Back to Journals » Cancer Management and Research » Volume 11
A multivariable model of BRAFV600E and ultrasonographic features for predicting the risk of central lymph node metastasis in cN0 papillary thyroid microcarcinoma
Authors Chen BD , Zhang Z, Wang KK, Shang MY, Zhao SS , Ding WB, Du R, Yu Z, Xu XM
Received 30 December 2018
Accepted for publication 4 July 2019
Published 30 July 2019 Volume 2019:11 Pages 7211—7217
DOI https://doi.org/10.2147/CMAR.S199921
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Beicheng Sun
Bao-Ding Chen,1,* Zheng Zhang,1,* Ke-Ke Wang,1 Meng-Yuan Shang,1 Shuang-Shuang Zhao,1 Wen-Bo Ding,2 Rui Du,1 Zhuan Yu,1 Xi-Ming Xu3
1Department of Medical Ultrasound, Affiliated Hospital of Jiangsu University, Zhenjiang 212000, People’s Republic of China; 2Department of Medical Ultrasound, Jiangsu Province Hospital on Integration of Chinese and Western Medicine, Nanjing 210028, People’s Republic of China; 3Department of Pharmaceutics, School of Pharmacy and Center for Drug/Gene Delivery and Tissue Engineering, Jiangsu University, Zhenjiang, Jiangsu 212001, People’s Republic of China
*These authors contributed equally to this work
Background: Prophylactic central lymph node dissection (CLND) in papillary thyroid microcarcinoma (PTMC) patients without clinical evidence of central lymph node metastasis (CLNM) remains controversial. The purpose of our study is to identify preoperative predictive factors for finding CLNM in Chinese PTMC patients, which may allow tailored CLND.
Methods: We retrospectively reviewed 182 consecutive Chinese PMTC patients with negative central lymph nodes who underwent total thyroidectomy plus central neck dissection from October 2015 to December 2017. Chi-squared and multivariate analysis were performed to evaluate the association of CLNM with ultrasonographic and clinicopathologic characteristics. Receiver operating characteristic (ROC) curve analysis was performed to evaluate the utility of markers in predicting CLNM.
Results: The CLNM was found in 39.0% (71 of 182) of cN0 PTMC patients. In multivariate analysis, tumor size>7 mm (OR: 3.636, 95% CI: 1.671–7.914), marked hypoechogenicity (OR: 2.686, 95% CI: 1.080–6.678), multifocality (OR: 4.184, 95% CI: 1.707–10.258) and BRAFV600E mutation (OR: 5.339, 95% CI: 2.529–11.272) were independent predictors of CLNM. In ROC analysis integrating these predictors, the sensitivity was 63.4% and specificity was 80.2%, and the area under the ROC (AUC) was 0.755.
Conclusion: In conclusion, we found tumor size>7 mm, marked hypoechogenicity, multifocality, and BRAFV600E mutation were risk factors for CLNM. In term of these preoperative risk factors for CLNM, prophylactic CLND should be cautiously performed in cN0 PTMC patients.
Keywords: central lymph node metastasis, prophylactic central lymph node dissection, papillary thyroid microcarcinoma, risk factor
Introduction
Papillary thyroid carcinoma (PTC) is the most common histological subtype of thyroid carcinoma, accounting for 85–90% of all thyroid malignancies, and its incidence is steadily increasing each year.1,2 Papillary thyroid microcarcinoma (PTMC) refers to a PTC with the greatest diameter of 10 mm or less.3 With the rapid development of high-resolution Ultrasonography and fine-needle aspiration biopsy (FNAB), impalpable PTMC has been frequently detected and diagnosed. Central lymph node metastasis (CLNM) is seen in 40–60% of PTMC patients.4 It is generally accepted that therapeutic central lymph node dissection (CLND) should be performed in patients with macroscopic CLNM. The revised American Thyroid Association (ATA) guidelines recommend that prophylactic CLND should be considered in patients with high-risk thyroid cancer.5 How to do with the low-risk PTMC patients? Recently, Agcaoglu et al recommends prophylactic CLND should not be operated for patients with tumors smaller than 5 mm and without evidence of nodal metastasis in preoperative neck ultrasonography.6 Whether patients without clinical evidence of CLNM need to receive routine prophylactic CLND remains controversial. It is the key to handle this question predicting the risk of central lymph node metastasis in cN0 PTMC validly.
