, the mixture was neutralized by triethanolamine to pH 7.4. In the same manner, the gel of free BDH was prepared. The final concentration of drug was 10 mg BDH/g gel.
In-vitro release and ex-vivo permeation studies of BDH ethosomal gel
The in-vitro release profile of the optimized BDH ethosomal gel was conducted and compared to the aqueous solution of BDH. In addition, the ex-vivo permeation studies of both BDH ethosomal and free BDH gels were performed. As described before, a volume or amount of gel equivalent to 10 mg BDH was accurately transferred to the tester and at predetermined time intervals along 16 h test period, 2 mL samples were withdrawn from the receptor medium and replaced by an equal volume of freshly prepared buffer solution to maintain a constant volume. Samples were filtered through 0.45 μm millipore filter and analyzed spectrophotometrically at 261 nm for the cumulative amount of BDH released (% release). All experiments were performed in triplicate and mean values ± SD were determined.
Pharmacodynamics
Animals
The study was carried out on adult female albino rats. Animals were obtained from the Modern Veterinary Office for Laboratory Animals (Cairo, Egypt). Animals were housed individually in chambers under standard temperature conditions (25°C±0.5°C) and relative humidity (55%±1%) with a 12 h light/dark cycles for 2 weeks for adaptation before being subjected to the experiment and were allowed free access to commercial diet and water.
The animal housing and handling were conducted in compliance with Beni-Suef University guidelines and in accordance with the research protocols established by the Animal Care Committee of the National Research Center (Cairo, Egypt) which followed the recommendation of the National Institute of Health Guide for Care and Use of Laboratory Animal (Publication No 85-23, revised 1985).
Experiment
Food intake and weight gain were recorded according to the method described by Kasaoka et al with minor modification.4,12 Briefly, after 2 weeks of adaptation, the rats were assigned to one of the following four groups (six rats per group) on the basis of body weight (average initial body weight =285 g, range =270–300 g):
Group A: the control group, not subjected to any application.
Group B: the placebo group, subjected to plain ethosomal gel application.
Group C: the free drug group, subjected to free BDH in gel base application.
Group D: the ethosomal drug group, subjected to BDH ethosomal gel application.
Each group was allowed free access to experimental diet listed in Table 2 based on the American Institute of Nutrition’s dietary allowance for rats (AIN-76) to maintain normal growth and weight gain.34 The placebo and BDH ethosomal gel were spread on the animal dorsal skin. The dose was calculated as each animal receives 24 mg BDH/kg/d of body weight. The experiment was designed to investigate the effect of different treatments on food intake and weight gain of the animals.
  | Table 2 Composition of experimental diet |
Food intake and weight gain studies
In the food intake study, each rat received 15 g of the experimental diet daily from the beginning of the experiment, and everyday food intake was recorded by subtracting the weight after feeding the experimental diet from that before feeding. The mean food intake per day for each group was calculated and presented as mean value ± SD. While in weight gain study, animal body weight was checked every 3 days and after 3 weeks, the final weights were measured. The difference in body weight for each animal was recorded and mean difference in body weight ± SD was expressed for each group.
Statistical analysis
All data were statistically analyzed by one-way ANOVA (P-value of ≤0.05) on GraphPad Prism software (version 6) followed by Tukey’s multiple comparison tests. The data were presented as a mean ± SD.
Histopathological study
For the histopathological study, the skin was cut out after animal scarification and kept in formalin solution (10% v/v). It was then fixed in paraffin and sections of 5 μm thickness were cut from each piece and stained with hematoxylin and eosin. The histological changes were examined under an optical microscope.35,36 The safety of BDH ethosomal gel on the skin was evaluated by naked eye observation.
Results and discussion
Preparation and evaluation of BDH ethosomes
Ethosomes are a vesicular system with hydrated bilayers. This system is mainly composed of phospholipid, ethanol, and water. The permeation property of ethosomes is attributed to the entire system.22
Previous studies that had compared permeation enhancement of drugs from ethosomal systems with hydroethanolic solutions showed that permeation enhancement from ethosomes was much greater than would be expected from ethanol alone.31,37–39
In our study, BDH-loaded ethosomes were prepared using various concentrations of PC, PG, and ethanol and characterized for appropriate physicochemical attributes. The Box–Behnken design was constructed to study the effect of independent variables on dependent ones (Table 1). The relationship between the dependent and the independent variables was further elucidated using 3D-response surface plots and regression equations (Table 3).
  | Table 3 Data of regression analysis for responses Y1, Y2, Y3, and Y4 for fitting to quadratic model |
A positive sign in the regression equation represents a synergistic effect, while a negative sign indicates an antagonistic one. The 3D-response surface plots exhibit the effect of two independent variables after fixing the third one at the medium-level value.
