Back to Journals » Journal of Inflammation Research » Volume 16
A Novel Predictor of Pathologic Complete Response for Neoadjuvant Immunochemotherapy in Resectable Locally Advanced Esophageal Squamous Cell Carcinoma
Authors Yang Y , Xin D , Wang H , Guan L, Meng X, Lu T, Bai X, Wang F
Received 2 November 2022
Accepted for publication 22 March 2023
Published 5 April 2023 Volume 2023:16 Pages 1443—1455
DOI https://doi.org/10.2147/JIR.S395231
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Yalan Yang,1,* Dao Xin,2,* Huike Wang,1,* Lulu Guan,1 Xiangrui Meng,1 Taiying Lu,1 Xiwen Bai,3 Feng Wang1
1Department of Oncology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, People’s Republic of China; 2Department of Medical Oncology, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, People’s Republic of China; 3Department of Translational Medicine, Nanchang University Queen Mary School, Nanchang, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Feng Wang, Department of Oncology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, People’s Republic of China, Email [email protected]
Purpose: Neoadjuvant immunochemotherapy (nICT) for resectable locally advanced esophageal squamous cell carcinoma (LA-ESCC) has attracted widespread attention recently, whose safety and clinical benefit was observed in clinical researches. This study aimed to develop and validate a novel predictor systemic inflammation-tumor markers index (SITI) to predict the pathological complete response (pCR) for resectable LA-ESCC patients receiving nICT.
Patients and Methods: A total of 147 LA-ESCC patients who underwent nICT followed by surgery from February 2020 to April 2022 were included in the study. The dynamic change of inflammatory indexes was compared at baseline, after two cycles of nICT and postoperative one month. Least absolute shrinkage and selection operator (LASSO) regression was performed to avoid collinearity and identify key indexes, with SITI constructed. After univariate and multivariate stepwise forward logistic analyses, a nomogram for pCR prediction was developed.
Results: 41(27.9%) patients achieved pCR among 147 resectable LA-ESCC patients received nICT. Compared with baseline, most inflammatory indexes were significantly decreased at postoperative one month. 5 key indexes were identified and then a predictive index named SITI was constructed. The result showed that lower SITI and earlier clinical tumor node metastasis (cTNM) stage were more likely to achieve pCR. The nomogram for pCR prediction had excellent discrimination performance (C-index = 0.791).
Conclusion: The SITI is an independent predictor for pCR in resectable LA-ESCC patients received nICT. To our knowledge, our nomogram is the first model using systemic inflammation-tumor markers for pCR prediction and may be a promising predictor to effectively differentiate pCR for nICT in LA-ESCC patients.
Keywords: esophageal squamous cell carcinoma, neoadjuvant therapy, immunotherapy, pathologic complete response, inflammation, nomogram
Introduction
Esophageal cancer is a global health challenge with high cancer incidence and cancer-related death worldwide.1,2 The majority of esophageal cancer cases are found in Asia, with 49% in China. Esophageal squamous cell carcinoma (ESCC) is the most common pathological type in China.3,4 Neoadjuvant chemotherapy (nCT) or chemoradiotherapy (nCRT) plus surgery has become the preferred treatment for locally advanced esophageal squamous cell carcinoma (LA-ESCC), although the long-time survival for which is still unsatisfactory.5–8
Recently, immunotherapy combined with chemotherapy has become the new standard of first-line treatment for advanced ESCC,9–13 which has brought widespread attention to attempts at neoadjuvant immunochemotherapy (nICT) for resectable LA-ESCC. At present, nICT is recommended for patients with LA-ESCC in clinical studies, and its safety and clinical benefit was observed.14–17 Pathological complete response (pCR) is considered to be an important therapeutic effect of neoadjuvant therapy and a good surrogate marker of postoperative survival. However, studies regarding reliable pCR prediction in resectable LA-ESCC patients receiving nICT are lacking. Although the expression of programmed cell death ligand-1 (PD-L1), tumor mutation load, microsatellite stability, and T cell function have been reported to be associated with immunotherapy efficacy,18,19 these tests remain difficult for some patients due to medical conditions and economic constraints. Therefore, it is of great significance to find more affordable, effective and accurate indicators and establish more practical predictive models for personalized pCR prediction for patients with LA-ESCC.
