Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 18
A Long-Term Study of Adverse Outcomes Associated With Oral Corticosteroid Use in COPD
Authors Tse G, Emmanuel B , Ariti C , Bafadhel M, Papi A , Carter V, Zhou J , Skinner D , Xu X, Müllerová H , Price D
Received 1 August 2023
Accepted for publication 1 November 2023
Published 15 November 2023 Volume 2023:18 Pages 2565—2580
DOI https://doi.org/10.2147/COPD.S433326
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jill Ohar
Gary Tse,1,2 Benjamin Emmanuel,3 Cono Ariti,1 Mona Bafadhel,4 Alberto Papi,5 Victoria Carter,1 Jiandong Zhou,1 Derek Skinner,1 Xiao Xu,3 Hana Müllerová,6 David Price1,7
1Observational and Pragmatic Research Institute, Singapore, Singapore; 2School of Nursing and Health Studies, Hong Kong Metropolitan University, Hong Kong, People’s Republic of China; 3AstraZeneca, Gaithersburg, MD, USA; 4Department of Immunobiology, School of Immunology and Microbial Sciences, Faculty of Life Sciences and Medicine, King’s College London, London, UK; 5Respiratory Medicine, Department of Translational Medicine, University of Ferrara, Ferrara, Italy; 6AstraZeneca, Cambridge, UK; 7Centre of Academic Primary Care, Division of Applied Health Sciences, University of Aberdeen, Aberdeen, UK
Correspondence: David Price, Observational and Pragmatic Research Institute, 22 Sin Ming Lane, #06-76, Midview City, 573969, Singapore, Tel +65 3105 1489, Email [email protected]
Background: Oral corticosteroids (OCS) are often prescribed for chronic obstructive pulmonary disease (COPD) exacerbations.
Methods: This observational, individually matched historical cohort study used electronic medical records (1987– 2019) from the UK Clinical Practice Research Datalink linked to English Hospital Episode Statistics (HES) to evaluate adverse outcomes in patients with COPD who used OCS (OCS cohort) and those not exposed to OCS (non-OCS cohort). Risk of 17 adverse outcomes was estimated using proportional hazard regression.
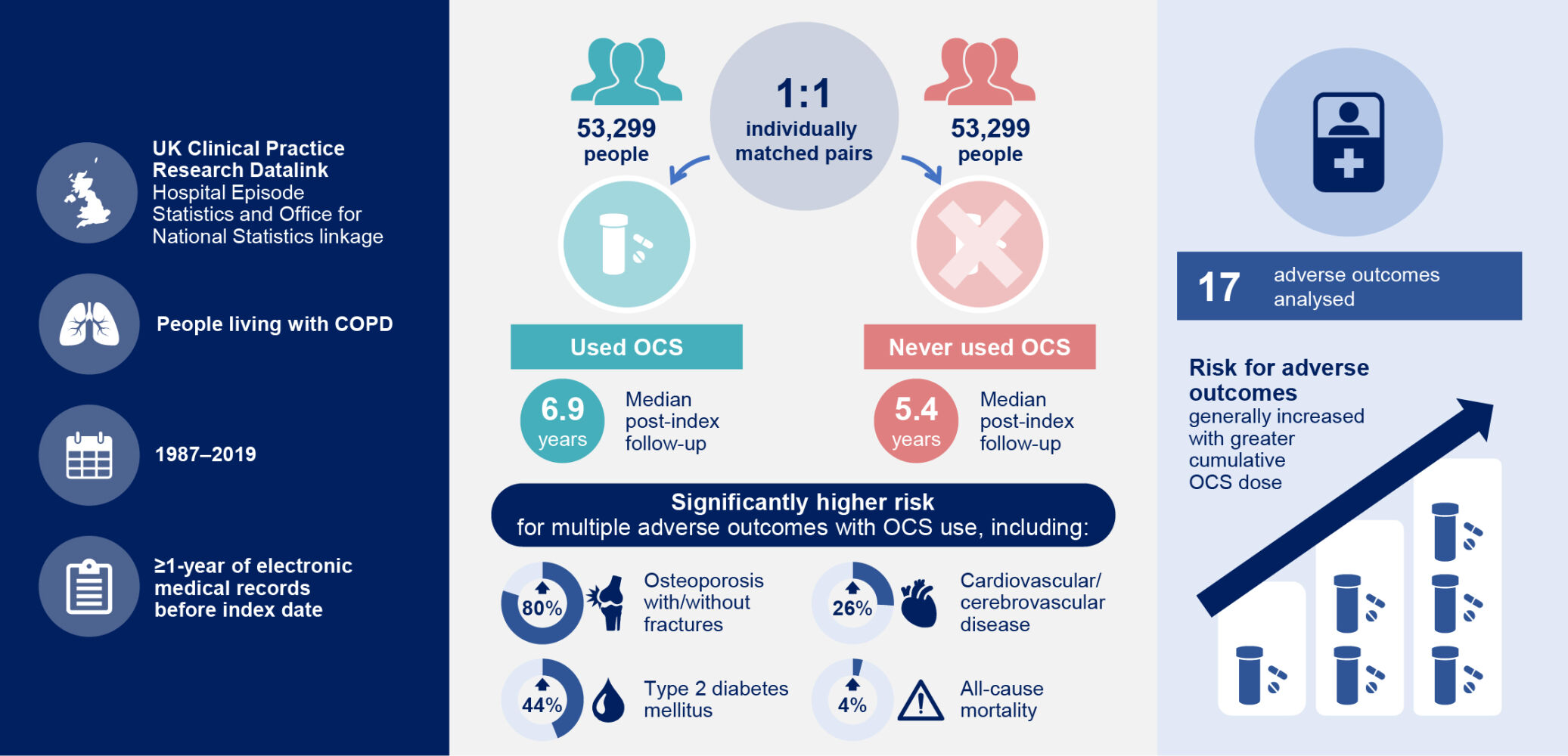
Results: Of 323,722 patients, 106,775 (33.0%) had COPD-related OCS prescriptions. Of the 106,775 patients in the overall cohort, 58,955 had HES linkage and were eligible for inclusion in the OCS cohort. The individual matching process identified 53,299 pairs of patients to form the OCS and non-OCS cohorts. Median follow-up post-index was 6.9 years (OCS cohort) and 5.4 years (non-OCS cohort). Adjusted risk of multiple adverse outcomes was higher for the OCS cohort versus the non-OCS cohort, including osteoporosis with/without fractures (adjusted hazard ratio [aHR] 1.80; 95% confidence interval [CI] 1.70– 1.92), type 2 diabetes mellitus (aHR 1.44; 95% CI 1.37– 1.51), cardiovascular/cerebrovascular disease (aHR 1.26; 95% CI 1.21– 1.30), and all-cause mortality (aHR 1.04; 95% CI 1.02– 1.07). In the OCS cohort, risk of most adverse outcomes increased with increasing categorized cumulative OCS dose. For example, risk of cardiovascular/cerebrovascular disease was 34% higher in the 1.0–< 2.5 g group versus the < 0.5 g group (HR 1.34; 95% CI 1.26– 1.42).