The vesicle size and EE% of various BDH ethosomes are presented in Table 1. Being hydrophilic, BDH would be incorporated into the internal aqueous core of vesicles as suggested by Lopes et al.37
The vesicle size range was 55.48–291.40 nm, while the EE% of BDH in different ethosomal formulations was in the range 82.31%–83.72% as shown in Table 1. An initial decrease in vesicle size and EE% was observed with increasing concentration of PG and ethanol, while there was a marked increase in the vesicle size and EE% with increasing PC concentration. These results were in agreement with the regression equations in Table 3.
The reason for size reduction with increasing ethanol and PG concentration could be attributed to the negative charge gained by both ethanol and PG, which caused electrostatic repulsion and some degree of steric stabilization.29
Another explanation can be due to the interaction of ethanol and PG with lipid bilayers, which reduced membrane thickness.38–41 The reduction in EE% with the increase in ethanol and PG content could be attributed to increasing the fluidity of vesicles membrane leading to drug leakage.41,42 The surface plots showing the effects of independent variables on vesicle size and EE% are illustrated in Figure 1.
  | Figure 1 3D-response surface plots showing the effect of independent variables on vesicle size and EE%. |
The Q6 h of BDH released from the different formulations lies in the range from 42.59% to 72.35%, while the results of drug flux ranged from 0.16 to 0.26 mg/cm2/h. It was also observed that increased concentrations of PC and PG had a negative relationship with % BDH released and flux, while ethanol showed a positive relationship with both responses. These findings may be attributed to the dual effect of ethanol on both the lipid bilayer in the stratum corneum and the vesicle lipid bilayer. Ethanol has a fluidizing effect on phospholipid bilayers, producing compact, soft, deformable vesicles, along with the interaction between ethanol and the polar head group region of the lipid molecule resulting in a decrease in transition temperature of stratum corneum lipids with a consequent increase in membrane fluidity and BDH ethosomes partitioning.21,43–47
As PG concentration increased the % released and flux were decreased due to increased viscosity of the ethosomal suspension, reducing vesicles fluidity and decreasing skin penetration; however, the use of PG with ethanol might improve ethosomes stability.48 The effects of the independent variables on % released and flux are illustrated in the surface plots in Figure 2 and in Table 3.
  | Figure 2 3D-response surface plots showing the effect of independent variables on % cumulative release (Q6 h) and flux. |
Our results agree with Dayan and Touitou who had studied the skin permeation of minoxidil and testosterone from ethosomal formulations.18 Also, they agree with Paolino et al, who had studied the inflammatory activity of ethosomal ammonium glycyrrhizinate for transdermal delivery.23 Also, they agree with the results of Dayan and others who studied the efficacy of ethosomes as carriers for skin delivery of trihexyphenidyl HCl.18
It was found that the experimental values fell close to unity lines when plotted against predicted dependent variables, indicating a good correlation between dependent and independent variables. Furthermore, the three independent variables were significant model terms.
The optimization process of the design was based on minimizing vesicle size and the release rate and, on the other hand, maximizing flux and EE% to ensure permeability of vesicles through the skin and longer stability. F16 was selected as optimized formulation, and comparing experimental and predicted values of F16 showed close resemblance with small relative prediction error (<11%), suggesting the validity of the generated regression equations. The values of size, EE%, Q6 h, and flux of the optimized formulation were 55.28±2.25 nm, 85.46%±0.50%, 46.50%±0.79%, and 0.21±0.02 mg/cm2/h, respectively.
As shown in Figure 3, the TEM images of the optimized BDH ethosomes formulation showed well-identified spherical vesicles with sealed nanovesicular structure. The zeta potential of the optimized formulation was −24 mV, which indicated stability of vesicles against aggregation and confirmed the effect of ethanol on vesicles repulsion.39
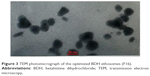  | Figure 3 TEM photomicrograph of the optimized BDH ethosomes (F16). |
Evaluation of BDH ethosomal gel
The in-vitro release profiles of BDH from BDH ethosomal gel in comparison with that of an aqueous solution as a control are represented graphically in Figure 4. About 37.5% was released from BDH ethosomal gel, compared to 99% released from an aqueous solution of BDH within 7 h.