Ultrasonography, based on the differences of reflection, absorption and attenuation of Ultrasonography waves in thyroid tissue and surrounding neck tissue, is a standard auxiliary examination for patients with thyroid cancer.7 Although preoperative Ultrasonography plays an important role in visualizing CLNM, neck Ultrasonography examination has a very low sensitivity to visualize the CLNM lesions.8
BRAFV600E mutation, which constitutively activates the MAPK signaling pathway, is a somatic alteration highly specific to PTMC.9,10 The MAPK signaling pathway plays a crucial role in the regulation of cell growth, division, and proliferation.11 In addition, many investigations have demonstrated that the BRAFV600E mutation is associated with aggressive clinicopathologic characteristics.12,13
Given that prophylactic CLND in PTMC patients without clinical evidence of CLNM is controversial, there is a great need for independent predictors for CLNM to allow targeted prophylactic CLND. The purpose of this study is to identify clinical and biological predictors of CLNM in Chinese patients with cN0 PTMC.
Methods and materials
Patients and thyroid cancer samples
This study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of the Affiliated Hospital of Jiangsu University (ethical review number: SWYXLL20190225-2). Written informed consent for the evaluation of BRAF status was obtained from participant patients prior to thyroidectomy.
This study was conducted in the Affiliated Hospital of Jiangsu University from October 2015 to December 2017. During this period, 232 patients underwent thyroidectomy with routine prophylactic CLND. All patients were diagnosed with PTMC preoperatively by FNAB or postoperative pathology, and the determination of CLNM confirmed with the surgery pathology. Among these patients, we set the following exclusion criteria for this study: (a) a previous history of thyroidectomy; (b) tumor size measurement>10 mm on Ultrasonography; (c) refusal of BRAFV600E analysis; and (d) absent or insufficient Ultrasonography image. The final dataset included 182 PTMC cases. We obtained demographic and clinical information including age, gender, BRAFV600E mutation, CLNM and chronic lymphocytic thyroiditis from electronic clinical and pathologic records.
Ultrasonography and image analysis
Every nodule was studied separately. Two experienced radiologists, who were unaware of clinicopathologic characteristics and BRAF results, independently interpreted all preoperative Ultrasonography features and recorded the Ultrasonography examination for each PTMC using standardized institutional protocols. When disagreements appeared between the two radiologists, the third senior radiologist reviewed the features and made the final decision. We selected the largest tumor as the target tumor when the preoperative Ultrasonography showed multifocality. Because vascularity on Ultrasonography was difficult to evaluate objectively even using color Doppler technique, vascularity was not assessed.
BRAF mutation analysis
According to published studies, the ability to detect BRAFV600E in FNAB cytologic specimens is not inferior to that in postoperative pathologic specimens.11,14 The polymerase chain reaction (PCR) conditions and primers for amplifying exon 15 of the BRAF, which contains V600E mutation, were established previously.15 Genomic DNA was extracted from FANB specimens using the QIAamp DNA FFPE Tissue Kit (QIAGEN) following the manufacturer’s instruction. For direct DNA sequencing, exon 15 was amplified by PCR, followed by the Big Dye terminator cycle sequencing reaction and sequence reading on an ABI PRISM 3730 genetic analyzer (Applied Biosystems, Foster City, CA).16
Statistical analysis
Statistical analysis was performed using SPSS software (ver. 19.0; SPSS Inc., Chicago, IL, USA). The Student’s t-test was used for comparison of continuous variables, and Pearson X2 or Fisher’s exact test was used for comparison of categorical variables. P-value≤0.05 was considered to be statistically significant. Multivariate logistic regression analysis was used to assess the relationship between the predicting factors and the presence of CLNM. A risk score for each patient was constructed based on the identified risk factors, including tumor size (1 for size>7 mm, 0 for size≤7 mm), marked hypoechogenicity (1 for presence, 0 for absence), multifocality (1 for positive, and 0 for negative) and BRAFV600E mutation (1 for positive and 0 for negative). Receiver operating characteristic (ROC) curve and area under the ROC curve (AUC) were used to estimate the predictive power.