Conclusion: Any OCS use was associated with higher risk of adverse outcomes in patients with COPD, with risk generally increasing with greater cumulative OCS dose.
Plain Language Summary: Many patients with chronic obstructive pulmonary disease (COPD) have occasions when their symptoms suddenly worsen, called flare-ups or exacerbations. To treat flare-ups, doctors might prescribe a course of steroid tablets (oral corticosteroids or OCS for short). Doctors might also prescribe “rescue packs” containing OCS and antibiotics, to keep at home and start taking when needed.
While OCS may speed up recovery from flare-ups, repeated use may have negative health effects. We studied effects of OCS use in patients with COPD, using anonymized electronic patient medical records in England. These databases are made available following a high-quality research proposal to research and ethics committees.
Of 323,722 patients with COPD, around one-third received OCS for flare-ups. We studied 17 outcomes including important medical diagnoses and death. We grouped patients into 53,299 pairs so that every patient who used OCS matched a similar patient (eg, the same age and sex) who never used OCS. The patients were followed for an average of 6.9 years (used OCS) and 5.4 years (never used OCS).
Most diagnoses, including diabetes, osteoporosis, cardiovascular/cerebrovascular disease, and death, were more likely in patients who used OCS than those who never used OCS. Patients using larger amounts of OCS over time were generally more likely to experience diagnoses or die.
These results show risks of using OCS, even occasionally, in patients with COPD. Flare-up prevention is important, for example with appropriate daily “maintenance” medication, vaccinations for infections, and quitting smoking, thereby reducing health effects from OCS use for flare-ups.
Keywords: chronic obstructive pulmonary disease, cohort study, COPD, corticosteroids, observational, primary care
Graphical Abstract:
Introduction
Systemic (oral or parenteral) corticosteroids are increasingly used by patients with chronic obstructive pulmonary disease (COPD). Findings presented at the 2021 British Thoracic Society Winter Meeting1 demonstrated that COPD was the second-leading contributor to total systemic corticosteroid dose among 27 conditions of interest in the UK, and systemic corticosteroid prescriptions in patients with COPD increased from 5.8% in 1990 to 34.8% in 2017. In 2020, a UK study reported that 44% of patients with COPD were prescribed oral corticosteroids (OCS).2 A patient’s COPD management plan may include a prescription of “rescue packs” of OCS and/or antibiotics to keep at home to self-administer if they begin to have an exacerbation.3,4 In patients experiencing exacerbations, OCS may reduce hospital admissions,5 shorten recovery time,6 and improve lung function,5–7 but there is not sufficient evidence to suggest an effect on mortality.5 Benefits of OCS have been reported to vary by blood eosinophil count, with higher blood eosinophil count predicting greater treatment response.8
However, OCS have been associated with short- and long-term adverse outcomes when prescribed for various conditions, including upper respiratory tract infections, spinal conditions, allergies, and asthma.9–12 In patients with asthma, OCS exposure has been associated with increased risk of cataracts, gastrointestinal ulcers/bleeds, hypertension, obesity, osteoporosis and fractures, and type 2 diabetes,10 and a dose–response relationship with OCS exposure has been described for many of these adverse outcomes.12,13 Given the increasing and common intermittent use of OCS in patients with COPD, it is important to understand potential long-term risks and dose-dependent relationships for different potentially OCS-related adverse outcomes.
In contrast to asthma and other chronic conditions, the potential short- and long-term risks associated with OCS use are not as well understood in patients with COPD.14 Adverse outcomes associated with short-term OCS use for COPD exacerbations have been described in several studies7,15,16 including weight gain and insomnia.7 Long-term OCS use is also an independent predictor of all-cause mortality in patients with COPD.17,18 A 4-year retrospective US claims analysis of patients newly diagnosed with COPD found that, compared with patients without OCS exposure, patients exposed to >1 g of OCS (prednisolone equivalent) in the 4 years after their COPD diagnosis experienced greater rates of cardiovascular disease, heart failure, hypertension, obesity, dyspepsia, infections, and depression/anxiety.14 However, the fragmented nature of health-care data in the United States and the relatively short-term duration of follow-up (average 37 months) complicated examination of long-term outcomes.14
An analysis of intermittent OCS use over a long-term follow-up period using robust, comprehensive real-world data is necessary to provide an extensive assessment of potential health risks of OCS in patients with COPD.14 Therefore, this study evaluated associations between COPD-related OCS exposure and adverse outcomes, including all-cause mortality, in a large English population using one of the largest databases of longitudinal medical records from primary care in the world.19 This is the first large-scale, long-term analysis of this kind in patients with COPD.
Methods
Study Design and Data Sources
This observational, individually matched historical cohort study of patients with a COPD diagnosis compared those who were exposed to OCS (the OCS cohort) with those who were never known to be exposed to any OCS (the non-OCS cohort) (Figure 1). Index date for patients in the OCS cohort was the date of their first recorded COPD-related OCS prescription.