  | Figure 4 Cumulative % released from aqueous solution of BDH and BDH ethosomal gel in phosphate buffer pH 7.4. |
The in-vitro release of BDH ethosomal gel followed zero-order kinetics (R2 =0.99) indicating a controlled release dosage form. This might be due to the surface active properties of poloxamer gel and would add further advantage to this system that is capable of delivering BDH at a controlled release rate.49
The ex-vivo permeation results of both free BDH in gel and BDH ethosomal gel in phosphate buffer pH 7.4 are shown in Figure 5. The retardation of drug release from BDH ethosomal gel compared to free BDH in gel could attribute to two main effects. Firstly, the viscosity of the gel and the reduction in the size of the extramicellar aqueous channels of poloxamer matrix through which the drug diffuses.50,51 Secondly, combining ethanol and PG in ethosomes can significantly enhance deposition of drugs on the skin.48 The cumulative % released and the cumulative amount permeated of the drug from the BDH ethosomal gel exceeded 99.5% over 16 h with a flux of 0.28 mg/cm2/h.
  | Figure 5 Ex-vivo permeation of both free BDH in gel and BDH ethosomal gel in phosphate buffer pH 7.4. |
Pharmacodynamic activity
With regard to weight gain results (Figure 6A), free BDH gel showed a significant difference from control and placebo (P<0.05), while ethosomal gel application showed a significant difference from control, placebo, and free BDH gel with P<0.05. This could be attributed to permeation enhancer effect of ethosomes, which delivers more BDH to systemic circulation than that delivered from BDH gel alone. The results obtained were in agreement with the two animal studies carried out by Rossi et al and Szelag et al, which have evaluated the effect of BDH on food intake in animals.13,14
Rossi investigated the effect of different doses of BDH on water and food intake in 12 pygmy goats and concluded that the injection of BDH (4 mg/kg body weight in power of 0.75) significantly reduced food intake at 1 h or during 6 h (8 mg/kg body weight in power of 0.75) post injection. Szelag studied the effect of systemic BDH on food intake of rats. Animals were divided into three experimental groups. The first was on a normal diet, the second one was deprived of food, and the third group was accustomed to “tasty” food. The results of the three experimental groups concluded that BDH at the dose of 24 mg/kg revealed stronger activity, reducing food intake.
Barak et al had studied the effect of BDH orally on reducing weight gain associated with olanzapine.52 In this study, 36 schizophrenic patients treated with olanzapine were randomized to BDH and matching placebo. The patients treated with BDH had less weight gain compared with the placebo group, but due to small sample size, the effect did not reach statistical significance. Furthermore, a study carried out by Zheng et al evaluated the effect of BDH on olanzapine-induced intestinal fat deposition using Caenorhabditis elegans as a model for obesity.53 This study proved that the combination of olanzapine with BDH reduced intestinal fat deposition compared with the olanzapine-treated group. Furthermore, the study indicated that the appropriate BDH dose to be used for this indication should be tripled due to the short half-life of BDH, so a sustained release formulation may have approved the effect.
Regarding food intake, application of BDH ethosomal gel significantly decreased the food intake of rats as compared with control and placebo and free BDH gel groups (Figure 6B).
The decrease in weight gain and food intake observed with BDH is due to the H3 antagonist action and H1 agonist action of BDH, which increase brain histamine concentration, inhibiting food intake and thus decreasing weight gain.3
Histopathological investigation
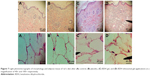
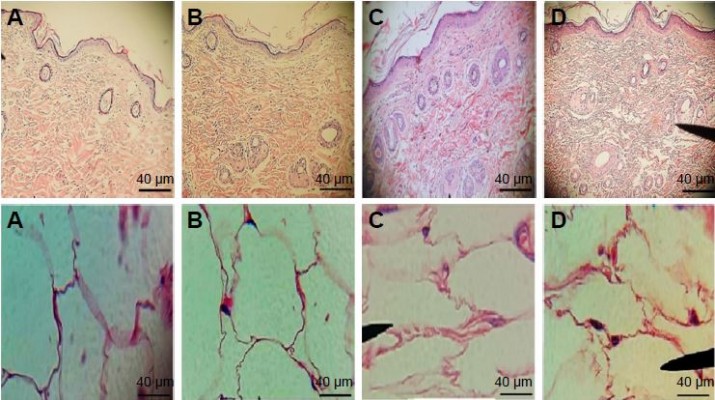
The histopathological investigation of skin and adipose tissue for the BDH ethosomal gel compared with control and placebo and free BDH gel are shown in Figure 7. The structure and the thickness of the horny layer of the BDH ethosomal gel-treated group did not show any differences compared with control and placebo. Additionally, no infiltration of inflammatory cells to the skin treated with BDH ethosomal gel was observed. These outcomes are in line with the safety and tolerability of the ethosomal system reported in the literature.22
A study carried out by Shumilov and Touitou,54 evaluated the safety of transdermal ethosomal system containing buspirone. The results showed no infiltration of inflammatory cells to the skin, and also no changes in the structure and thickness of the horny layer.