Several inflammatory indexes have been reported to be associated with pCR and prognosis after neoadjuvant therapy for esophageal cancer, such as lymphocyte (LY),20,21 neutrophil to lymphocyte ratio (NLR),21,22 platelet to lymphocyte ratio (PLR),21,23 lymphocyte to monocyte ratio (LMR),24 and systemic immune inflammation index (SII).23 In this study, we explored the dynamics of inflammatory indexes at baseline, after two cycles of nICT and postoperative one month. Based on LASSO regression analysis, 5 key markers were identified, including CA125, CEA and neutrophil (NEUT) before nICT, monocyte (MONO) and LMR after nICT. Then we developed a novel predictor named systemic inflammation-tumor markers index (SITI) for pCR. Finally, SITI and clinical tumor node metastasis (cTNM) stage were identified as the independent significant predictors for pCR and used to develop a novel nomogram to accurately and effectively differentiate pCR for nICT in resectable LA-ESCC patients.
Materials and Methods
Patients
In this retrospective single-center study, we collected data from 168 patients with esophageal cancer who underwent nICT followed by surgery at the First Affiliated Hospital of Zhengzhou University from February 2020 to April 2022. The inclusion criteria were as follows: (1) diagnosis of ESCC based on preoperative pathological examination; (2) ESCC confirmed with clinical stage II–IVA based on computed tomography (CT) and endoscopic ultrasonography (EUS); (3) Surgery after nICT, with R0 resection; (4) age ≤80 years, with a good general condition and normal cardiopulmonary and other organ function; and (5) well-documented medical records and follow-up. The exclusion criteria were as follows: (1) infection, autoimmune disease or hematologic disease; (2) other synchronous or previous malignancy; and (3) immunotherapy could not be administered due to a serious adverse event. We excluded 6 patients who received nICT in combination with radiotherapy or targeted therapy. In addition, only 7 patients received 1 cycle, 5 patients received 3 cycles, and 3 patients received 4 cycles of nICT. We ended up enrolling 147 patients in this study.
Treatment
Patients received neoadjuvant chemotherapy combined with immunotherapy every 3 weeks. Neoadjuvant chemotherapy has two regimens: (1) docetaxel plus nedaplatin; (2) paclitaxel plus cisplatin or nedaplatin. Immunotherapy (pembrolizumab, camrelizumab, tislelizumab or sintilimab 200 mg) was performed on day 1 of each cycle. After 2 cycles of nICT, the laparo-thoracoscopic McKeown surgery was performed. Pathological examination of the surgically removed tissue specimens was performed, and the tumor and lymph nodes were independently evaluated by two pathologists from our hospital after neoadjuvant treatment. The histological type of esophageal cancer was determined according to the 2019 edition of the World Health Organization (WHO) Classification of Tumors of the Digestive System. The tumor regression grade (TRG) was based on the criteria of the College of American Pathologists (CAP), which is in line with the approach recommended by the National Comprehensive Cancer Network (NCCN) guidelines for the management of esophageal cancer.4 The TNM stage was determined according to the International Union Against Cancer (UICC)/American Joint Committee on Cancer (AJCC) TNM Staging System (8th edition, 2017).25
Outcomes and Follow Up
The primary clinical endpoint was the pathological response of the primary tumor. Patients without residual tumor cells of the resected tumor specimen and lymph node metastasis (ypT0N0) were considered to have achieved pCR. After treatment, patients were regularly checked, including physical examinations, tumor markers tests and contrast CT examinations. The last follow-up time was completed in October 2022.