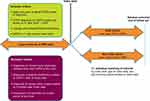 |
Figure 1 Study design. Data included in this analysis spanned from 1987 to 2019. *Date that primary care practitioners entered into a government contract providing additional payments for high-quality COPD care to aid with the diagnostic Quality and Outcomes Framework.20 †OCS cohort: date of first COPD-related OCS prescription; non-OCS cohort, nearest primary care visit to the matched OCS patient index date. ‡Each patient was followed from index date until the first occurrence of an adverse outcome of interest or the end of the patient’s available records (reasons for the last record included death, leaving the primary care practice, or last data extracted). §Index date and sex matching criteria were used per similar studies of systemic (oral or parenteral) corticosteroid use in patients with asthma;12,21 as a COPD study, age and smoking status criteria were included to fully ensure similar covariate distribution. Abbreviations: COPD, chronic obstructive pulmonary disease; EMR, electronic medical record; OCS, oral corticosteroid(s). |
To ensure characteristics between the cohorts were balanced, and to minimize bias due to confounding, patients in the non-OCS cohort were individually matched (1:1) to patients in the OCS cohort based on index date, age at index date, sex, and smoking status closest to index date. Index date and sex matching criteria were used per similar studies of systemic (oral or parenteral) corticosteroid use in patients with asthma;12,21 as a COPD study, age and smoking status criteria were included. Due to the nature of UK primary care data and incentives related to the UK Quality and Outcomes Framework (QOF),20 missingness is extremely rare for these variables. Index dates and patients in the non-OCS cohort were selected from a pool of available primary care consultation dates at random to be closest to the index date for the OCS cohort. Patients could only contribute once as a control in the non-OCS cohort.
Data sources were anonymized, longitudinal primary care practice electronic medical records from the UK Clinical Practice Research Datalink (CPRD) GOLD database linked to hospital-admitted patient care (Hospital Episode Statistics [HES]) and mortality statistics from the UK Office for National Statistics (ONS). Data included in this analysis spanned from 1987 to 2019. The CPRD includes routinely collected data from a large number of patients from UK primary care practices, providing a longitudinal, representative UK population health dataset,19 with 18 million patients registered as of 2023.22 In addition to containing information on diagnoses, symptoms, tests, and prescriptions, the CPRD also contains key lifestyle data such as smoking status.19 A patient’s COPD is principally managed through primary care and by one practice, and the majority of prescribing outside of hospital-treated events is in primary care. A subset of CPRD patients (those from England) has linkage to HES, which records complete and detailed information on inpatient hospital admissions.23 CPRD records are also linked to the UK ONS Mortality registry, which records all mortality data registered by age, sex, and selected underlying cause of death.24
The study population consisted of patients who had a diagnostic code for COPD or had a record of COPD monitoring on or after 1 April 2003, and who were registered at primary care practices in the UK that provide data to the CPRD. If patients had a first diagnostic code before 1 April 2003 but the diagnosis of COPD was reaffirmed after this date, they were also included. This date was chosen because at that date, primary care practitioners entered into a contract with the UK government that provided additional payments for high-quality care for patients with COPD, including use of post-bronchodilator spirometry to aid with diagnoses (the Quality and Outcomes Framework).20 Eligible patients were aged ≥40 years at latest COPD review or diagnosis, had HES linkage (applicable to patients included in the matched OCS and non-OCS cohorts), and electronic medical record data for ≥1 year before index date. Patients were excluded if the records contained a diagnosis of chronic lower respiratory disease other than COPD at any time, adrenal insufficiency before or within 1 year of index date, or cancer within 5 years before or 3 months after index date. Patients were also excluded if they had been prescribed tamoxifen for breast cancer at any time.
The sample size and power calculation are described in the Supplementary Material.
The study protocol was approved by the CPRD Independent Scientific Advisory Committee (reference number 20_159R), and the study was registered with the European Network of Centres for Pharmacoepidemiology and Pharmacovigilance (ENCePP; EUPAS35975). No patient-identifying information was accessible during the study.
Study Variables and Outcomes
For the OCS cohort, a first-recorded COPD-related OCS prescription was defined as an oral prednisolone prescription in patients with a contemporary diagnostic code for COPD, a broad acute respiratory code (including chest infection, cough, wheezing, or breathlessness), or a prescription for antibiotics on the same day as the oral prednisolone prescription. After a patient’s first COPD-related OCS prescription, all-cause OCS use was captured. OCS prescriptions outside of primary care were not captured in the database.
Cumulative OCS dose (grams) was estimated as the total OCS dose prescribed to a patient from index date to the occurrence of the adverse outcome of interest. The number of acute courses of OCS was calculated based on a single course being 30 mg daily for 5 to 10 days (0.15–0.30 g).
Pre-specified corticosteroid-related adverse outcomes were identified using varying approaches based on the persistence of the diagnoses or conditions, as summarized in Table 1. Separate, subsequent, risk cohorts were used for each adverse outcome assessment, where diagnoses or conditions were only included for patients with no prior history of the specific adverse outcome before index date. For example, a patient with a diagnosis of diabetes prior to index date was excluded from the risk cohort for analyzing risk of onset of diabetes. Each patient was followed from index date until the first occurrence of an adverse outcome of interest or the end of the patient’s available records (reasons for the last record included death, leaving the primary care practice, or last data extracted).
 |
Table 1 Adverse Outcomes Evaluated After Index Date and Exclusion Criteria Used for Each Specific Outcome (Risk) Cohort |
Statistical Analysis
Descriptive statistics for pre-index characteristics are reported for the matched cohorts. The quality of matching was evaluated using the standardized mean difference,25 with values >0.2 considered to indicate relevant covariate imbalance. There was no imputation for missing data.
The incidence rate for each adverse outcome in the OCS and non-OCS cohorts (patients with events per 100 patient-years [PY] of follow-up) was calculated and then compared using the incidence rate difference and the incidence rate ratio (IRR) with 95% confidence intervals (CIs).