Regarding the adipose histopathological investigation, a marked decrease in adipose tissue with the use of a BDH ethosomal gel for 3 weeks was observed; however, large fat cells of adipose tissue were seen with both the control and the placebo groups with no significant difference in size.
Conclusion
A novel ethosomal gel of BDH with satisfactory release characteristics was successfully prepared. A mathematical model was developed connecting the independent variables with the measured responses. The quadratic regression modeling was a proper way for selecting the best drug ethosomal formulation based on minimizing vesicle size as well as the release rate and maximizing flux and EE% to ensure permeability of vesicles through the skin and longer stability. Poloxamer gel had offered a further advantage in delivering BDH at a controlled release rate. BDH ethosomal gel showed effective, sustained transdermal absorption and central action in decreasing food intake and, consequently, the weight gain.
Disclosure
The authors report no conflicts of interest in this work.
References
Kaplan NM. The deadly quartet: upper-body obesity, glucose intolerance, hypertriglyceridemia, and hypertension. Arch Intern Med. 1989;149(7):1514–1520. | ||
Sakata T, Yoshimatsu H, Kurokawa M. Hypothalamic neuronal histamine: implications of its homeostatic control of energy metabolism. Nutrition. 1997;13(5):403–411. | ||
Masaki T, Yoshimatsu H, Chiba S, Watanabe T, Sakata T. Central infusion of histamine reduces fat accumulation and upregulates UCP family in leptin-resistant obese mice. Diabetes. 2001;50(2):376–384. | ||
Kasaoka S, Tsuboyama-Kasaoka N, Kawahara Y, et al. Histidine supplementation suppresses food intake and fat accumulation in rats. Nutrition. 2004;20(11):991–996. | ||
Yoshimatsu H, Hidaka S, Niijima A, Sakata T. Histamine neurons down-regulate ob gene expression in rat white adipose tissue. Inflamm Res. 2001;50(Suppl 2):S72–S73. | ||
Tighilet B, Trottier S, Lacour M. Dose- and duration-dependent effects of betahistine dihydrochloride treatment on histamine turnover in the cat. Eur J Pharmacol. 2005;523(1–3):54–63. | ||
Jain R, Yadav RK, Rather JA. Voltammetric assay of anti-vertigo drug betahistine hydrochloride in sodium lauryl sulphate. Colloids Surf A Physicochem Eng Asp. 2010;366(1–3):63–67. | ||
Hathout RM, Nasr M. Transdermal delivery of betahistine hydrochloride using microemulsions: physical characterization, biophysical assessment, confocal imaging and permeation studies. Colloids Surf B Biointerfaces. 2013;110:254–260. | ||
Tighilet B, Trottier S, Mourre C, Chotard C, Lacour M. Betahistine dihydrochloride interaction with the histaminergic system in the cat: neurochemical and molecular mechanisms. Eur J Pharmacol. 2002;446(1–3):63–73. | ||
Barak N, Greenway FL, Fujioka K, Aronne LJ, Kushner RF. Effect of histaminergic manipulation on weight in obese adults: a randomized placebo controlled trial. Int J Obes. 2008;32(10):1559–1565. | ||
Ali AH, Yanoff LB, Stern EA, et al. Acute effects of betahistine hydrochloride on food intake and appetite in obese women: a randomized, placebo-controlled trial. Am J Clin Nutr. 2010;92(6):1290–1297. | ||
Kasaoka S, Kawahara Y, Inoue S, et al. Gender effects in dietary histidine-induced anorexia. Nutrition. 2005;21(7–8):855–858. | ||
Rossi R, Del Prete E, Scharrer E. Effect of the H 1-histamine receptor agonist betahistine on drinking and eating behavior in pygmy goats. Physiol Behav. 1999;66(3):517–521. | ||
Szelg A, Trocha M, Merwid-Lad A. Betahistine inhibits food intake in rats. Pol J Pharmacol. 2001;53(6):701–707. | ||
