Back to Journals » OncoTargets and Therapy » Volume 16
A Nomogram Prognostic Model for Advanced Hepatocellular Carcinoma Based on the Interaction Between CD8+T Cell Counts and Age
Authors Wu Y , Liu X, Wang X , Yu L , Yan H , Xie Y, Pu Q, Cai X, Kong Y, Yang Z
Received 16 June 2023
Accepted for publication 6 September 2023
Published 21 September 2023 Volume 2023:16 Pages 753—766
DOI https://doi.org/10.2147/OTT.S426195
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Arseniy Yuzhalin
Yuan Wu,1,* Xiaoli Liu,1,* Xinhui Wang,2 Lihua Yu,1 Huiwen Yan,1 Yuqing Xie,1 Qing Pu,1 Xue Cai,1 Yaxian Kong,3 Zhiyun Yang1
1Center of Integrative Medicine, Beijing Ditan Hospital, Capital Medical University, Beijing, 100015, People’s Republic of China; 2Department of Chinese Medicine, National Center for Children’s Health, Beijing Children’s Hospital, Capital Medical University, Beijing, 100045, People’s Republic of China; 3Beijing Key Laboratory of Emerging Infectious Diseases, Institute of Infectious Diseases, Beijing Ditan Hospital, Capital Medical University, Beijing, 100015, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yaxian Kong, Beijing Key Laboratory of Emerging Infectious Diseases, Institute of Infectious Diseases, Beijing Ditan Hospital, Capital Medical University, Beijing, 100015, People’s Republic of China, Email [email protected] Zhiyun Yang, Center of Integrative Medicine, Beijing Ditan Hospital, Capital Medical University, Beijing, 100015, People’s Republic of China, Email [email protected]
Objective: CD8+T cells are essential components of the adaptive immune system and are crucial in the body’s immune system. This study aimed to investigate how the prognosis of patients with advanced hepatocellular carcinoma (HCC) was affected by their CD8+ T cell counts and age and established an effective nomogram model to predict the overall survival (OS).
Methods: A total of 427 patients with advanced HCC from Beijing Ditan Hospital, Capital Medical University, were enrolled in this study and randomly divided into training and validation groups, with 300 and 127 individuals in each group, respectively. Cox regression analysis was used to screen for independent risk factors for advanced HCC, and the interactive relationship between CD8+T cells and patient age was examined to establish a nomogram prediction model.
Results: Cox multivariate regression and interaction analyses indicated that tumor number, tumor size, aspartate aminotransferase (AST), C-reactive protein (CRP), relationship of CD8+T cell counts and age were independent predictors of 6-month OS in patients with advanced HCC, and the nomogram model was established based on these factors. The area under the receiver operating characteristic curve (AUC) of the nomogram model for predicting the 3-month, 6-month, and 12-month OS rates were 0.821, 0.802, and 0.756, respectively. Moreover, in clinical practice, patients with true-positive survival benefit more than true-positive death, therefore, we selected 25% as the clinical decision threshold probability based on probability density functions (PDFs) and clinical utility curves (CUCs), which can distinguish approximately 92% of patients who died and 37% of patients who survived.
Conclusion: The nomogram model based on CD8+T cell counts and age accurately assessed the prognosis of patients with advanced HCC and suggested that high CD8+T cell levels are beneficial to the survival of patients with advanced HCC.
Keywords: advanced hepatocellular carcinoma, nomogram, CD8+T cell counts, age, overall survival
Introduction
According to global cancer statistics for 2020,1 primary liver cancer has a high incidence and mortality rate, accounting for the sixth and third of new cancer cases and deaths worldwide, respectively. In 2020, approximately 906,000 new cases and 830,000 deaths occurred worldwide, and hepatocellular carcinoma (HCC) accounted for 75%–85% of primary liver cancer cases. Approximately 50% of HCC cases are diagnosed at an advanced diagnosis.2 The Barcelona Clinic Liver Cancer (BCLC) staging system is the most commonly used staging system for HCC in clinic.3 According to the European Society of Oncology (EASL) clinical practice guidelines, advanced HCC is defined as BCLC stage C, specifically referring to patients with HCC with macrovascular invasion (either segmental or portal invasion) or extrahepatic spread (lymph node involvement or metastases)2,4,5 and well-compensated liver function (Child-Pugh A or B).