Results
Demographic variables
Among the 182 patients, 71 (39.0%) were CLNM positive. There were 145 (79.7%) female and 37 (20.3%) male. The mean age was 42.0 years and 110 (60.4%) were younger than 45 years. The mean tumor size was 7.21 mm and 89 (48.9%) were larger than 7 mm in diameter. Multifocal PTMC was observed in 34 (18.9%) cases and bilateral PTMC in 29 (15.9%) cases. Suspicious Ultrasonography features including solid component, marked hypoechogenicity, microcalcifications, irregular/lobulated margins, and non-parallel orientation were presented in 98.9%, 17.6%, 59.3%, 69.8%, and 45.6% of PTMCs, respectively. Fifty-five (30.2%) cases had concomitant chronic lymphocytic thyroiditis. BRAFV600E mutation was observed in 87 (47.8%) patients.
Distribution of CLNM among cN0 PTMC patients with different clinicopathologic and ultrasonography features
As shown in Table 1, patients younger than 45 years appeared to have a higher prevalence of CLNM than those 45 years or older (66.2% vs 33.8%), but did not reach statistical significant (P=0.204). The presence of CLNM in male and female was similar (P=0.831). BRAFV600E mutation carriers were more likely to be CLNM positive (P<0.001).
 |
Table 1 Characteristics of the patients with cN0 PTMC |
Among Ultrasonography features of PTMC, tumor size (P=0.027), multifocality (P=0.009), and marked hypoechogenicity (P=0.028) were significantly associated with the presence of CLNM. Other Ultrasonography features, including solid component, microcalcification, irregular/lobulated margins, and non-parallel orientation, were not associated with CLNM (all P>0.05).
Multivariate logistic analysis for CLNM of PTMC
We next used multivariate logistic regression analysis to examine the independent associations between clinicopathologic and Ultrasonography features and the risk of having positive CLNM. Tumor size>7 mm (OR =3.636, 95% CI, 1.671–7.914, P=0.001), marked hypoechogenicity (OR =2.686, 95% CI, 1.080–6.678, P=0.002), multifocality (OR =4.184, 95% CI, 1.707–10.258, P=0.002) and BRAFV600E mutation (OR =5.339, 95% CI, 2.529–11.272, P<0.001) turned out to be independent risk factors for finding CLNM in clinically negative PTMC patients (Table 2).
 |
Table 2 Multivariate analyses of factors for predicting CLNM in cN0 PTMC |
Association between risk factors and CLNM in the score system
Finally, we computed a risk score for each patient based on the above-identified significant predictors and constructed a ROC curve using the risk score. The AUC was 0.755 (Figure 1). A cut-off point of 0.44 resulted in a sensitivity of 63.4% and a specificity of 80.2% for prediction.
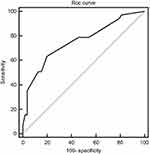 |
Figure 1 Receiver-operating characteristic (ROC) curve for the scoring system. The area under the ROC curve (AUC) was 0.755. |
Discussion
PTMC belongs to the low-risk PTC group of thyroid carcinomas, which are rarely life-threatening. CLNM is common in PTMC patients with an incidence of 40–60%.17 PTMC patients with clinically positive CLNM are usually treated with therapeutic CLND. However, how to manage cN0 PTMC patients is still a matter of debate because of the limited survival benefits and the CLND-associated complications, such as hypoparathyroidism and recurrent laryngeal nerve injury. Using preoperative Ultrasonography to visualize metastatic lymph node in the central compartment is not very accurate. Previous studies have attempted to identify predictive factors of CLNM in cN0 PTMC patients, but the results were inconsistent. Therefore, we set to investigate the preoperative predictive factors for occult CLNM in Chinese cN0 PTMC patients.
The current system to predict cN0 CLNM primarily depends on histopathologic features, such as extrathyroidal extension (ETE), tumor subtype, and advanced T stage (T3 and/or T4), all of which are only available from postoperative pathology. Consequently, recent efforts have focused on the preoperative clinical features to predict subclinical CLNM. Preoperative risk factors for subclinical CLNM in PTMC patients were not well defined. Our study found several clinicopathologic and ultrasonographic characteristics, including large tumor size (>7 mm), marked hypoechogenicity, multifocality, and BRAFV600E mutation, which were available preoperatively, as potential risk factors for CLNM.
An age of 45 years old as the cut-off point is common to be a clinical marker for prognosis. Several previous studies have shown that age<45 years exhibited a poorer prognosis.18 Consistently, in our study, there were apparently higher percentage of CLNM in patients younger than 45 years than those 45 years or older, although the difference did not reach statistical significance.