Sweetman SC. Martindale The Complete Drug Reference. Thirty-sixth edition ed: Pharmaceutical Press; 2009. | ||
Heda AA, Sonawane AR, Naranje GH, Somani VG, Puranik PK. Development and in vitro evaluation of betahistine adhesive-type transdermal delivery system. Trop J Pharm Res. 2010;9(6):516–524. | ||
El-Nabarawi MA, Ali AA, Aboud HM, Hassan AH, Godah AH. Transbuccal delivery of betahistine dihydrochloride from mucoadhesive tablets with a unidirectional drug flow: in vitro, ex vivo and in vivo evaluation. Drug Des Devel Ther. 2016;10:4031–4045. | ||
Dayan N, Touitou E. Carriers for skin delivery of trihexyphenidyl HCl: ethosomes vs. liposomes. Biomaterials. 2000;21(18):1879–1885. | ||
Touitou E, Godin B, Dayan N, Weiss C, Piliponsky A, Levi-Schaffer F. Intracellular delivery mediated by an ethosomal carrier. Biomaterials. 2001;22(22):3053–3059. | ||
Touitou E, Godin B. Ethosomes for skin delivery. J Drug Deliv Sci Technol. 2007;17(5):303–308. | ||
Scognamiglio I, De Stefano D, Campani V, et al. Nanocarriers for topical administration of resveratrol: a comparative study. Int J Pharm. 2013;440(2):179–187. | ||
Ainbinder D, Paolino D, Fresta M, Touitou E. Drug delivery applications with ethosomes. J Biomed Nanotechnol. 2010;6(5):558–568. | ||
Paolino D, Lucania G, Mardente D, Alhaique F, Fresta M. Ethosomes for skin delivery of ammonium glycyrrhizinate: in vitro percutaneous permeation through human skin and in vivo anti-inflammatory activity on human volunteers. J Control Release. 2005;106(1–2):99–110. | ||
Aboud HM, Ali AA, El-Menshawe SF, Elbary AA. Nanotransfersomes of carvedilol for intranasal delivery: formulation, characterization and in vivo evaluation. Drug Deliv. 2016;23(7):2471–2481. | ||
Zaki RM, Ali AA, El Menshawi SF, Bary AA. Effect of binary and ternary solid dispersions prepared by fusion method on the dissolution of poorly water soluble diacerein. Int J Drug Deliv. 2013;5(1):99–109. | ||
Shamma RN, Basalious EB, Shoukri R. Development of novel sustained release matrix pellets of betahistine dihydrochloride: effect of lipophilic surfactants and co-surfactants. Pharm Dev Technol. 2012;17(5):583–593. | ||
Mishra GP, Kinser R, Wierzbicki IH, Alany RG, Alani AW. In situ gelling polyvalerolactone-based thermosensitive hydrogel for sustained drug delivery. Eur J Pharm Biopharm. 2014;88(2):397–405. | ||
Qian S, Wong YC, Zuo Z. Development, characterization and application of in situ gel systems for intranasal delivery of tacrine. Int J Pharm. 2014;468(1–2):272–282. | ||
Ahad A, Aqil M, Kohli K, Sultana Y, Mujeeb M. Enhanced transdermal delivery of an anti-hypertensive agent via nanoethosomes: statistical optimization, characterization and pharmacokinetic assessment. Int J Pharm. 2013;443(1–2):26–38. | ||
Gupta A, Aggarwal G, Singla S, Arora R. Transfersomes: a novel vesicular carrier for enhanced transdermal delivery of sertraline: development, characterization, and performance evaluation. Sci Pharm. 2012;80(4):1061–1080. | ||
Nipun TS, Ashraful Islam SM. SEDDS of gliclazide: preparation and characterization by in-vitro, ex-vivo and in-vivo techniques. Saudi Pharm J. 2014;22(4):343–348. | ||
Trompeta AF, Koklioti MA, Perivoliotis DK, Lynch I, Charitidis CA. Towards a holistic environmental impact assessment of carbon nanotube growth through chemical vapour deposition. J Clean Prod. 2016;129:384–394. | ||
Moawad FA, Ali AA, Salem HF. Nanotransfersomes-loaded thermosensitive in situ gel as a rectal delivery system of tizanidine HCl: preparation, in vitro and in vivo performance. Drug Deliv. 2017;24(1):252–260. | ||
Diet CB, Diet U, Diet NP. Report of the American Institute of Nutrition Ad Hoc Committee on Standards for Nutritional Studies. J Nutr. 1977;107:1340. | ||