Risk of adverse outcomes was compared for the OCS and non-OCS cohorts using univariable and multivariable Cox proportional hazard regressions. The multivariable analyses of all adverse outcomes were adjusted for the following confounders estimated at or prior to index date: sex, age, type of inhaler use in the 12 months before index date (an inhaled corticosteroid [ICS]; an ICS and a long-acting β2-agonist [LABA]; an ICS, a LABA, and a long-acting muscarinic antagonist [LAMA]; and a short-acting β2-agonist [SABA] with or without a short-acting muscarinic antagonist [SAMA]), and number of exacerbations in the 12 months before index date. Data are presented using unadjusted HRs or adjusted hazard ratios (aHRs) and 95% CIs.
Within the OCS cohort, further analyses were conducted to examine risk of adverse outcome occurrence with increasing cumulative OCS dose. Risk of type 2 diabetes mellitus worsening, osteoporosis worsening, and pneumonia recurrence (as defined in Supplementary Table 1) were also analyzed by increasing cumulative OCS dose. Univariable Cox proportional hazard regression was used to compare risk of adverse outcomes across pre-specified cumulative dose categories (<0.5 g [reference dose] vs 0.5–<1.0 g, 1.0–<2.5 g, 2.5–<5.0 g, 5.0–<10.0 g, and ≥10.0 g). This analysis treated cumulative dose as a time-varying measure to account for the fact that patients with longer follow-up time accumulate more OCS exposure; this analysis allows for different hazard ratios to be generated over time in patients with comparable follow-up periods. Data are presented using HRs and 95% CIs.
Statistical analyses were conducted using Stata SE version 16 (StataCorp, College Station, TX), Python version 3.9.0 (Python Software Foundation), or RStudio Version 1.4.1717 (R Core Team, 2021).
Results
Patients
Of 323,722 patients with a diagnosis of COPD in the UK CPRD GOLD database (Supplementary Figure 1), 106,775 (33.0%) had ≥1 COPD-related OCS prescription and were included in the overall cohort. The median (interquartile range [IQR]) number of acute OCS courses was eight (3–24); 36.7% of patients (39,159/106,775) had ≤4 courses, 17.9% (19,107/106,775) had 5–9 courses, and 45.4% (48,509/106,755) had ≥10 courses. Longer-term OCS use (≥30-day continuous exposure) was reported in 8.1% (8637/106,755) of patients receiving OCS ever.
Of 106,775 patients in the overall cohort, 58,955 had HES linkage and were eligible for inclusion in the OCS cohort. The individual matching process identified 53,299 pairs of patients forming the OCS cohort and the non-OCS cohort (Supplementary Figure 1 and Table 2). Median (IQR) duration of data availability before index date was 15.0 (6.5–28.7) years (OCS cohort) and 12.2 (4.5–25.4) years (non-OCS cohort) and median (IQR) follow-up duration after index date was 6.9 (3.0–12.1) years (OCS cohort) and 5.4 (1.8–10.6) years (non-OCS cohort). Table 2 reports demographic and clinical characteristics. In the OCS cohort, the mean (SD) cumulative OCS dose was 3.4 (7.1) g and the median (IQR) cumulative OCS dose was 1.1 (0.4–3.3) g.
 |
Table 2 Demographic and Clinical Characteristics of the Matched Treatment Cohorts |
Risk of Adverse Outcomes with OCS Use
In the unadjusted analysis of the matched cohorts, incidence rates (Supplementary Table 2) and IRRs (Supplementary Figure 2) were higher for all adverse outcomes except psychosis in the OCS cohort versus the non-OCS cohort.
Adjusted risk was numerically higher for all adverse outcomes and significantly higher for all except psychosis and hospitalized infections in the OCS cohort versus the non-OCS cohort (Figure 2). Of particular clinical importance, adjusted risk in the OCS cohort versus the non-OCS cohort was 190% greater for pneumonia (aHR 2.90 [95% CI 2.77–3.03]), 80% greater for osteoporosis with/without fractures (aHR 1.80 [95% CI 1.70–1.92]), 44% greater for type 2 diabetes mellitus (aHR 1.44 [95% CI 1.37–1.51]), and 26% greater for cardiovascular/cerebrovascular disease (aHR 1.26 [95% CI 1.21–1.30]).
Cumulative OCS Dose and Adverse Outcome Risk in the OCS Cohort
Positive dose–response associations were observed for risk of most adverse outcomes by categorized, cumulative OCS dose from as low as 0.5–<1.0 g and with more significant associations with doses ≥1.0 g (Figure 3). Risk of osteoporosis with/without fractures was 45% higher for the 0.5–<1.0 g dose category versus the <0.5 g reference group (HR 1.45 [95% CI 1.30–1.62]) and increased to 89% higher for the 1.0–<2.5 g dose category versus the <0.5 g reference group (HR 1.89 [95% CI 1.70–2.11]). Similar dose–response associations were seen for sleep apnea, pneumonia, type 2 diabetes mellitus, weight gain, glaucoma, hospitalized infections, anxiety/depression, cataract, sleep disorders, cardiovascular/cerebrovascular disease, peptic ulcer, and chronic kidney disease.
Additionally, positive dose–response associations were observed for risk of pneumonia recurrence and type 2 diabetes mellitus worsening; a positive dose–response trend was observed for osteoporosis worsening, but the 95% CIs were wide and overlapping (Figure 4).
Risk of All-Cause Mortality
Unadjusted incidence rates for all-cause mortality were 3.32 and 2.88 per 100 PY in the matched OCS and non-OCS cohorts, respectively (Supplementary Table 2). Adjusted all-cause mortality risk was 4% higher in the OCS cohort versus the non-OCS cohort (aHR 1.04 [95% CI 1.02–1.07]; Figure 2). All-cause mortality risk increased as cumulative OCS dose increased (Figure 3). In the 0.5–<1.0 g dose category, all-cause mortality risk was 74% higher versus the <0.5 g reference group (HR 1.74 [95% CI 1.65–1.83]) and increased to 145% higher in the 1.0–<2.5 g dose category (HR 2.45 [95% CI 2.33–2.58]; Figure 3).