As expected, tumor size was confirmed as a prognostic feature in PTMC in our study. We observed that tumor size>7 mm presented a 3.6-fold increased risk of CLNM in cN0 patients. Likewise, Zhou et al reported that tumor size>7 mm was a risk factor of CLNM.19 Two other studies also demonstrated that larger tumor size of PTMC enhanced tumor aggressiveness and worsened survival of patients.20,21
Multifocality in PTMC is an indication for increased risk of tumor recurrence and CLNM. A previous study has shown multifocality may be associated with clone selection from a preneoplastic field and spread throughout the thyroid gland.22 In a meta-analysis, Sun et al found multifocal PTMC was also an independent predictor of CLNM.8 Our study found that multifocality was also an independent predictive factor for CLNM with an OR of 4.2.
Whether suspicious ultrasonographic features are associated with CLNM in cN0 PTMC remains controversial. Some studies have reported on the association between CLNM and selected preoperative ultrasonographic features, such as the presence of calcification.23 whereas other studies reported null results for the associations of CLNM with several ultrasonographic features including solid component, marked hypoechogenicity, microcalcification, microlobulated or irregular margin, and non-parallel orientation.15,24 We defined marked hypoechogenicity as decreased echogenicity when compared with the surrounding strap muscle.11 In our study, we found marked hypoechogenicity as an independent predictor for CLNM, but not other ultrasonographic features including microcacification, microlobulated or irregular margin and non-parallel orientation. Further studies are needed to clarify the value of ultrasonographic features in predicting CLNM.
The prevalence of concomitant chronic lymphocytic thyroiditis (CLT) in PTC has been reported to range from 10% to 58%.15 Only a handful of investigations have reported the effects of concomitant CLT with occult CLNM. Loh et al showed that PTC patients with concomitant CLT had a good prognosis, due to the low frequency of extrathyroid extension (ETE), lymph node metastasis and distant metastasis.25,26 In our study, the incidence of CLT was less frequent in node-positive patients than node-negative patients (33.8% vs 66.2%), which did not reach statistical significance (P=0.400). Likewise, Xiang et al also did not find significant association between concomitant CLT and CLNM.17
Over the last decade, the relationship between BRAFV600E mutation and clinicopathological characteristics in PTC has been extensively studied. BRAFV600E mutation was closely related to a poor outcome and could lead to an increase in tumor recurrence and cancer-related mortality.27 BRAF mutation-related molecular alterations, including overexpression of tumor-promoting genes, silencing of tumor suppressor genes, and down-regulation of thyroid iodide-handling genes, plays a fundamental role in the formation, progression, and aggressiveness of PTMC.28,29 Though BRAFV600E mutation has been considered as an important clinical marker of adverse prognosis, its value as a predictor of CLNM is still debatable. Virk et al found that PTMC patients with BRAF mutation were more likely to present cervical lymph node metastasis characteristics.30 but a recent study showed that BRAF mutation was not a predictor for CLNM in cN0 PTMC patients.31 We examined BRAFV600E mutation on FNAB tissues preoperatively and found BRAFV600E mutation as a strong independent risk factor for CLNM (OR=5.339) in multivariate analysis.
In our ROC curve analysis combining all the identified predictors, the AUC reached 0.755, demonstrating a strong prediction efficiency. A cut-off point of 0.44, resulted in a sensitivity of 63.4% and a specificity of 80.2%. Moreover, the predictors in this scoring system mainly come from preoperative ultrasonographic features, which is advantageous to postoperative markers for predicting occult CLNM. These results may aid surgeons to tailor the follow-up treatment of each PTMC patient.
There are several limitations to our study. Firstly, our study was a retrospective observational study and there may be selection bias. Secondly, the 232 patients of our studies are all Chinese, and whether the identified factors can predict CLNM in other races needs further investigation. Thirdly, locoregional recurrence and disease-specific survival were not investigated in this study due to short follow-up time and low events. Fourthly, some risk factors for CLNM such as family history and behaviors, have not been investigated in our study. Future prospective, multicenter, large sample size, and long-term follow-up studies are warranted to evaluate the impact of occult CLNM on prognosis.