Clinical Features and Inflammatory Indexes
The data of clinical characteristics, clinical staging and hematological indexes, were retrospectively collected and arranged. Hematological indicators of patients extracted from the medical record system included NEUT, LY, MONO, NLR, PLR, LMR, AISI, and SII before nICT treatment, after 2 cycles of nICT treatment, and 1 month after surgery. The 4 hematological indexes, including platelet (PLT), NEUT, LY and MONO, were obtained before nICT, after 2 cycles of nICT and postoperative one month. The NLR, PLR and LMR were defined as NEUTs divided by LYs, PLTs divided by LYs and LYs divided by MONOs, respectively. According to previously published study, variables were calculated by the following formula: aggregate index of systemic inflammation (AISI) = MONO × PLT× NEUT/LY,26 SII = PLT × NEUT/LY.27
Statistical Analysis
R software (version 4.2.0) and IBM SPSS 26.0 were carried out to conduct all statistical analyses. Continuous variables were performed by t-tests and categorical variables were analyzed by Chi-square or Fisher’s exact tests. Based on the ratio of hematological markers before and after nICT, the least absolute shrinkage and selection operator (LASSO) regression was performed to avoid collinearity by using the “glmnet” package in R. The receiver operating characteristic curve (ROC) analysis was performed to group 147 patients by the best cutoff value and patients was divided into high and low groups.28 Univariate logistic analysis was performed to determine whether the indicator is positive or negative. The high indicator group of adverse factors is defined as a risk score of 1, and the high indicator group of favorable factors is defined as a score of 0. We calculated the cumulative score of difficulty to achieve pCR, and the formula is as follows: Cumulative Score = pre-NEUT Score (0/1) + pre-CA125 Score (0/1) + pre-CEA Score (0/1) + post-MONO Score (0/1) + post-LMR Score (0/1). Referring to the predictive ability of cumulative scores on pCR, patients with cumulative scores of 0–1, 2, and 3–5 were then defined as the three grades of SITI. Univariate and multivariate stepwise forward logistic regression analyses were used to identify the predictors of pCR, with the odds ratios (ORs) and 95% confidence intervals (CIs) calculated. Then, independent predictive factors of pCR prediction were selected to establish and validate a nomogram. The calibration curve, ROC curve, decision curve analyses (DCA) and clinical impact curve were used to assess the discriminative ability of pCR prediction. All probabilities were two-tailed, and the level of significance was set at 0.05.
Results
Patient Characteristics
Ultimately, there were 147 patients with LA-ESCC who underwent nICT followed by radical resection in this study (Figure 1). The study enrolled 105 (71.4%) men and 42 (28.6%) women with a mean age of 63.4 ± 7.0 years. Most patients were diagnosed at the stage of cT3 (47.6%), cN0 (59.2%), and cTNM II (55.1%), respectively. Among the patients, 41 (27.9%) cases achieved pCR. The detailed baseline clinical characteristics of the patients are summarized in Table 1.
 |
Table 1 Characteristics for 147 LA-ESCC Patients |
 |
Figure 1 The flow diagram of selection of eligible LA-ESCC patients who received nICT plus radical resection. |
Inflammatory and Nutritional Indexes Grouped by pCR
Figure 2 illustrates dynamic changes in inflammatory indexes during baseline, after two cycles of nICT, and postoperative one month for 147 LA-ESCC patients. Compared with baseline, most indicators except for PLR were significantly decreased at postoperative one month, while only NEUT, LY and LMR were significantly decreased after nICT. Consistent results were also observed in 41 patients with pCR in Supplemental Figure 1. Further, these inflammatory indicators were assessed based on pCR, respectively (Table 2). There were no statistically significant differences in all indexes at baseline and postoperative one month between two groups. Interestingly, the pCR group had a higher MONO and lower LMR than the non-pCR group after nICT treatment.
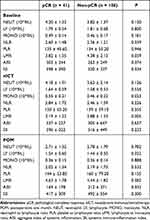 |
Table 2 Inflammatory and Nutritional Scores at Different Time Points |
Identification of Key Indexes and Establishment of a Classifier
In Figure 3, a correlation heatmap is shown for 8 inflammatory indexes before and after nICT and tumor markers at baseline in 147 LA-ESCC patients. In order to avoid collinearity, LASSO regression was performed and 5 key markers were identified (Figure 3A–C). The ROC analysis indicated that the optimal cutoff value for CA125, CEA, pre-NEUT, post-MONO and post-LMR was 8.90 U/mL, 1.11 U/mL, 3.44*10^9/L, 0.455*10^9/L and 4.194 (Table 3), and the area under the curve (AUC) was 0.605, 0.600, 0.584, 0.662 and 0.635 (Figure 3D), respectively. Then 147 patients were divided into high and low groups based on the cutoff value for ROC analysis. And finally a scoring system was developed, with a larger AUC (0.734) for pCR (Figure 3D). Thus, the patients were divided into three groups based on the SITI for further analysis. Table 4 revealed that baseline clinical characteristics grouped by SITI were significantly associated with pCR (P < 0.001).
