Back to Journals » Stem Cells and Cloning: Advances and Applications » Volume 11
A functional human motor unit platform engineered from human embryonic stem cells and immortalized skeletal myoblasts
Authors Abd Al Samid M , McPhee JS , Saini J , McKay TR, Fitzpatrick LM, Mamchaoui K , Bigot A, Mouly V , Butler-Browne G, Al-Shanti N
Received 28 June 2018
Accepted for publication 31 August 2018
Published 9 November 2018 Volume 2018:11 Pages 85—93
DOI https://doi.org/10.2147/SCCAA.S178562
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Bernard Binetruy
Supplementary video presented by Samid et al.
Views: 215
Marwah Abd Al Samid,1 Jamie S McPhee,2 Jasdeep Saini,1 Tristan R McKay,1 Lorna M Fitzpatrick,1 Kamel Mamchaoui,3 Anne Bigot,3 Vincent Mouly,3 Gillian Butler-Browne,3 Nasser Al-Shanti1
1Healthcare Science Research Institute, School of Healthcare Science, Manchester Metropolitan University, Manchester, UK; 2Department of Sport and Exercise Science, Manchester Metropolitan University, Manchester, UK; 3Center for Research in Myology, Sorbonne Université-INSERM, Paris, France
Background: Although considerable research on neuromuscular junctions (NMJs) has been conducted, the prospect of in vivo NMJ studies is limited and these studies are challenging to implement. Therefore, there is a clear unmet need to develop a feasible, robust, and physiologically relevant in vitro NMJ model.
Objective: We aimed to establish a novel functional human NMJs platform, which is serum and neural complex media/neural growth factor-free, using human immortalized myoblasts and human embryonic stem cells (hESCs)-derived neural progenitor cells (NPCs) that can be used to understand the mechanisms of NMJ development and degeneration.
Methods: Immortalized human myoblasts were co-cultured with hESCs derived committed NPCs. Over the course of the 7 days myoblasts differentiated into myotubes and NPCs differentiated into motor neurons.
Results: Neuronal axon sprouting branched to form multiple NMJ innervation sites along the myotubes and the myotubes showed extensive, spontaneous contractile activity. Choline acetyltransferase and βIII-tubulin immunostaining confirmed that the NPCs had matured into cholinergic motor neurons. Postsynaptic site of NMJs was further characterized by staining dihydropyridine receptors, ryanodine receptors, and acetylcholine receptors by α-bungarotoxin.
Conclusion: We established a functional human motor unit platform for in vitro investigations. Thus, this co-culture system can be used as a novel platform for 1) drug discovery in the treatment of neuromuscular disorders, 2) deciphering vital features of NMJ formation, regulation, maintenance, and repair, and 3) exploring neuromuscular diseases, age-associated degeneration of the NMJ, muscle aging, and diabetic neuropathy and myopathy.
Keywords: motor unit, neuromuscular junctions, human embryonic stem cells, neuronal progenitor cells, human myoblasts
Introduction
Neuromuscular junctions (NMJs) serve as the interface between nerves and skeletal muscles. Maintenance, structure, and formation of NMJs depend on the bidirectional molecular interaction between the muscle and motor neuron.1 The NMJ consists of a presynaptic motor neuron terminal, a postsynaptic motor end plate and a synaptic cleft. If chemical or molecular communication is disrupted, NMJ deterioration can follow. This involves axon degeneration, synapse disruption, impaired NMJ transmission, and muscle fiber degradation2 which are the features of neuromuscular diseases, myopathies, and age-associated neuromuscular impairments.3
Despite decades of intensive research to characterize the structure and function of NMJs by utilizing animals and ex vivo models,4 effective treatment of neuromuscular and neurodegenerative diseases remains a significant unmet clinical need. This is mainly due to the failure of experimental animal models to reflect complex processes of human aging and disease progression.5 In order to advance this field, novel, alternative, experimental models are needed.
There has been recent progress toward the development of in vitro co-culture models using human induced pluripotent stem cells (iPSCs);6,7 mouse,8 rat,9 and human primary myoblasts;10,11 and human embryonic stem cells (hESCs)12–14 and cross-species models.15,16 However, existing in vitro motor neuron and skeletal muscle co-culture systems typically require a complex neural growth medium that contains serum and cocktails of around 15 neural growth factors (some of which are derived from animals).11,12,17 This further complicates drug discovery and toxicology studies due to possible cross-communication of the novel compound with factors contained within the added media, possibly explaining why many promising therapies do not translate to clinics. Another issue with existing models is that muscle contraction is induced by applied electrical or chemical stimulation, which does not replicate the native physiological stimulation required for muscle contractions.8,17–19 Recent innovation in the use of iPSCs offers the potential to derive myoblasts and motor neurons for use with in vitro NMJ models. However, cells derived from iPSCs may exhibit genetic inconsistency and genetic modification, which limit their use.20 Recent human iPSC-based studies have failed to recapitulate the severe neuronal loss observed in human neurodegenerative diseases.21–23 Human skeletal myoblasts which were used in some of the abovementioned models10,11 were obtained from primary cells (eg, muscle biopsy or surgical samples), but their life span is limited to just a few passages which restricts experimentation and necessitates repeated supply of the primary cells.24,25 Furthermore, primary cells have varied cell purity26 and experience phenotypic changes when expanded, rendering primary myoblasts a problematic choice for a consistently reproducible co-culture system.24,25 Therefore, there is a clear need for a more relevant human experimental model to study motor units and NMJs to overcome the limitations of existing models.