Singh RM, Kumar A, Pathak K. Thermally triggered mucoadhesive in situ gel of loratadine: β-cyclodextrin complex for nasal delivery. AAPS Pharm Sci Tech. 2013;14(1):412–424. | ||
Tuntiyasawasdikul S, Limpongsa E, Jaipakdee N, Sripanidkulchai B. A monolithic drug-in-adhesive patch of methoxyflavones from Kaempferia parviflora: in vitro and in vivo evaluation. Int J Pharm. 2015;478(2):486–495. | ||
Lopes LB, Scarpa MV, Silva GV, Rodrigues DC, Santilli CV, Oliveira AG. Studies on the encapsulation of diclofenac in small unilamellar liposomes of soya phosphatidylcholine. Colloids Surf B Biointerfaces. 2004;39(4):151–158. | ||
Dubey V, Mishra D, Dutta T, Nahar M, Saraf DK, Jain NK. Dermal and transdermal delivery of an anti-psoriatic agent via ethanolic liposomes. J Control Release. 2007;123(2):148–154. | ||
Verma P, Pathak K. Nanosized ethanolic vesicles loaded with econazole nitrate for the treatment of deep fungal infections through topical gel formulation. Nanomedicine. 2012;8(4):489–496. | ||
Tanriverdi ST, Özer Ö. Novel topical formulations of Terbinafine-HCl for treatment of onychomycosis. Eur J Pharm Sci. 2013;48(4–5):628–636. | ||
Ahad A, Raish M, Al-Mohizea AM, Al-Jenoobi FI, Alam MA. Enhanced anti-inflammatory activity of carbopol loaded meloxicam nanoethosomes gel. Int J Biol Macromol. 2014;67:99–104. | ||
López-Pinto JM, González-Rodríguez ML, Rabasco AM. Effect of cholesterol and ethanol on dermal delivery from DPPC liposomes. Int J Pharm. 2005;298(1):1–12. | ||
Godin B, Touitou E. Mechanism of bacitracin permeation enhancement through the skin and cellular membranes from an ethosomal carrier. J Control Release. 2004;94(2–3):365–379. | ||
Elsayed MM, Abdallah OY, Naggar VF, Khalafallah NM. Lipid vesicles for skin delivery of drugs: reviewing three decades of research. Int J Pharm. 2007;332(1–2):1–16. | ||
Fan C, Li X, Zhou Y, et al. Enhanced topical delivery of tetrandrine by ethosomes for treatment of arthritis. Biomed Res Int. 2013;2013:161943. | ||
Zhang YT, Shen LN, Wu ZH, Zhao JH, Feng NP. Comparison of ethosomes and liposomes for skin delivery of psoralen for psoriasis therapy. Int J Pharm. 2014;471(1–2):449–452. | ||
Yu X, Du L, Li Y, Fu G, Jin Y. Improved anti-melanoma effect of a transdermal mitoxantrone ethosome gel. Biomed Pharmacother. 2015;73:6–11. | ||
Shen LN, Zhang YT, Wang Q, Xu L, Feng NP. Enhanced in vitro and in vivo skin deposition of apigenin delivered using ethosomes. Int J Pharm. 2014;460(1–2):280–288. | ||
Bendas ER, Tadros MI. Enhanced transdermal delivery of salbutamol sulfate via ethosomes. AAPS Pharm Sci Tech. 2007;8(4):E107. | ||
Abdelkader H, Pierscionek B, Alany RG. Novel in situ gelling ocular films for the opioid growth factor-receptor antagonist-naltrexone hydrochloride: fabrication, mechanical properties, mucoadhesion, tolerability and stability studies. Int J Pharm. 2014;477(1–2):631–642. | ||
Hani U, Shivakumar H. Development of miconazole nitrate thermosensitive bioadhesive vaignal gel for vaginal candidiasis. Am J Adv Drug Deliv. 2013;1(3):358–368. | ||
Barak N, Beck Y, Albeck JH. A randomized, double-blind, placebo-controlled pilot study of betahistine to counteract olanzapine-associated weight gain. J Clin Psychopharmacol. 2016;36(3):253–256. | ||
Zheng J, Vasselli JR, King JF, et al. Using caenorhabditis elegans as a model for obesity pharmacology development. Am J Ther. 2016;23(6):e1363–e1370. | ||
Shumilov M, Touitou E. Buspirone transdermal administration for menopausal syndromes, in vitro and in animal model studies. Int J Pharm. 2010;387(1–2):26–33. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.