Currently, sorafenib and lenvatinib systematic therapies for advanced HCC have a progression-free survival (PFS) of 3.7–7.4 months.6,7 With the development of immunotherapy, clinical trials have proven the efficacy of immune checkpoint inhibitors, such as anti-programmed cell death-1 (PD-1), anti-programmed cell death-ligand 1 (PD-L1), and anti-cytotoxic T lymphocyte-associated antigen-4 (CTLA-4) antibodies, in the treatment of advanced HCC. These drugs promote T-cell activation and proliferation by targeting immunological checkpoint molecules on the surface of cancer cells and relieving the immunosuppression of T cells, thus recognizing and attacking cancer cells.8–10 Notably, the greater the number and variety of T cells, the more specific receptors that can be provided to fight against different types of cancer cells and comprehensively kill cancer cells.11
Currently, the tumor node metastasis (TNM) stage,12 Okuda staging,13 model for end-stage liver disease (MELD),14 Cancer of the Liver Italian Program (CLIP) score,15 the Chinese University Prognostic Index (CUPI),16 and Japan Integrated Staging (JIS)17 are some of the most commonly used tumor prediction models. These models lack immune-related factors and consider only clinically relevant factors.
Our team previously found that T and NK cell counts could help predict the long-term survival of patients with HCC.18,19 Moreover, some studies have found that the prognosis of patients with various tumors, such as kidney, prostate, and bladder cancers, strongly correlates with CD8+T cell expression. If the infiltration level of CD8+T cells in the tumor is < 2.2%, the risk of disease progression is four times higher (hazard ratio (HR) = 3.84).20 Moreover, previous studies have confirmed that immune function weakens with age, particularly in T-cell defects.21–23 However, it remains unclear whether the prognosis of patients with advanced HCC is affected by the relationship between T cells and age. Therefore, we conducted an interaction analysis and established an advanced HCC prognostic model combined with clinically relevant factors to evaluate prognostic risk in patients with advanced HCC.
Materials and Methods
Patients Information
This was a retrospective cohort study. A total of 427 patients with advanced HCC were consecutively admitted to Capital Medical University-Affiliated Beijing Ditan Hospital between June 2009 and December 2019. This study was approved by the Beijing Ditan Hospital Ethics Committee. The patient inclusion criteria were as follows: (1) diagnosis of advanced HCC; (2) age (18–75 years old); and (3) availability of complete clinical information. The exclusion criteria were as follows: (1) cholangiocarcinoma; (2) metastatic liver cancer; (3) other types of cancer; (4) lost to follow-up; (5) incomplete clinical data. Finally, 427 patients were randomly divided into training and validation groups (300 and 127 patients, respectively) at a ratio of 7:3. The diagnostic criteria for advanced HCC were based on the EASL Clinical Practice Guidelines.4
We collected important clinical data including age; sex; family history; personal life history (history of smoking and alcohol consumption); aetiology of HCC; HBV-related indicators (hepatitis B virus (HBV) DNA, HBeAg); tumor-related indicators (tumor number, tumor size, portal vein tumor thrombus (PVTT), extrahepatic spread); and laboratory parameters (white blood cells (WBC), alanine aminotransferase (ALT), aspartate aminotransferase (AST), neutrophil-lymphocyte ratio (NLR), total bilirubin (TBIL), platelet counts (PLT), albumin (ALB), γ-glutamyl transferase (γ-GGT), lactate dehydrogenase (LDH), alkaline phosphatase (ALP), creatinine, prothrombin time activity (PTA), C-reactive protein (CRP), International Normalized Ratio (INR), alpha fetoprotein (AFP), Child-Pugh stage, and T lymphocytes).
Follow-Up and Endpoint
Overall survival was the primary outcome measure and was determined as the interval between the date of the initial advanced HCC diagnosis and the conclusion of follow-up in December 2021, or death from any cause. Our follow-up methods included regular reviews of hospital records and telephone follow-ups.
Statistical Analysis
IBM SPSS Statistics 25.0 and R software 4.0.5 were used for the statistical analysis. Continuous variables were compared using the t-test or Mann–Whitney U-test, whereas categorical variables were compared using the chi-square test or Fisher’s exact test. Patients with advanced HCC have limited treatment options and a poor prognosis, with a 6-month mortality rate as high as 50% after diagnosis.24,25 Therefore, we used Cox proportional hazards regression analysis to screen candidate variables for 6-month overall survival with advanced HCC patients. The nonlinear relationships were studied using restricted cubic splines. Two-way interactions between some factors of the multivariate analysis and CD8+ T-cell counts were investigated and considered in the model. Finally, based on the results of the Cox multivariate and interaction term analyses, a nomogram model was established using the rms package in R project version 4.0.5. The areas under the receiver operating characteristic (ROC) and calibration curves were used to evaluate the discrimination and calibration of the nomogram model, respectively. Moreover, probability density functions (PDFs) and clinical utility curves (CUCs) were plotted to select an appropriate cutoff point for clinical practice by comparing the net benefits of various thresholds.