Discussion
This was the first long-term comprehensive analysis of adverse outcomes associated with OCS use in patients with COPD. Patients with COPD exposed to any OCS experienced a significantly higher risk for onset of multiple pre-specified adverse outcomes versus patients without any OCS exposure. Risk of most adverse outcomes increased with increasing cumulative OCS dose. At cumulative OCS doses of ≥1.0 g, significant increases in risk were seen for 14 of the 17 assessed adverse outcomes and two of the three assessed worsening or recurrence of adverse outcomes. These included clinically important adverse outcomes such as pneumonia and recurrence of the condition, osteoporosis with/without fractures, cardiovascular/cerebrovascular disease, type 2 diabetes mellitus and worsening of the condition, and all-cause mortality. Importantly, even relatively low OCS doses (0.5–<1.0 g) were associated with higher risk of many of the adverse outcomes versus the <0.5 g OCS reference group.
To place these findings in context, the recommended OCS dose for COPD exacerbations in the UK is 30 mg daily for 5 days (equivalent to 0.15 g),26 although this dose may be taken for up to 14 days.27 In this study, the median number of OCS courses in the overall cohort was eight (equivalent to 1.2 g), with more than 60% of patients having ≥5 OCS courses. As the cumulative dose analysis findings indicate as few as four OCS courses within current UK recommendations (equivalent to 0.6 g) increase adverse outcome risk, our findings suggest a large proportion of patients with COPD who use OCS for exacerbations are exposed to doses that could substantially increase their risk of adverse outcomes.
These findings on the long-term risks and dose-dependent relationships of OCS in COPD are important considering OCS are commonly and increasingly prescribed.1,2 Of particular importance are OCS-containing rescue packs, which patients keep at home to self-administer if they begin to experience an exacerbation.4 Physicians must identify whether rescue packs are suitable for a patient, including assessment of the patient’s risk for OCS-related comorbidities, and patients should be educated on appropriate rescue pack usage and potential long-term risks of OCS.4 Physicians should also consider OCS-sparing strategies, as the triggers and mechanisms of exacerbations are heterogeneous and there is evidence to suggest that not all exacerbations require, nor are responsive to, OCS.28 Additional proposed actions to minimize long-term risks of OCS rescue use include clinical review of a patient after a course of OCS rescue medication and specialist referral after a threshold number of OCS rescue courses is reached. Exacerbation risk must be carefully managed in order to stabilize and control COPD, which will thereby reduce exposure to OCS. In addition to managing a patient’s COPD with appropriate maintenance medication, other key factors to minimize risk of exacerbations include smoking cessation and vaccinations to prevent respiratory infections.26,29,30
COPD is associated with an increased risk of cardiovascular disease and cardiovascular-related mortality.31,32 Use of appropriate and effective maintenance therapy for COPD should reduce the frequency of exacerbations,6 thereby reducing the need to use OCS that could further increase overall cardiovascular disease risk.33 The ETHOS and IMPACT studies both reported reduced exacerbation rates34,35 and fewer deaths due to cardiovascular causes35,36 in patients with COPD receiving fixed-dose triple ICS/LAMA/LABA therapy versus LAMA/LABA dual therapy. It is plausible that the reduced risk of cardiovascular death observed with ICS/LAMA/LABA therapy versus LAMA/LABA therapy in ETHOS and IMPACT could, at least in part, be related to a reduced need for OCS to treat exacerbations. Indeed, findings of the current study indicate that risk of cardiovascular/cerebrovascular disease in patients with COPD could be significantly reduced through OCS dose-sparing strategies. For example, in patients with a cumulative OCS dose of <0.5 g, risk of cardiovascular/cerebrovascular disease was 34% lower versus patients with a cumulative OCS dose of 1.0–<2.5 g and 93% lower versus patients with a cumulative OCS dose of ≥10 g.
The findings of this study are generally consistent with the increased risk for adverse outcomes reported in prior studies in patients with asthma.12,13 In patients who initiated systemic corticosteroids, dose–response relationships for most adverse outcomes were observed to start at cumulative corticosteroid doses as low as 1.0–<2.5 g.12 In patients with intermittent OCS use, dose–response relationships were observed to begin from cumulative doses of 0.5–<1.0 g for almost all adverse outcomes.13
Similarly, a Cochrane database review reported evidence for increased likelihood of hyperglycemia, weight gain, and insomnia with systemic (oral or parenteral) corticosteroid use for COPD exacerbations.15 However, the studies included in the review only assessed short-term effects (10 days to 6 months) in patients who participated in randomized clinical trials.7,16,37–39 Additionally, a retrospective US claims analysis found that patients treated with >1 g of prednisolone-equivalent OCS had a higher risk of adverse outcomes including cardiovascular disease, heart failure, hypertension, obesity, dyspepsia, infections, and depression/anxiety, compared with patients with no OCS use.14 However, this analysis was limited to patients newly diagnosed with COPD and mean follow-up was 37 months.14 In contrast, a strength of the current study is that it followed patients over a longer duration (median follow-up of 6.9 and 5.4 years in the OCS cohort and non-OCS cohort, respectively), enabling a more in-depth study of the association between OCS exposure and the risk of multiple adverse outcomes.
Further strengths include that the CPRD GOLD database is large and well established, with high-quality long-term electronic medical record data from a broad, representative patient population,19 supporting a generalizable interpretation of the current findings. Additionally, data pertaining to OCS use and disease diagnoses are recorded prospectively and not influenced by recall bias. Finally, a major strength is that, based on availability of data before index date, it was possible to confirm that patients were OCS-naive when entering the observation period, as evidenced by median pre-index data availability of almost 16 years in the OCS cohort.