In summary, we presented several independent predictive factors for CLNM in patients with cN0 PTMC. We constructed a risk prediction model consisting of tumor size>7 mm, marked hypoechogenicity, multifocality and BRAFV600E mutation that may guide surgeons to evaluate the nodal status in cN0 PTMC and perform tailored prophylactic CLND.
Acknowledgments
This study was supported by the Zhenjiang Social Development Fund (SH2018035), Zhenjiang Key Research and Development Plan Fund (SH2016036) and the Doctoral Start-up Fund of Affiliated Hospital of Jiangsu University (jdfyRC2017013).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Vuong H, Altibi A, Duong U, Hassell L. Prognostic implication of BRAF and TERT promoter mutation combination in papillary thyroid carcinoma-A meta-analysis. Clin Endocrinol (Oxf). 2017;87(5):411–417. doi:10.1111/cen.13413
2. La VC, Malvezzi M, Bosetti C, et al. Thyroid cancer mortality and incidence: a global overview. Int J Cancer Suppl. 2015;136(9):2187.
3. Zuo H, Tang W, Yasuoka H, et al. A review of 227 cases of small papillary thyroid carcinoma. Eur J Surg Oncol. 2007;33(3):370–375.
4. Shindo M, Wu JC, Park EE, Tanzella F. The importance of central compartment elective lymph node excision in the staging and treatment of papillary thyroid cancer. Arch Otolaryngol Head Neck Surg. 2006;132(6):650–654. doi:10.1001/archotol.132.6.650
5. Cooper D, Doherty G, Haugen B, et al. Revised American thyroid association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19(11):1167–1214. doi:10.1089/thy.2009.0110
6. Agcaoglu O, Sengun B, Ozoran E, et al. Should we perform routine prophylactic central neck dissection in patients with thyroid papillary microcarcinoma? Ann Ital Chir. 2018;89(undefined):485–488.
7. Sun QH, Zhang L, Yang JB, et al. [Related factors analysis for lymph node metastasis in papillary thyroid carcinoma: a series of 2 073 patients]. Zhonghua Wai Ke Za Zhi. 2017;55(8):592–598. doi:10.3760/cma.j.issn.0529-5815.2017.08.008
8. Sun W, Lan X, Zhang H, et al. Risk factors for central lymph node metastasis in CN0 papillary thyroid carcinoma: a systematic review and meta-analysis. PLoS One. 2015;10(10):e0139021. doi:10.1371/journal.pone.0139021
9. Xing M, Alzahrani AS, Carson KA, et al. Association between BRAF V600E mutation and recurrence of papillary thyroid cancer. J Clin Oncol. 2014;33(1):42–50. doi:10.1200/JCO.2014.56.8253
10. Moon HJ, Kwak JY, Kim EK, et al. The role of BRAFV600E mutation and ultrasonography for the surgical management of a thyroid nodule suspicious for papillary thyroid carcinoma on cytology. Ann Surg Oncol. 2009;16(11):3125–3131. doi:10.1245/s10434-009-0644-9
11. Kwak JY, Kim EK, Chung WY, Moon HJ, Kim MJ, Choi JR. Association of BRAFV600E mutation with poor clinical prognostic factors and US features in Korean patients with papillary thyroid microcarcinoma. Radiology. 2009;253(3):854–860. doi:10.1148/radiol.2533090471
12. Zheng X, Wei S, Han Y, et al. Papillary microcarcinoma of the thyroid: clinical characteristics and BRAF(V600E) mutational status of 977 cases. Ann Surg Oncol. 2013;20(7):2266. doi:10.1245/s10434-012-2851-z
13. Liu D, Liu Z, Condouris S, Xing M. BRAF V600E maintains proliferation, transformation, and tumorigenicity of BRAF-mutant papillary thyroid cancer cells. J Clin Endocrinol Metab. 2007;92(6):2264–2271. doi:10.1210/jc.2006-1613
14. Kim TY, Kim WB, Song JY, et al. The BRAF mutation is not associated with poor prognostic factors in Korean patients with conventional papillary thyroid microcarcinoma. Clin Endocrinol (Oxf). 2010;63(5):588–593. doi:10.1111/j.1365-2265.2005.02389.x