Results
Patient Characteristics
In this study, 427 patients with advanced HCC were randomly divided into training (n = 300) and validation (n = 127) sets using 70% and 30% proportions, respectively. Table 1 presents patient characteristics. Among all patients, 344 (80.6%) were males and 83 (19.4%) were females, with an average age of 57.36 years. There were 359 (84.1%) patients with hepatitis B virus (HBV) infection, including a case of chronic alcohol consumption complications and two cases of hepatitis C virus (HCV) infection. Additionally, 28 patients (6.6%) had hepatitis C infections, with consequences from chronic alcohol use occurring in two of them. Forty-five (10.5%) patients had HCC caused by alcoholic liver disease. Twenty-seven (6.3%) patients had other causes, including five cases of autoimmune liver disease and two cases of nonalcoholic fatty liver disease. Regarding tumor characteristics, 341 (79.9%) patients had portal vein tumor thrombus (PVTT), and 169 (39.6%) had extrahepatic spread. Among the types of treatment, 250 (58.5%) patients with advanced HCC received local minimally invasive therapy, and 177 (41.5%) received palliative treatment. Generally, no significant differences occurred between the training and validation cohorts (p > 0.05).
 |
Table 1 Baseline Characteristics of Patients with Advanced Hepatocellular Carcinoma |
Development of Nomogram Model
Risk Factors Associated with Advanced HCC
Table 2 displays the results of univariate and multivariate Cox regression analyses for the 6-month overall survival of patients with advanced HCC. Univariate analysis indicated that HBV-DNA, HBeAg positive, tumor size ≥ 5 cm, tumor numbers ≥ 2, Child-Pugh Stage B, WBC, NLR > 2.41, AST > 40 U/L, ALT > 50 U/L, γ-GGT, LDH, ALP, CRP > 5 mg/L, AFP ≥ 400 ng/mL, T cell ≥ 1027 cells/μL, CD8+T cell ≥ 320 cells/μL, CD4+T cell ≥ 706 cells/μL, CD4/CD8 ≥ 1.5, and types of treatment were significantly related to the 6-month overall survival of advanced HCC in the training set (p < 0.05). Subsequently, these factors were placed into cox multivariate analysis, indicating that tumor size ≥ 5 cm (hazard ratio [HR] = 1.549, 95% confidence interval [CI]: 1.095–2.193, p = 0.014), tumor numbers ≥ 2 (HR = 1.677, 95% CI: 1.170–2.405, p = 0.005), AST (> 40 U/L; HR = 1.769, 95% CI: 1.156–2.706, p = 0.009), CRP (> 5 mg/L, HR = 3.004, 95% CI: 1.777–5.077, p < 0.001), and CD8+T cell counts (> 320 cells/μL, HR = 0.371, 95% CI: 0.244–0.565, p < 0.001) were the independent prognostic factors of advanced HCC (Table 2). These factors were incorporated into the nomogram models.
 |
Table 2 Univariate and Multivariate Cox Regression Analyses for 6-Month Overall Survival in Patients with Advanced Hepatocellular Carcinoma |
Interaction Term Analysis
We tested whether the interaction term between CD8+T cell counts and the other predictors was significant in the Cox regression analysis for advanced HCC (Table 3) and found a significant interaction between age or tumor number and CD8+T cell counts. Second, we plotted restricted cubic splines and demonstrated the relationship between CD8+T cell counts and the hazard ratio for survival in patients with advanced HCC stratified by age, tumor number, and tumor size (Figure 1). Regardless of the patient group, the number of CD8+T cells was greater than a certain threshold, which had a protective effect on the patient. Moreover, no matter the CD8+T cell count, the HR in patients of age < 50 years was always lower than that in patients of age ≥ 50 years (Figure 1A), and patients with tumor number < 2 always lower than patients with tumor numbers ≥ 2 (Figure 1B); however, the HR in patients with tumors smaller than 5 cm was getting closer to that of patients with tumors larger than 5 cm (Figure 1C). Therefore, no significant difference occurred between patients with tumors < 5 cm and those with tumors ≥ 5 cm. Finally, based on the interaction of these factors, we further investigated the relationship between CD8+T cell counts and median survival times in patients with advanced HCC in different sublayers. In Figure S1, as a cutoff value for the CD8+T cell count, patients were divided into two groups according to a maximum Youden index of 320 cells/μL. We then compared the effect of high (CD8+ T cell ≥ 320 cells/μL) and low (CD8+ T cell < 320 cells/μL) CD8+T cell groups on the median survival time in different groups and found that high CD8+T cells have a protective effect on patients with high or low age, single or multiple tumors, and tumor size ≥ 5 cm, but no significant protective effect on patients with tumor size < 5 cm.