 |
Table 3 Cut off Value Based on ROC Analysis and Scoring System Constructed |
 |
Table 4 The Clinical Characteristics Grouped by SITI |
Evaluation of the SITI as an Independent Factor
To determine independent predicted factors for pCR, univariate and multivariate logistic regression analyses were performed for 147 LA-ESCC patients among the SITI and other clinical characteristics. Table 5 shows that predictive factors associated with pCR in univariate logistic regression included cN Stage, cTNM and the SITI. According to the multivariate stepwise forward logistic regression analyses, the results indicated that the SITI remained an independent predictor of pCR in resectable LA-ESCC patients treated with nICT (2 vs 1: OR=0.361, 95% CI = 0.134–0.968, P = 0.0437; 3 vs 1: OR=0.104, 95% CI=0.038–0.290, P < 0.0001). Besides, cTNM was also an independent predictor of pCR (III vs II: OR=0.330, 95% CI=0.115–0.942, P = 0.0388; IVA vs II: OR=0.158, 95% CI = 0.041–0.614, P = 0.0076).
 |
Table 5 Logistic Univariate and Multivariate Analysis of Predictors for pCR |
Nomogram Constructed to Predict pCR and Validated
Finally, we established a nomogram to predict pCR according to the independent predictors (SITI and cTNM) identified in the multivariate stepwise forward logistic regression analyses (Figure 4A). To better understand the predictive value of the nomogram, the calibration slope and the ROC were also plotted to graphically assess calibration and discrimination, respectively (Figure 4B and C). The decision curve (Figure 4D) and the clinical impact curve (Figure 4E) for this model was performed and indicated a good clinical applicability in predicting the probability of pCR. These results confirmed that the SITI-based nomogram may serve as a simple and potential model for predicting pCR in resectable LA-ESCC treated with nICT.
Discussion
nICT is currently recommended only for clinical studies of esophageal cancer.7,8,15–17 In clinical practice, nICT brings higher pCR rates, but lacks effective markers. Therefore, it is of great significance to find more reliable indicators and establish more practical predictive models to guide personalized therapy for LA-ESCC patients. Evidence have suggested that several inflammatory indexes at baseline, including LY, NLR, PLR, LMR, and SII, were associated with pCR and prognosis after neoadjuvant therapy for esophageal cancer.20–22,24 We are the first to explore the dynamic changes of inflammatory indexes in resectable LA-ESCC patients before treatment, after nICT, and after surgical treatment. We found that the six included indicators, NEUT, LY, MONO, NLR, AISI and SII, were significantly decreased after surgery compared with those before treatment. Then the same phenomena were also observed in patients who achieved pCR. Moreover, the results showed that LMR significantly decreased after nICT and increased after surgery in patients achieving pCR.
Considering pCR as an important therapeutic effect and prognostic indicators of neoadjuvant therapy, further research was conducted on the predictive value of inflammatory indicators for pCR. We observed no significant differences in inflammation and nutritional markers between the pCR and non-pCR groups at baseline and after surgery. An interesting result, however, was that the pCR group had lower LMR and higher MONO after nICT treatment. Pre-treatment monocytes have been reported to be associated with immunotherapy.29 At present, we have no previous literature reports to support this result in our study. MONO is known to participate in phagocytosis, the removal of injured and senescent cells, as well as immune response and antigen presentation, which may explain this phenomenon to some extent.30,31 Tumor markers, including CA125,32 CEA,33,34 and CA155,34 have been reported to be associated with poor prognosis in esophageal carcinoma. Further, we attempted to use hematological indicators before treatment and after nICT to developed a novel scoring system based on inflammatory and tumor markers. Our results indicated that both SITI and cTNM were independent predictors of pCR in resectable LA-ESCC patients treated with nICT. Finally, we constructed a new nomogram based on SITI and cTNM that had good predictive ability for pCR.