15. Yang Y, Chen C, Chen Z, et al. Prediction of central compartment lymph node metastasis in papillary thyroid microcarcinoma. Clin Endocrinol (Oxf). 2014;81(2):282–288. doi:10.1111/cen.12417
16. Fukushima T, Suzuki S, Mashiko M, et al. BRAF mutations in papillary carcinomas of the thyroid. Oncogene. 2003;22(41):6455–6457. doi:10.1038/sj.onc.1206739
17. Xiang Y, Lin K, Dong S, Qiao LI, Qiuxiang HE, Zhang X. Prediction of central lymph node metastasis in 392 patients with cervical lymph node-negative papillary thyroid carcinoma in Eastern China. Oncol Lett. 2015;10(4):2559–2564. doi:10.3892/ol.2015.3544
18. Kai Q, Lin T, Shan J. Risk Factors for central compartment lymph nodemetastasis in papillary thyriod microcarcinoma. Med J Wuhan Univ. 2015;36(04):558–561. doi:10.14188/j.1671-8852.2015.04.014
19. Zhou Y, Gao E, Zhang W, et al. Factors predictive of papillary thyroid micro-carcinoma with bilateral involvement and central lymph node metastasis: a retrospective study. World J Surg Oncol. 2012;10:67. doi:10.1186/1477-7819-10-198
20. Machens A, Holzhausen HJ, Dralle H. The prognostic value of primary tumor size in papillary and follicular thyroid carcinoma. Cancer. 2005;103(11):2269–2273. doi:10.1002/cncr.21055
21. Rossi R, Trasforini G, Bertelli F, et al. Clinical and histological characteristics of papillary thyroid microcarcinoma: results of a retrospective study in 243 patients. J Clin Endocrinol Metab. 2006;91(6):2171–2178. doi:10.1210/jc.2005-2372
22. Zhang L, Liu Z, Liu Y, Gao W, Zheng C. Risk factors for nodal metastasis in cN0 papillary thyroid microcarcinoma. Asian Pac J Cancer Prev. 2015;16(8):3361–3363. doi:10.7314/apjcp.2015.16.8.3361
23. Gao Y, Qu N, Zhang L, Chen JY, Ji QH. Preoperative ultrasonography and serum thyroid-stimulating hormone on predicting central lymph node metastasis in thyroid nodules as or suspicious for papillary thyroid microcarcinoma. Tumour Bio. 2015;37(6):7453–7459. doi:10.1007/s13277-015-4535-3
24. Kim KE, Kim EK, Yoon JH, Han KH, Moon HJ, Kwak JY. Preoperative prediction of central lymph node metastasis in thyroid papillary microcarcinoma using clinicopathologic and sonographic features. World J Surg. 2013;37(2):385–391. doi:10.1007/s00268-012-1826-3
25. Loh KC, Greenspan FS, Dong F, Miller TR, Yeo PP. Influence of lymphocytic thyroiditis on the prognostic outcome of patients with papillary thyroid carcinoma. J Clin Endocrinol Metab. 1999;84(2):458–463. doi:10.1210/jcem.84.2.5443
26. Choi SY, Park H, Kang MK, et al. The relationship between the BRAF(V600E) mutation in papillary thyroid microcarcinoma and clinicopathologic factors. World J Surg Oncol. 2013;11(undefined):291. doi:10.1186/1477-7819-11-291
27. Abubaker J, Jehan Z, Bavi P, et al. Clinicopathological analysis of papillary thyroid cancer with PIK3CA alterations in a Middle Eastern population. J Clin Endocrinol Metab. 2008;93(2):611–618. doi:10.1210/jc.2007-1717
28. Park AY, Son EJ, Kim JA, et al. Associations of the BRAF(V600E) mutation with sonographic features and clinicopathologic characteristics in a large population with conventional papillary thyroid carcinoma. PLoS One. 2014;9(10):e110868. doi:10.1371/journal.pone.0110868
29. Kim SK, Park I, Woo JW, et al. Predicting factors for bilaterality in papillary thyroid carcinoma with tumor size <4 cm. Thyroid. 2017;27:2.
30. Virk RK, Dyke ALV, Finkelstein A, et al. BRAFV600E mutation in papillary thyroid microcarcinoma: a genotype|[ndash]|phenotype correlation. Mod Pathol. 2013;26(1):62. doi:10.1038/modpathol.2012.152
31. Li M, Zhu XY, Lv J, et al. Risk factors for predicting centrallymph node metastasis in papillary thyroid microcarcinoma (CN0):a study of 273 resections. Eur Rev Med Pharmacol Sci. 2017;21(17):3801–3807.
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