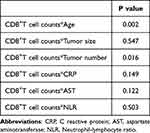 |
Table 3 Interaction Terms |
Nomogram Model Establishment
We combined the interaction term factors, such as CD8+T cell counts and age or tumor number, with the factors obtained from Cox multivariate analysis and used backward stepwise Cox regression analysis to establish the best nomogram model by comparing the Akaike Information Criterion (AIC) (lower AIC value indicates better outcomes) and C-statistic (higher C-statistic indicates better outcomes). The final model included the following factors: age, tumor number, tumor size, AST, CRP, and CD8+ T-cell count (Figure S2). A nomogram model was established to show the 3-month, 6-month, and 12-month overall survival (OS) rates of patients with advanced HCC. As shown in Figure 2, based on each factor’s risk score in the nomogram model, CRP level was the most important factor for predicting overall survival in patients with advanced HCC, followed by CD8+T cell count; the remaining factors had moderate predictive value. Compared with patients of age < 50 years, low CD8+T cell counts accounted for a greater point in patients of age ≥ 50 years (the points 51 vs 46), indicating that CD8+T cell were effective in both older and younger patients, but had a more prominent effect on older patients. Each factor’s risk score can be read on a relevant point scale, and the total points can then be determined. Based on the 3-month, 6-month, and 12-month OS total-point scales, predicting the survival of patients with advanced HCC is easy.
 |
Figure 2 Nomogram predicted the survival risks of patients with advanced HCC. |
Evaluation and Application of This Nomogram
Performance of the Model
The areas under the ROC and calibration curves were used to evaluate the performance of the model. The AUC values of the nomogram model for predicting the 3-, 6-, and 12-month survival of patients with advanced HCC in the training set were 0.821, 0.802, and 0.756, respectively (Figure 3A). In addition, they were 0.839, 0.795, and 0.807, respectively, in the validation set (Figure 3B). Calibration curves demonstrated that the 3-month, 6-month, and 12-month OS probabilities predicted by the nomogram model fit the actual data in the training and validation sets with a high degree of accuracy. (Figure 3C–H). Therefore, the nomogram model performed satisfactorily.
Application in Clinic
As shown in Figure 4, we used PDFs and CUCs to select the optimal nomogram model threshold. As shown in Figure 4A, some of the PDF curves of the surviving and dead patients overlapped. The percentages of surviving and undetected dead patients at any probability threshold are shown in Figure 4B. From a clinical point of view, we considered that additional intensive treatment would be more beneficial for patients who survive than for those who die; therefore, a threshold probability of 25% was chosen as the clinical decision. In this study, we could distinguish approximately 92% of patients who died (blue area in Figure 4A) and approximately 37% of surviving patients (red area in Figure 4A).
 |
Figure 4 Probability density functions (A) and clinical utility curves (B) of the nomogram model. |
Risk Stratification
Patients were divided into low-risk (< 170 points) and high-risk (≥ 170 points) groups based on the maximum Youden index value of the nomogram model score. The nomogram model could discriminate between high- and low-risk patients in the training and validation sets according to the Kaplan–Meier survival curves. (Figure 5A and B) and in patients aged ≥ 50 years and < 50 years (Figure 5C and D) (p< 0.0001).
Prognostic Value of CD8+T Cell Counts in Advanced HCC Patients
Based on the cut-off value of 320 cells /μL for CD8+T cell count, the patients were split into two groups. The Kaplan–Meier survival curve was used to analyze the 12-month overall survival rate. We found that in both training and validation sets, advanced HCC patients with a high level of CD8+T cells had a significantly reduced risk of death (death risk: HR = 0.74, 95% CI: 0.55–0.98, P = 0.036 and HR = 0.50, 95% CI: 0.30–0.81, P = 0.004 in training and validation cohorts separately). (Figure 6A and B). We further divided the patients into different subgroups based on clinical factors to explore whether CD8+ T-cell counts were closely associated with the overall survival of patients with advanced HCC. We found that patients in age ≥ 50 years, age < 50 years, tumor multiple, tumor solitary, tumor size ≥ 5 cm, tumor size < 5 cm, Child-Pugh A, Child-Pugh B, with HBV, and male subgroups, advanced HCC patients with a high CD8+T cell count had a higher survival rate than those with a low CD8+T cell count (Figure 6C–K and M). However, no statistically significant difference occurred in patients without HBV infection or in the female subgroups (p = 0.34 and p = 0.24, respectively) (Figures 6L and N). Therefore, the high expression of CD8+T cells can improve the survival rate of patients with advanced HCC.