Study limitations include that the datasets represent information collected for clinical and routine use rather than for research. OCS exposure was estimated based on the number of prescriptions over time, and correct administration of all OCS doses as prescribed is not guaranteed, particularly in the case of OCS in “rescue packs” for a patient to use as-needed at home. Additionally, study patients might have been exposed to OCS earlier than 1987, from a period when prescription records were not available in electronic medical records. OCS prescriptions outside primary care were not captured in the database. However, COPD is typically managed at primary care level in the UK. Whilst the analysis of adverse outcomes in the OCS cohort versus the non-OCS cohort was adjusted for key confounders (sex, age, type of inhaler use, and exacerbation history), data were not available to adjust for other possible confounders such as socioeconomic status, frailty, and primary care practice. Additionally, the disease and its severity are probably the strongest confounders of the relationship between OCS and adverse outcomes. Matching patients based on baseline COPD severity can be difficult since many patients use OCS before their official COPD diagnosis. Based on standardized mean differences, the OCS and non-OCS cohorts were generally well-matched in terms of disease severity (assessed by airflow limitation), with the exception of the proportion of patients with severe airflow limitation. However, as matched pairs were employed and the analysis of adverse outcomes in the OCS cohort versus the non-OCS cohort was adjusted for exacerbation history, the current findings would not be expected to change after accounting for disease severity. Whilst the standardized mean difference for baseline FEV1 predicted was >0.2, indicating a degree of imbalance between cohorts, the imbalance was considered sufficiently minimal that, combined with evidence that FEV1 alone has limited ability to predict exacerbations,40 it was decided to not adjust the analyses for FEV1. Finally, whilst there was some missing data for a number of baseline characteristics, there was minimal missing data for most key characteristics owing to the nature of UK medical record data.
Conclusions
In this large population of patients with COPD followed for a median post-index period of 6.9 years (OCS cohort) and 5.4 years (non-OCS cohort), exposure to even low OCS doses increased the risk of multiple corticosteroid-related adverse outcomes, including all-cause mortality, pneumonia, osteoporosis with/without fractures, cardiovascular/cerebrovascular disease, and type 2 diabetes mellitus. Moreover, risk of most adverse outcomes, including all-cause mortality, increased with increasing cumulative OCS dose. These findings highlight the importance of improving awareness of OCS-related adverse outcomes in patients with COPD, particularly as these patients are often older41 and already at increased risk for comorbidities that overlap with the adverse outcomes in this study.42–45 Furthermore, these findings underscore the need to evaluate individual patients and identify treatments to manage and reduce exacerbation risk, and, hence, reduce exposure to OCS.
Data Sharing Statement
Per the Clinical Practice Research Datalink Independent Scientific Advisory Committee guidance, the data will not be made available for sharing.
Ethical Approval
The study protocol was approved by the CPRD Independent Scientific Advisory Committee (reference number 20_159R), and the study was registered with the European Network of Centres for Pharmacoepidemiology and Pharmacovigilance (ENCePP; EUPAS35975). No patient-identifying information was accessible during the study.
Acknowledgments
The authors thank Hilda de Jong, PhD (the PHARMO Institute, Utrecht, the Netherlands) for her assistance in developing the study protocol. The authors also thank Sharen Lee (Cardiovascular Analytics Group, Hong Kong) for her technical assistance in generating figures for the dose-dependent relationship between OCS dose and adverse outcomes. The authors also thank Colin Bonner, Linda Stotsky, Mario Marinazzo, and Philomena Britto, for their review of the plain language summary as people living with COPD; these individuals were compensated for their time. Medical writing support, under the guidance of the authors, was provided by Sara Cameron, MPhil, and Sarah Piggott, MChem, CMC Connect, a division of IPG Health Medical Communications, funded by AstraZeneca, in accordance with Good Publication Practice (GPP 2022) guidelines.46
Author Contributions
All authors made a significant contribution to the work reported. DP and VC contributed to study conception or design, data acquisition, data analysis, and data interpretation. MB and AP contributed to data interpretation. GT, CA, and JZ contributed to data analysis and data interpretation. DS contributed to data acquisition, data analysis, and data interpretation. XX, HM, and BE contributed to study conception or design and data interpretation. All authors took part in drafting, revising, or critically reviewing the article, gave final approval for the version to be published, have agreed on the journal to which the article has been submitted, and agree to be accountable for all aspects of the work.
Funding
This study was sponsored by AstraZeneca. All authors, including those employed by the funder of the study, were involved in the interpretation of data, and writing or revising the report, had final responsibility for the decision to submit for publication, and take responsibility for the integrity of the data and the accuracy of the data analysis.
Disclosure
GT and CA are former employees of the Observational and Pragmatic Research Institute (OPRI), which was funded by AstraZeneca to conduct this study. VC, JZ, and DS are employees of OPRI, which was funded by AstraZeneca to conduct this study. BE, XX, and HM are employees of AstraZeneca and hold stock and/or stock options in the company. MB has received research grants to her institution from AstraZeneca; honoraria to her institution from AstraZeneca, Chiesi, and GlaxoSmithKline; and is an advisory board member for Albus Health and ProAxsis. AP has received scientific grants to his institution from Agenzia Italiana del Farmaco (AIFA), AstraZeneca, Chiesi, GlaxoSmithKline, and Sanofi; has received consulting fees from AstraZeneca, Avillion, Chiesi, ELPEN Pharmaceuticals, GlaxoSmithKline, Novartis, and Sanofi; has received payment or honoraria for lectures, presentations, speaker bureaus, or educational events from AstraZeneca, Avillion, Chiesi, Edmond Pharma, ELPEN Pharmaceuticals, Moderna, GlaxoSmithKline, IQVIA, Menarini, Mundipharma, Novartis, Sanofi, and Zambon; and is an advisory board member for AstraZeneca, Avillion, Chiesi, ELPEN Pharmaceuticals, GlaxoSmithKline, IQVIA, MSD, Novartis, and Sanofi. DP is an employee of OPRI, which was funded by AstraZeneca to conduct this study; has advisory board membership with AstraZeneca, Boehringer Ingelheim, Chiesi, Mylan, Novartis, Regeneron Pharmaceuticals, Sanofi Genzyme, and Thermo Fisher; has consultancy agreements with Airway Vista Secretariat, AstraZeneca, Boehringer Ingelheim, Chiesi, EPG Communication Holdings Ltd, FIECON, Fieldwork International, GlaxoSmithKline, Mundipharma, Mylan, Novartis, OM Pharma SA, PeerVoice, Phadia AB, Spirosure Inc, Strategic North Limited, Synapse Research Management Partners S.L., Talos Health Solutions, Theravance, and WebMD Global LLC; has received grants and unrestricted funding for investigator-initiated studies (conducted through OPRI) from AstraZeneca, Boehringer Ingelheim, Chiesi, Mylan, Novartis, Regeneron Pharmaceuticals, Respiratory Effectiveness Group, Sanofi Genzyme, Theravance, and UK National Health Service; has received payment for lectures/speaking engagements from AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, GlaxoSmithKline, Kyorin, Mundipharma, Mylan, Novartis, Regeneron Pharmaceuticals, and Sanofi Genzyme; has received payment for travel/accommodation/meeting expenses from AstraZeneca, Boehringer Ingelheim, Mundipharma, Mylan, Novartis, and Thermo Fisher; has stock/stock options from AKL Research and Development Ltd, which produces phytopharmaceuticals; owns 74% of the social enterprise Optimum Patient Care Ltd (Australia and UK) and 92.61% of OPRI (Singapore); has a 5% shareholding in Timestamp, which develops adherence monitoring technology; is peer reviewer for grant committees of the UK Efficacy and Mechanism Evaluation Programme and Health Technology Assessment; and has been an expert witness for GlaxoSmithKline. The authors report no other conflicts of interest in this work.