With the advent of a new era of precision immunotherapy, immunocheckpoint inhibitor therapy targeting the PD-1/PD-L1 pathway has revolutionized the treatment of esophageal cancer. Multiple Phase III clinical studies have established the role of immunotherapy in the first-line or second-line treatment of advanced esophageal squamous cell carcinoma.35,36 Immunotherapy combined with chemotherapy as neoadjuvant therapy has also been tried to be applied to locally advanced esophageal squamous cell carcinoma, in which efficacy and safety have also been observed.16,37–39 PDL1 expression, tumor mutation load, microsatellite stability, and T cell function were reported to be correlated with the efficacy of immunotherapy.18,19 However, due to medical conditions and economic factors, these tests are still difficult for some patients. Currently, finding the effective markers to predict treatment response is critical to precision therapy. Reports has aroused our concern that inflammatory markers, which are economical and convenient, are related to pCR and prognosis after neoadjuvant therapy in malignant tumors.20–23 Currently, there are few studies using inflammatory indicators to predict the pCR for nICT in LA-ESCC. We compared the predictive ability of our model to Feng Jifeng’s model.40 They constructed an IINS with eight indicators at baseline that the AUC for pCR was 0.7. SITI, which we constructed based on five indicators before and after nICT, seemed to have a higher predictive power for pCR (AUC=0.734). And our nomogram can predict pCR for nICT in resectable LA-ESCC (C-index=0.79), excellently. Another study, involving 64 patients, focused only on the predictive power of inflammation and nutritional indexes for pCR at baseline and after different treatment cycles.41 Compared to the other study,24 our study considered changes in hematological indicators before and after nICT treatment and constructed an entirely new model.
Furthermore, our study still has some limitations. As a single-center retrospective study, there is a potential for bias in data collection. In this study, we mainly included 147 patients with resectable LA-ESCC who received 2 cycles of neoadjuvant therapy. As currently neoadjuvant immunochemotherapy is only recommended in clinical studies, the number of patients applying this regimen is limited, which also leads to the lack of external validation in this study. Although two studies found that prolonged nICT may lead to better pathological responses.42,43 However, Hongsheng Deng et al also found in the study that for the patients with stable disease/progressive disease (SD/PD) after neoadjuvant therapy, the benefit of pathological remission from additional cycles was relatively insignificant compared with the patients with significant therapeutic effect and partial response.42 In current clinical practice and research, the number of treatment cycles of nICT for patients with resectable LA-ESCC is still controversial, and it is unclear whether giving more treatment cycles is associated with better prognosis. Too few treatment cycles may lead to poor curative effect. Longer treatment cycles not only lead to higher treatment costs, but also may increase surgical risks. We ultimately decided to include the population who received 2 cycles of nICT, but this may have resulted in a certain selection bias. In the future we look forward to gaining more evidence to explore the best neoadjuvant therapy cycle. Additionally, due to the limited or absence of hematological markers in our cohort, we were unsuccessful in replicating other models. And the mechanisms affecting inflammation and nutritional indicators need further investigation. Despite the above limitations, the SITI-based nomogram may serve as a promising predictor to accurately and effectively differentiate pCR for nICT in resectable LA-ESCC patients.
Conclusion
In summary, we are the first to explore the dynamic changes of inflammatory indexes in resectable LA-ESCC patients before treatment, after nICT, and after surgical treatment. We identified key indexes before and after nICT and constructed a predictive index named SITI. The SITI is an independent predictor for pCR in resectable LA-ESCC patients received nICT. The SITI-based nomogram may be a promising predictor to effectively differentiate pCR for nICT in resectable LA-ESCC patients, which has important implications for individualized treatment strategies.
Ethics Approval and Consent to Participate
This study was approved by the Ethics Committee of scientific research and clinical trial of the First Affiliated Hospital of Zhengzhou University (KY-2022-0362). All patients provided written-informed consent for the collection and publication of their medical information at the first visit to our center, which was filed in their medical records, and the ethics committees approved this consent procedure. The study complied with the Declaration of Helsinki.
Acknowledgments
The authors thank the participating patients for all their help in enabling completion of this study.