Discussion
Recently, nomogram prediction models have been widely used in clinical settings. It can be used to make clinical decisions based on a single variable or combination of variables in the model.26 Nomogram models have been shown to help patients with breast cancer, nasopharyngeal carcinoma, and hepatocellular carcinoma make accurate clinical predictions, including disease progression and overall survival.27–29 Although some models associated with the prognosis of advanced HCC have been reported, there is a lack of prognostic models that combine immunological and clinical indicators. Such as Ke Su et al performed PD-1 inhibitors combined with radiotherapy and antiangiogenic therapy in advanced HCC, it was found that PD-L1 expression on circulating tumor cells, Child-Pugh classA/B, the number of tumors and AFP could predict the OS and PFS of advanced HCC.30 Besides, Han Li et al evaluated the 3-year overall survival of HCC patients treated with TACE or TACE combined with systemic therapy, and found that ALR scores (AST and lymphocyte rate) could predict the prognosis of patients and constructed a prognostic nomogram model.31 Thus, this study retrospectively enrolled patients with advanced HCC, collected complete clinical data including the expression of circulating T cells in peripheral blood, and explored the factors that may affect the survival of patients with advanced HCC.
In recent years, with the deepening of research on tumor immunity and the continuous development of immunology and molecular biology, tumor immunotherapy has shown good clinical value and advantages in clinical practice. At present, the treatment of HCC mainly includes sorafenib, PD-1 inhibitors, anti-angiogenic therapy, intensity-modulated radiotherapy (IMRT), transcatheter arterial chemoembolization (TACE) and hepatic artery infusion chemotherapy. In the study of patients with advanced HCC, Ke Su et al found that PD-1 inhibitors combined with anti-angiogenic therapy and IMRT had better OS and PFS than PD-1 inhibitors combined with anti-angiogenic therapy.32 And Han Li et al conducted a meta-analysis to compare the efficacy of different treatments for unresectable HCC and found that sorafenib combined with and external radiotherapy had longer OS and PFS and fewer the adverse events.33 So, we have to admit that immunotherapy is more and more helpful for cancer patients. CD8+T cells play a key role in anti-tumor immune response and immune checkpoint inhibitor therapy and it can selectively kill tumor cells. Tumor-associated antigens include specific neoantigens and autoantigens produced by tumor mutations. CD8+T cells can recognize these antigens and induce antitumor responses.34 CD8+T cells can also be detected in human and mouse tumor-infiltrating lymphocytes, including melanoma and ovarian, breast, and lung cancers.35 A systematic review and meta-analysis indicated that high levels of CD8+ T-cell infiltration are linked to better outcomes in breast cancer patients,36 which was consistent with the results of this study. CD8+T cells kill tumor cells in three main ways. First, they bind with tumor cells and use perforin and granzyme B to directly kill tumor cells. Second, activated CD8+T cells express FAS-L and bind to FAS on the surface of tumor cells to induce tumor cell apoptosis. Third, tumor cells can be indirectly killed by releasing cytokines such as TNF-α and IFN-γ.37,38 Therefore, this study focused on the effect of CD8+T cells on the prognosis of patients with advanced HCC.