References
1. Voorham J, Menzies-Gow AN, Tran TN, et al. S29 Longitudinal systemic corticosteroid utilisation for asthma and other diseases in the United Kingdom from 1990 to 2018: a population-based cohort analysis. Thorax. 2021;76(Suppl 1):A21.
2. Price D, Chen S, Kerkhof M, et al. Disease burden for patients with chronic obstructive pulmonary disease receiving maintenance therapy. Am J Resp Crit Care Med. 2020;201:A2553.
3. Asthma and Lung UK. Managing COPD flare-ups; 2022. Available from: https://www.blf.org.uk/support-for-you/copd/flare-ups.
4. Robinson F, Hurst J. The appropriate use of rescue packs. Primary Care Respiratory Update. 2020; (21) :41–43.
5. Wedzicha JA, Miravitlles M, Hurst JR, et al. Management of COPD exacerbations: a European Respiratory Society/American Thoracic Society guideline. Eur Respir J. 2017;49(3):1600791. doi:10.1183/13993003.00791-2016
6. Global Initiative for Chronic Obstructive Lung Disease. GOLD Report. Global strategy for prevention, diagnosis and management of COPD; 2023. Available from: https://goldcopd.org/2023-gold-report-2/.
7. Aaron SD, Vandemheen KL, Hebert P, et al. Outpatient oral prednisone after emergency treatment of chronic obstructive pulmonary disease. N Engl J Med. 2003;348(26):2618–2625. doi:10.1056/NEJMoa023161
8. Kerkhof M, Chaudhry I, Pavord ID, et al. Blood eosinophil count predicts treatment failure and hospital readmission for COPD. ERJ Open Res. 2020;6(4):00188–02020. doi:10.1183/23120541.00188-2020
9. Waljee AK, Rogers MAM, Lin P, et al. Short term use of oral corticosteroids and related harms among adults in the United States: population based cohort study. BMJ. 2017;357:j1415. doi:10.1136/bmj.j1415
10. Sullivan PW, Ghushchyan VH, Globe G, Schatz M. Oral corticosteroid exposure and adverse effects in asthmatic patients. J Allergy Clin Immunol. 2018;141(1):110–116.e117. doi:10.1016/j.jaci.2017.04.009
11. van Staa TP, Leufkens HGM, Abenhaim L, Zhang B, Cooper C. Oral corticosteroids and fracture risk: relationship to daily and cumulative doses. Rheumatology. 2000;39(12):1383–1389. doi:10.1093/rheumatology/39.12.1383
12. Price DB, Trudo F, Voorham J, et al. Adverse outcomes from initiation of systemic corticosteroids for asthma: long-term observational study. J Asthma Allergy. 2018;11:193–204. doi:10.2147/JAA.S176026
13. Heatley H, Tran TN, Bourdin A, et al. Observational UK cohort study to describe intermittent oral corticosteroid prescribing patterns and their association with adverse outcomes in asthma. Thorax. 2022;78(9):860–867. doi:10.1136/thorax-2022-219642
14. Bazell C, Pollack M, Comellas AP, et al. A 4-year retrospective claims analysis of oral corticosteroid use and health conditions in newly diagnosed Medicare FFS patients with COPD. Int J Chron Obstruct Pulmon Dis. 2022;17:2635–2652. doi:10.2147/COPD.S373590
15. Walters JAE, Tan DJ, White CJ, Gibson PG, Wood‐Baker R, Walters EH. Systemic corticosteroids for acute exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2014;(9):CD001288. doi:10.1002/14651858.CD001288.pub4
16. Maltais F, Ostinelli J, Bourbeau J, et al. Comparison of nebulized budesonide and oral prednisolone with placebo in the treatment of acute exacerbations of chronic obstructive pulmonary disease: a randomized controlled trial. Am J Respir Crit Care Med. 2002;165(5):698–703. doi:10.1164/ajrccm.165.5.2109093
17. Schols AMWJ, Wesseling G, Kester ADM, et al. Dose dependent increased mortality risk in COPD patients treated with oral glucocorticoids. Eur Respir J. 2001;17(3):337–342. doi:10.1183/09031936.01.17303370
18. Sivapalan P, Ingebrigtsen TS, Rasmussen DB, et al. COPD exacerbations: the impact of long versus short courses of oral corticosteroids on mortality and pneumonia: nationwide data on 67 000 patients with COPD followed for 12 months. BMJ Open Respir Res. 2019;6(1):e000407. doi:10.1136/bmjresp-2019-000407
19. Herrett E, Gallagher AM, Bhaskaran K, et al. Data resource profile: Clinical Practice Research Datalink (CPRD). Int J Epidemiol. 2015;44(3):827–836. doi:10.1093/ije/dyv098
20. Roland M. Linking physicians’ pay to the quality of care—a major experiment in the United Kingdom. N Engl J Med. 2004;351(14):1448–1454. doi:10.1056/NEJMhpr041294
21. Voorham J, Xu X, Price DB, et al. Healthcare resource utilization and costs associated with incremental systemic corticosteroid exposure in asthma. Allergy. 2019;74(2):273–283. doi:10.1111/all.13556
22. Medicines and Healthcare products Regulatory Agency and the National Institute for Health Research. Clinical Practice Research Datalink; 2023. Available from: http://www.cprd.com/home/.