Funding
This work was supported by the General program of National Natural Science Foundation of China (81672442), Natural Science Foundation of Henan Province (222300420557), Beijing Xisike Clinical Oncology Research Foundation (Y-HR2018-219).
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Lagergren J, Smyth E, Cunningham D, Lagergren P. Oesophageal cancer. Lancet. 2017;390(10110):2383–2396. doi:10.1016/s0140-6736(17)31462-9
2. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
3. Uhlenhopp DJ, Then EO, Sunkara T, Gaduputi V. Epidemiology of esophageal cancer: update in global trends, etiology and risk factors. Clin J Gastroenterol. 2020;13(6):1010–1021. doi:10.1007/s12328-020-01237-x
4. Ajani JA, D’Amico TA, Bentrem DJ, et al. Esophageal and esophagogastric junction cancers, version 2.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2019;17(7):855–883. doi:10.6004/jnccn.2019.0033
5. Hou S, Pan Z, Hao X, Hang Q, Ding Y. Recent progress in the neoadjuvant treatment strategy for locally advanced esophageal cancer. Cancers. 2021;13(20):5162. doi:10.3390/cancers13205162
6. Yang H, Liu H, Chen Y, et al. Long-term efficacy of neoadjuvant chemoradiotherapy plus surgery for the treatment of locally advanced esophageal squamous cell carcinoma: the NEOCRTEC5010 randomized clinical trial. JAMA Surg. 2021;156(8):721–729. doi:10.1001/jamasurg.2021.2373
7. Yang H, Liu H, Chen Y, et al. Neoadjuvant chemoradiotherapy followed by surgery versus surgery alone for locally advanced squamous cell carcinoma of the esophagus (NEOCRTEC5010): a phase III multicenter, randomized, open-label clinical trial. J Clin Oncol. 2018;36(27):2796–2803. doi:10.1200/jco.2018.79.1483
8. Wang H, Tang H, Fang Y, et al. Morbidity and mortality of patients who underwent minimally invasive esophagectomy after neoadjuvant chemoradiotherapy vs neoadjuvant chemotherapy for locally advanced esophageal squamous cell carcinoma: a randomized clinical trial. JAMA Surg. 2021;156(5):444–451. doi:10.1001/jamasurg.2021.0133
9. Doki Y, Ajani JA, Kato K, et al. Nivolumab combination therapy in advanced esophageal squamous-cell carcinoma. N Engl J Med. 2022;386(5):449–462. doi:10.1056/NEJMoa2111380
10. Luo H, Lu J, Bai Y, et al. Effect of camrelizumab vs placebo added to chemotherapy on survival and progression-free survival in patients with advanced or metastatic esophageal squamous cell carcinoma: the ESCORT-1st randomized clinical trial. JAMA. 2021;326(10):916–925. doi:10.1001/jama.2021.12836
11. Wang ZX, Cui C, Yao J, et al. Toripalimab plus chemotherapy in treatment-naïve, advanced esophageal squamous cell carcinoma (Jupiter-06): a multi-center Phase 3 trial. Cancer Cell. 2022;40(3):277–288.e3. doi:10.1016/j.ccell.2022.02.007
12. Lu Z, Wang J, Shu Y, et al. Sintilimab versus placebo in combination with chemotherapy as first line treatment for locally advanced or metastatic oesophageal squamous cell carcinoma (ORIENT-15): multicentre, randomised, double blind, phase 3 trial. BMJ. 2022;377:e068714. doi:10.1136/bmj-2021-068714
13. Sun JM, Shen L, Shah MA, et al. Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): a randomised, placebo-controlled, phase 3 study. Lancet. 2021;398(10302):759–771. doi:10.1016/s0140-6736(21)01234-4
14. Wu Z, Zheng Q, Chen H, et al. Efficacy and safety of neoadjuvant chemotherapy and immunotherapy in locally resectable advanced esophageal squamous cell carcinoma. J Thorac Dis. 2021;13(6):3518–3528. doi:10.21037/jtd-21-340