In this study, we identified five important factors (tumor size, tumor number, AST, CRP, and CD8+T cell counts) affecting the 6-month overall survival of patients with advanced HCC using Cox univariate and multivariate regression analyses. Then according to previous studies, it was found that the expression of CD8+T cells changed with age, and the elderly were more susceptible to infectious diseases because of their aging immune system.35,39 According to statistics, the median age of HCC patients was 55 years in China,40 which was consistent with the age of our enrolled patients. And HCC was mainly caused by hepatitis B virus (HBV) in China,41 which most from mother to child transmission.42 Although the elderly was more susceptible to infectious diseases, the viral infections that caused HCC in our population may not be affected by age. In addition, cox univariate and multivariate regression analysis showed that age had no independent effect on the mortality of patients with advanced HCC. Furthermore, some studies have confirmed that some factors affect the expression of CD8+T cells in cancer patients, such as aging, inflammation, and immunosuppression, due to the tumor burden.43 Therefore, we assessed the interaction terms between CD8+T cell count, tumor factors, infection factors, and age. The results indicated that although age was not an independent prognostic factor for advanced HCC, the interaction between age and CD8+ T-cell count had a significant effect on the prognosis of advanced HCC. We then established the final nomogram model by combining and comparing the results of the Cox regression and interaction term analyses. A model with only the results of Cox regression analysis vs a model combined with Cox regression analysis and an interaction term was fairly similar but with a small advantage for the latter (C-statistic 0.73 vs 0.75, AIC 1500.1 vs 1498.3). Therefore, the final nomogram model included age, tumor number, tumor size, AST, CRP levels, and CD8+T cell count. The area under the receiver operating characteristic (ROC) curve (AUC) and calibration curve were used to assess the nomogram model’s performance, and it was found that both the model’s calibration and discrimination were good. Moreover, 25% was chosen as the threshold probability for clinical decision-making in this study. As correctly detecting a patient’s risk of death is crucial, with a threshold probability of 25%, we could identify approximately 92% of the patients who died. Thus, it can help doctors choose the appropriate treatment methods in clinical practice. In fact, in clinical practice, doctors can choose different cutoff values for PDFs and CUCs according to different clinical situations to choose a more suitable treatment for patients.
This study has certain limitations, such as a small sample size. Although we performed internal verification, there was a lack of external verification. In the future, we will need to use more samples to further verify the predictive accuracy of this nomogram model and compare it with other models.
Conclusion
In conclusion, we developed a prognostic nomogram model for patients with advanced HCC based on CD8+ T-cell count and age. It can predict the overall survival of patients accurately and conveniently. High expression of CD8+T cells protects against advanced HCC.
Abbreviations
HBV, hepatitis B virus; HCV, hepatitis C virus; WBC, white blood cells; ALT, alanine aminotransferase; AST, aspartate aminotransferase; NLR, Neutrophil‐lymphocyte ratio; TBIL, total bilirubin; γ-GGT, γ-glutamyl transferase, LDH, lactate dehydrogenase; ALP, alkaline phosphatase; PTA, prothrombin time activity; INR, international normalized ratio; CRP, C reactive protein; AFP, alpha-fetoprotein; ALB, albumin; SD, standard deviation.
Ethics Approval and Consent to Participate
This study is in accordance with the Declaration of Helsinki and has been approved by the ethical committee of Beijing Ditan Hospital, Capital Medical University. This study was a retrospective research. The ethics committee approved it to be used in this study while ensuring patient privacy, not interfering with the patient’s treatment methods, and not bringing risks to the patient’s physiology.
Acknowledgments
This study was supported by the National Science Foundation of China (No. 82274479, No. 82104781), High-level Public Health Technical Personnel Construction Project (Subject leaders-02-16), Dengfeng Talent Support Program of Beijing Municipal Administration of Hospitals (No. DFL20191803), Beijing Hospitals Authority Clinical Medicine Development of Special Funding Support (No. ZYLX202127), the Special Fund of Capital Health Research and Development (No. 2020-2-2173), Fund of Beijing Ditan Hospital, Capital Medical University (DTYM-202113), Beijing Hospitals Authority Youth Programme (QML20231801).