23. Padmanabhan S, Carty L, Cameron E, Ghosh RE, Williams R, Strongman H. Approach to record linkage of primary care data from Clinical Practice Research Datalink to other health-related patient data: overview and implications. Eur J Epidemiol. 2019;34(1):91–99. doi:10.1007/s10654-018-0442-4
24. Delmestri A, Prieto-Alhambra D. CPRD GOLD and linked ONS mortality records: reconciling guidelines. Int J Med Inform. 2020;136:104038. doi:10.1016/j.ijmedinf.2019.104038
25. Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28(25):3083–3107. doi:10.1002/sim.3697
26. National Institute for Health and Care Excellence. Chronic obstructive pulmonary disease in over 16s: diagnosis and management. NICE guideline [NG115]; 2018. Available from: https://www.nice.org.uk/guidance/ng115.
27. National Institute for Health and Care Excellence. British National Formulary. Prednisolone; 2021. Available from: https://bnf.nice.org.uk/drug/prednisolone.html#indicationsAndDoses.
28. Wedzicha JA. Mechanisms of chronic obstructive pulmonary disease exacerbations. Ann Am Thorac Soc. 2015;2(12 Suppl 2):S157–159. doi:10.1513/AnnalsATS.201507-427AW
29. Hurst JR, Han MK, Singh B, et al. Prognostic risk factors for moderate-to-severe exacerbations in patients with chronic obstructive pulmonary disease: a systematic literature review. Respir Res. 2022;23(1):213. doi:10.1186/s12931-022-02123-5
30. Ji Z, Jareño-Esteban JJ, de Miguel-Díez J. Role of vaccines in COPD patients. Open Respir Arch. 2022;4(3):100191. doi:10.1016/j.opresp.2022.100191
31. André S, Conde B, Fragoso E, Boléo-Tomé JP, Areias V, Cardoso J. COPD and cardiovascular disease. Pulmonology. 2019;25(3):168–176. doi:10.1016/j.pulmoe.2018.09.006
32. Morgan AD, Zakeri R, Quint JK. Defining the relationship between COPD and CVD: what are the implications for clinical practice? Ther Adv Respir Dis. 2018;12:1753465817750524. doi:10.1177/1753465817750524
33. Souverein PC, Berard A, Van Staa TP, et al. Use of oral glucocorticoids and risk of cardiovascular and cerebrovascular disease in a population based case-control study. Heart. 2004;90(8):859–865. doi:10.1136/hrt.2003.020180
34. Rabe KF, Martinez FJ, Ferguson GT, et al. Triple inhaled therapy at two glucocorticoid doses in moderate-to-very-severe COPD. N Engl J Med. 2020;383(1):35–48. doi:10.1056/NEJMoa1916046
35. Lipson DA, Barnhart F, Brealey N, et al. Once-daily single-inhaler triple versus dual therapy in patients with COPD. N Engl J Med. 2018;378(18):1671–1680. doi:10.1056/NEJMoa1713901
36. Martinez FJ, Rabe KF, Ferguson GT, et al. Reduced all-cause mortality in the ETHOS trial of budesonide/glycopyrrolate/formoterol for chronic obstructive pulmonary disease. A randomized, double-blind, multicenter, parallel-group study. Am J Respir Crit Care Med. 2021;203(5):553–564. doi:10.1164/rccm.202006-2618OC
37. Niewoehner DE, Erbland ML, Deupree RH, et al. Effect of systemic glucocorticoids on exacerbations of chronic obstructive pulmonary disease. N Engl J Med. 1999;340(25):1941–1947. doi:10.1056/NEJM199906243402502
38. Alía I, de la Cal MA, Esteban A, et al. Efficacy of corticosteroid therapy in patients with an acute exacerbation of chronic obstructive pulmonary disease receiving ventilatory support. Arch Intern Med. 2011;171(21):1939–1946. doi:10.1001/archinternmed.2011.530
39. Abroug F, Ouanes-Besbes L, Fkih-Hassen M, et al. Prednisone in COPD exacerbation requiring ventilatory support: an open-label randomised evaluation. Eur Respir J. 2014;43(3):717–724. doi:10.1183/09031936.00002913
40. Kerkhof M, Freeman D, Jones R, Chisholm A, Price DB, Respiratory Effectiveness Group. Predicting frequent COPD exacerbations using primary care data. Int J Chron Obstruct Pulmon Dis. 2015;10:2439–2450. doi:10.2147/COPD.S94259
41. Raherison C, Girodet P-O. Epidemiology of COPD. Eur Respir Rev. 2009;18(114):213–221. doi:10.1183/09059180.00003609
42. Coughlan T, Dockery F. Osteoporosis and fracture risk in older people. Clin Med. 2014;14(2):187–191.
43. Crowley K. Sleep and sleep disorders in older adults. Neuropsychol Rev. 2011;21(1):41–53.
44. Rodgers JL, Jones J, Bolleddu SI, et al. Cardiovascular risks associated with gender and aging. J Cardiovasc Dev Dis. 2019;6(2):19. doi:10.3390/jcdd6020019
45. van der Maarel-Wierink CD, Vanobbergen JNO, Bronkhorst EM, Schols JMGA, de Baat C. Risk factors for aspiration pneumonia in frail older people: a systematic literature review. J Am Med Dir Assoc. 2011;12(5):344–354.
46. DeTora LM, Toroser D, Sykes A, et al. Good Publication Practice (GPP) guidelines for company-sponsored biomedical research: 2022 update. Ann Intern Med. 2022;175(9):1298–1304. doi:10.7326/M22-1460
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