15. Liu J, Yang Y, Liu Z, et al. Multicenter, single-arm, Phase II trial of camrelizumab and chemotherapy as neoadjuvant treatment for locally advanced esophageal squamous cell carcinoma. J Immunother Cancer. 2022;10(3). doi:10.1136/jitc-2021-004291
16. Liu J, Li J, Lin W, et al. Neoadjuvant camrelizumab plus chemotherapy for resectable, locally advanced esophageal squamous cell carcinoma (NIC-ESCC2019): a multicenter, Phase 2 study. Int J Cancer. 2022;151(1):128–137. doi:10.1002/ijc.33976
17. Xing W, Zhao L, Zheng Y, et al. The sequence of chemotherapy and toripalimab might influence the efficacy of neoadjuvant chemoimmunotherapy in locally advanced esophageal squamous cell cancer-a phase II study. Front Immunol. 2021;12:772450. doi:10.3389/fimmu.2021.772450
18. Deng H, Zhao Y, Cai X, et al. PD-L1 expression and tumor mutation burden as pathological response biomarkers of neoadjuvant immunotherapy for early-stage non-small cell lung cancer: a systematic review and meta-analysis. Crit Rev Oncol Hematol. 2022;170:103582. doi:10.1016/j.critrevonc.2022.103582
19. Lefler DS, Snook AE, Bashir B. Immune checkpoint inhibitors in luminal gastrointestinal malignancies: going beyond MSI-H/dMMR, TMB and PD-L1. Immunotherapy. 2022;14(11):885–902. doi:10.2217/imt-2022-0012
20. Li Q, Zhou S, Liu S, et al. Treatment-related lymphopenia predicts pathologic complete response and recurrence in esophageal squamous cell carcinoma undergoing neoadjuvant chemoradiotherapy. Ann Surg Oncol. 2019;26(9):2882–2889. doi:10.1245/s10434-019-07334-7
21. Wu Y, Chen J, Zhao L, et al. Prediction of pathologic response to neoadjuvant chemoradiotherapy in patients with esophageal squamous cell carcinoma incorporating hematological biomarkers. Cancer Res Treat. 2021;53(1):172–183. doi:10.4143/crt.2020.594
22. Li C, Lin JW, Yeh HL, Chuang CY, Chen CC. Good prediction of treatment responses to neoadjuvant chemoradiotherapy for esophageal cancer based on preoperative inflammatory status and tumor glucose metabolism. Sci Rep. 2021;11(1):11626. doi:10.1038/s41598-021-90753-y
23. Cai G, Yu J, Meng X. Predicting prognosis and adverse events by hematologic markers in patients with locally advanced esophageal squamous cell carcinoma treated with neoadjuvant chemoradiotherapy. Cancer Manag Res. 2020;12:8497–8507. doi:10.2147/cmar.S257058
24. Feng J, Wang L, Yang X, Chen Q, Cheng X. Prediction of pathologic complete response prediction in patients with locally advanced esophageal squamous cell carcinoma treated with neoadjuvant immunochemotherapy: a real-world study. Bosn J Basic Med Sci. 2022. doi:10.17305/bjbms.2022.7696
25. Rice TW, Patil DT, Blackstone EH. 8th edition AJCC/UICC staging of cancers of the esophagus and esophagogastric junction: application to clinical practice. Ann Cardiothorac Surg. 2017;6(2):119–130. doi:10.21037/acs.2017.03.14
26. Zinellu A, Collu C, Nasser M, et al. The Aggregate Index of Systemic Inflammation (AISI): a novel prognostic biomarker in idiopathic pulmonary fibrosis. J Clin Med. 2021;10(18):4134. doi:10.3390/jcm10184134
27. Li X, Zhang S, Lu J, Li C, Li N. The prognostic value of systemic immune-inflammation index in surgical esophageal cancer patients: an updated meta-analysis. Front Surg. 2022;9:922595. doi:10.3389/fsurg.2022.922595
28. Budczies J, Klauschen F, Sinn BV, et al. Cutoff Finder: a comprehensive and straightforward Web application enabling rapid biomarker cutoff optimization. PLoS One. 2012;7(12):e51862. doi:10.1371/journal.pone.0051862
29. Shao Y, Lin S, Zhang P, et al. Baseline monocyte and its classical subtype may predict efficacy of PD-1/PD-L1 inhibitor in cancers. Biosci Rep. 2021;41(1). doi:10.1042/bsr20202613