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
2. Llovet JM, Kelley RK, Villanueva A, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7(1):6. doi:10.1038/s41572-020-00240-3
3. Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19(3):329–338. doi:10.1055/s-2007-1007122
4. European Association For The Study Of The Liver. EASL Clinical Practice Guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182–236. doi:10.1016/j.jhep.2018.03.019
5. Ali R, Gabr A, Abouchaleh N, et al. Survival Analysis of Advanced HCC Treated with Radioembolization: comparing Impact of Clinical Performance Status Versus Vascular Invasion/Metastases. Cardiovasc Intervent Radiol. 2018;41(2):260–269. doi:10.1007/s00270-017-1791-1
6. Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–390. doi:10.1056/NEJMoa0708857
7. Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised Phase 3 non-inferiority trial. Lancet. 2018;391(10126):1163–1173. doi:10.1016/S0140-6736(18)30207-1
8. Wong KM, King GG, Harris WP. The Treatment Landscape of Advanced Hepatocellular Carcinoma. Curr Oncol Rep. 2022;24(7):917–927. doi:10.1007/s11912-022-01247-7
9. Llovet JM, Castet F, Heikenwalder M, et al. Immunotherapies for hepatocellular carcinoma. Nat Rev Clin Oncol. 2022;19(3):151–172. doi:10.1038/s41571-021-00573-2
10. Waldman AD, Fritz JM, Lenardo MJ. A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nat Rev Immunol. 2020;20(11):651–668. doi:10.1038/s41577-020-0306-5
11. Woolaver RA, Wang X, Krinsky AL, et al. Differences in TCR repertoire and T cell activation underlie the divergent outcomes of antitumor immune responses in tumor-eradicating versus tumor-progressing hosts. J Immunother Cancer. 2021;9(1):e001615. doi:10.1136/jitc-2020-001615
12. Minagawa M, Ikai I, Matsuyama Y, et al. Staging of hepatocellular carcinoma: assessment of the Japanese TNM and AJCC/UICC TNM systems in a cohort of 13,772 patients in Japan. Ann Surg. 2007;245(6):909–922. doi:10.1097/01.sla.0000254368.65878.da
13. Okuda K, Obata H, Nakajima Y, et al. Prognosis of primary hepatocellular carcinoma. Hepatology. 1984;4(1 Suppl):3s–6s. doi:10.1002/hep.1840040703
14. Wiesner RH, Freeman RB, Mulligan DC. Liver transplantation for hepatocellular cancer: the impact of the MELD allocation policy. Gastroenterology. 2004;127(5 Suppl 1):S261–267. doi:10.1053/j.gastro.2004.09.040
15. CLIP. A new prognostic system for hepatocellular carcinoma: a retrospective study of 435 patients: the Cancer of the Liver Italian Program (CLIP) investigators. Hepatology. 1998;28(3):751–755. doi:10.1002/hep.510280322
16. Leung TW, Tang AM, Zee B, et al. Construction of the Chinese University Prognostic Index for hepatocellular carcinoma and comparison with the TNM staging system, the Okuda staging system, and the Cancer of the Liver Italian Program staging system: a study based on 926 patients. Cancer. 2002;94(6):1760–1769. doi:10.1002/cncr.10384
17. Kudo M, Chung H, Osaki Y. Prognostic staging system for hepatocellular carcinoma (CLIP score): its value and limitations, and a proposal for a new staging system, the Japan Integrated Staging Score (JIS score). J Gastroenterol. 2003;38(3):207–215. doi:10.1007/s005350300038
18. Liu X, Wang X, Yu L, et al. A Novel Prognostic Score Based on Artificial Intelligence in Hepatocellular Carcinoma: a Long-Term Follow-Up Analysis. Front Oncol. 2022;12:817853. doi:10.3389/fonc.2022.817853
19. Yu L, Liu X, Wang X, et al. Nomogram for prediction of long-term survival with hepatocellular carcinoma based on NK cell counts. Ann Hepatol. 2022;27(2):100672. doi:10.1016/j.aohep.2022.100672
20. Jansen CS, Prokhnevska N, Master VA, et al. An intra-tumoral niche maintains and differentiates stem-like CD8 T cells. Nature. 2019;576(7787):465–470. doi:10.1038/s41586-019-1836-5
21. Song Y, Wang B, Song R, et al. T-cell Immunoglobulin and ITIM Domain Contributes to CD8 + T-cell Immunosenescence. Aging Cell. 2018;17(2):e12716. doi:10.1111/acel.12716
22. Linton PJ, Dorshkind K. Age-related changes in lymphocyte development and function. Nat Immunol. 2004;5(2):133–139. doi:10.1038/ni1033
23. Shaw AC, Goldstein DR, Montgomery RR. Age-dependent dysregulation of innate immunity. Nat Rev Immunol. 2013;13(12):875–887. doi:10.1038/nri3547
24. Vogel A, Martinelli E, Vogel A. Updated treatment recommendations for hepatocellular carcinoma (HCC) from the ESMO Clinical Practice Guidelines. Ann Oncol. 2021;32(6):801–805. doi:10.1016/j.annonc.2021.02.014
25. Giannini EG, Farinati F, Ciccarese F, et al. Prognosis of untreated hepatocellular carcinoma. Hepatology. 2015;61(1):184–190. doi:10.1002/hep.27443
26. Iasonos A, Schrag D, Raj GV, et al. How to build and interpret a nomogram for cancer prognosis. J Clin Oncol. 2008;26(8):1364–1370. doi:10.1200/JCO.2007.12.9791
27. Xiao BB, Lin DF, Sun XS, et al. Nomogram for the prediction of primary distant metastasis of nasopharyngeal carcinoma to guide individualized application of FDG PET/CT. Eur J Nucl Med Mol Imaging. 2021;48(8):2586–2598. doi:10.1007/s00259-020-05128-8
28. Lyu MH, Ma YZ, Tian PQ, et al. Development and validation of a nomogram for predicting survival of breast cancer patients with ipsilateral supraclavicular lymph node metastasis. Chin Med J. 2021;134(22):2692–2699. doi:10.1097/CM9.0000000000001755
29. Liu Q, Li J, Liu F, et al. A radiomics nomogram for the prediction of overall survival in patients with hepatocellular carcinoma after hepatectomy. Cancer Imaging. 2020;20(1):82. doi:10.1186/s40644-020-00360-9
30. Su K, Guo L, He K, et al. PD-L1 expression on circulating tumor cells can be a predictive biomarker to PD-1 inhibitors combined with radiotherapy and antiangiogenic therapy in advanced hepatocellular carcinoma. Front Oncol. 2022;12:873830. doi:10.3389/fonc.2022.873830
31. Li H, Guo L, Su K, et al. Construction and Validation of TACE Therapeutic Efficacy by ALR Score and Nomogram: a Large, Multicenter Study. J Hepatocell Carcinoma. 2023;10:1009–1017. doi:10.2147/JHC.S414926
32. Su K, Guo L, Ma W, et al. PD-1 inhibitors plus anti-angiogenic therapy with or without intensity-modulated radiotherapy for advanced hepatocellular carcinoma: a propensity score matching study. Front Immunol. 2022;13:972503. doi:10.3389/fimmu.2022.972503
33. Li H, Wu Z, Chen J, et al. External radiotherapy combined with sorafenib has better efficacy in unresectable hepatocellular carcinoma: a systematic review and meta-analysis. Clin Exp Med. 2022;23(5):1537–1549. doi:10.1007/s10238-022-00972-4
34. Philip M, Schietinger A. CD8(+) T cell differentiation and dysfunction in cancer. Nat Rev Immunol. 2022;22(4):209–223. doi:10.1038/s41577-021-00574-3
35. St Paul M, Ohashi PS. The Roles of CD8(+) T Cell Subsets in Antitumor Immunity. Trends Cell Biol. 2020;30(9):695–704. doi:10.1016/j.tcb.2020.06.003
36. Sun YP, Ke YL, Li X. Prognostic value of CD8(+) tumor-infiltrating T cells in patients with breast cancer: a systematic review and meta-analysis. Oncol Lett. 2023;25(1):39. doi:10.3892/ol.2022.13625
37. van der Leun AM, Thommen DS, Schumacher TN. CD8(+) T cell states in human cancer: insights from single-cell analysis. Nat Rev Cancer. 2020;20(4):218–232. doi:10.1038/s41568-019-0235-4
38. Qin S, Xu L, Yi M, et al. Novel immune checkpoint targets: moving beyond PD-1 and CTLA-4. Mol Cancer. 2019;18(1):155. doi:10.1186/s12943-019-1091-2
39. Yousefzadeh MJ, Flores RR, Zhu Y, et al. An aged immune system drives senescence and ageing of solid organs. Nature. 2021;594(7861):100–105. doi:10.1038/s41586-021-03547-7
40. Wang S, Shi H, Liu T, et al. Mutation profile and its correlation with clinicopathology in Chinese hepatocellular carcinoma patients. Hepatobiliary Surg Nutr. 2021;10(2):172–179. doi:10.21037/hbsn.2019.09.17
41. Li Q, Cao M, Lei L, et al. Burden of liver cancer: from epidemiology to prevention. Chin J Cancer Res. 2022;34(6):554–566. doi:10.21147/j.issn.1000-9604.2022.06.02
42. Liu J, Wang X, Wang Q, et al. Hepatitis B virus infection among 90 million pregnant women in 2853 Chinese counties, 2015-2020: a national observational study. Lancet Reg Health West Pac. 2021;16:100267. doi:10.1016/j.lanwpc.2021.100267
43. Saeidi A, Zandi K, Cheok YY, et al. T-Cell Exhaustion in Chronic Infections: reversing the State of Exhaustion and Reinvigorating Optimal Protective Immune Responses. Front Immunol. 2018;9:2569. doi:10.3389/fimmu.2018.02569
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