30. Wang L, Simons DL, Lu X, et al. Breast cancer induces systemic immune changes on cytokine signaling in peripheral blood monocytes and lymphocytes. EBioMedicine. 2020;52:102631. doi:10.1016/j.ebiom.2020.102631
31. Olingy CE, Dinh HQ, Hedrick CC. Monocyte heterogeneity and functions in cancer. J Leukoc Biol. 2019;106(2):309–322. doi:10.1002/jlb.4ri0818-311r
32. Zhao H, Chen W, Wu J, Wang L, Mao W. Clinical significance of preoperative serum tumor markers in esophageal squamous cell carcinoma. J Cancer Res Ther. 2014;10(Suppl):C179–85. doi:10.4103/0973-1482.145863
33. Hong Z, Huang Z, Chen Z, Kang M. Prognostic value of carcinoembryonic antigen changes before and after operation for esophageal squamous cell carcinoma. World J Surg. 2022;46(11):2725–2732. doi:10.1007/s00268-022-06672-0
34. Yang Y, Huang X, Zhou L, et al. Clinical use of tumor biomarkers in prediction for prognosis and chemotherapeutic effect in esophageal squamous cell carcinoma. BMC Cancer. 2019;19(1):526. doi:10.1186/s12885-019-5755-5
35. Li ZC, Sun YT, Lai MY, Zhou YX, Qiu MZ. Efficacy and safety of PD-1 inhibitors combined with chemotherapy as first-line therapy for advanced esophageal cancer: a systematic review and network meta-analysis. Int Immunopharmacol. 2022;109:108790. doi:10.1016/j.intimp.2022.108790
36. Zhu X, Shanzhou Q, Li D, Pang X, Ma D. PD-1 inhibitors versus chemotherapy as second-line treatment for advanced esophageal squamous cell carcinoma: a meta-analysis. BMC Cancer. 2021;21(1):1195. doi:10.1186/s12885-021-08958-3
37. Shen D, Chen Q, Wu J, Li J, Tao K, Jiang Y. The safety and efficacy of neoadjuvant PD-1 inhibitor with chemotherapy for locally advanced esophageal squamous cell carcinoma. J Gastrointest Oncol. 2021;12(1):1–10. doi:10.21037/jgo-20-599
38. Yang P, Zhou X, Yang X, et al. Neoadjuvant camrelizumab plus chemotherapy in treating locally advanced esophageal squamous cell carcinoma patients: a pilot study. World J Surg Oncol. 2021;19(1):333. doi:10.1186/s12957-021-02446-5
39. Lv H, Tian Y, Li J, et al. Neoadjuvant sintilimab plus chemotherapy in resectable locally advanced esophageal squamous cell carcinoma. Front Oncol. 2022;12:864533. doi:10.3389/fonc.2022.864533
40. Feng J, Wang L, Yang X, Chen Q, Cheng X. Pathologic complete response prediction to neoadjuvant immunotherapy combined with chemotherapy in resectable locally advanced esophageal squamous cell carcinoma: real-world evidence from integrative inflammatory and nutritional scores. J Inflamm Res. 2022;15:3783–3796. doi:10.2147/jir.S367964
41. Zhang X, Gari A, Li M, et al. Combining serum inflammation indexes at baseline and post treatment could predict pathological efficacy to anti‑PD‑1 combined with neoadjuvant chemotherapy in esophageal squamous cell carcinoma. J Transl Med. 2022;20(1):61. doi:10.1186/s12967-022-03252-7
42. Deng H, Liang H, Chen J, et al. Preoperative immunochemotherapy for locally advanced non-small cell lung cancer: an analysis of the clinical outcomes, optimal number of cycles, and peripheral immune markers. Transl Lung Cancer Res. 2022;11(12):2364–2381. doi:10.21037/tlcr-22-439
43. Qiu F, Fan J, Shao M, et al.Two cycles versus three cycles of neoadjuvant sintilimab plus platinum-doublet chemotherapy in patients with resectable non-small-cell lung cancer (neoSCORE): a randomized, single center, two-arm phase II trial. ASCO Annual Meeting. 2022;2022. doi:10.1200/JCO.2022.40.16_suppl.8500
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