Methods
Human immortalized myoblast cultures
The human immortalized myoblasts cell line (“C25”) was obtained from the Institute of Myology.27 This cell line was established using a biopsy of semi-tendinosis from a 25-year-old male (obtained anonymously from Myobank, a tissue bank affiliated to EuroBioBank which is authorized by the French Ministry of Research [authorization AC-2013-1868]). After attaining 80% confluence, cells were seeded in six-well plates recoated with gelatin (0.5%) at a concentration of 1.5×105 cells/mL in growth media. The growth media was supplemented with DMEM from Lonza (Basel, Switzerland), 60% (v/v) Medium 199 with Earle’s Balanced Salt Solution from Lonza, 20% (v/v) heat-inactivated FBS from Thermo Fisher Scientific (Waltham, MA, USA), 20% (v/v) L-glutamine from Lonza, 1% (v/v) fetuin from FBS from Sigma-Aldrich (St Louis, MO, USA) 25 µg/mL, recombinant human basic fibroblast growth factor from Thermo Fisher Scientific 0.5 ng/mL, recombinant human EGF from Thermo Fisher Scientific 5 ng/mL, recombinant human hepatocyte growth factor from Sino Biological Inc. (Beijing, China) 2.5 ng/mL, recombinant human insulin from Sigma-Aldrich 5 µg/mL, dexamethasone from Sigma-Aldrich 0.2 µg/mL and gentamicin from Thermo Fisher Scientific.
Neural differentiation of hESCs
Induction of neuroepithelial clusters (NECs)
Mouse embryonic fibroblasts (MEFs; Cell Biolabs, San Diego, CA, USA) were cultured within MEF growth media (summarized in Table 1) and were passaged at a 1:4 ratio. At passage 4 (p4), MEFs were inactivated mitotically using 0.1 µg/mL mitomycin C (Sigma-Aldrich). The Shef3 hESC line was obtained from the UK StemCell Bank under the project SCSC10-48 and maintained on mitotically inactivated MEFs in hESC medium (summarized in Table 2) in 96-well plates. hESCs were mechanically passaged every 5–7 days and then conditioned to feeder-free culture by TrypLE Express (Thermo Fisher Scientific) enzyme dissociation and plated at a high density (1:1) onto hESC-qualified Matrigel® (Corning)-coated flasks in mTESR (STEMCELL Technologies). For neural induction, feeder-free hESCs were dissociated with TrypLE and replated in neural induction medium (NIM; summarized in Table 3) in uncoated, V-shaped 96-well plates at a density of 1×104 cells/well. Within 24 hours, NECs formed.
  | Table 1 MEF growth media Abbreviation: MEF, mouse embryonic fibroblast. |
  | Table 2 hESC media for cells on MEFs Abbreviations: bFGF, basic fibroblast growth factor; hESC, human embryonic stem cell; MEF, mouse embryonic fibroblast; MEM, Minimum Essential Medium. |
  | Table 3 Neural induction medium Abbreviations: bFGF, basic fibroblast growth factor; MEM, Minimum Essential Medium; NIM, neural induction medium. |
Generation of neural rosette-forming progenitor cells (NRPCs)
Medium was replaced daily for 5 days. On day 6, aggregates were replated in 96-well plates in NIM onto 20 µg/mL laminin (Millipore)-coated dishes to allow neural rosette formation (2–3 days).
Expansion of neural progenitor cells
Neural rosette clusters were mechanically isolated and replated in 96-well plates onto laminin-coated dishes in neural expansion medium (NIM plus 1X B27 supplement; Thermo Fisher Scientific). Early- and late-passage neural progenitor cells (NPCs) were cultured in the same conditions and passaged at a 1:3 ratio using TrypLE. To monitor NPCs differentiation into motor neurons, some of the NPCs were transfected with a GFP-reporter lentivirus system.
Immunohistochemistry of NECs, NRPCs, and NPCs
For immunostaining, at each stage of neural differentiation (NECs and NRPCs), cells were sectioned onto glass slides. Sectioned NECs and NRPCs and cultured NPCs in six-well plates were fixed with 4% paraformaldehyde. Fixed sections and NPCs were permeabilized with 0.3% Triton X in PBS and then subsequently blocked with 2% BSA in PBS/Tween 20. The slides were then incubated overnight at 4°C with a primary antibody (MAB2736; R&D Systems). The following day, sections and NPCs were washed with PBS and incubated for 1 hour at room temperature (RT) with goat anti-mouse secondary antibody (Alexa Flour 568-red for NECs, NRPCs and NPCs; Thermo Fisher Scientific) and imaged under a fluorescent microscope (Leica CTR 6000; Leica Microsystems, Wetzlar, Germany).
Co-culture of NPCs and human myoblasts
Human myoblasts were incubated over 24 hours at 37°C within a 5% CO2 environment in six-well plates. Then, the growth media was replaced with co-culture media supplemented with DMEM (500 mL), 10 µg/mL recombinant human insulin, and 10 µg/mL gentamicin. NPCs were present at a concentration of 25×103 cells/mL and incubated at 37°C with 5% CO2 for up to 7 days. At day 7, myotube contractions were observed.
Immunohistochemistry
At day 7, cells were stained with α-Bungarotoxin (α-BTX) (T0195, 1/200; Sigma-Aldrich), and then fixed with 4% paraformaldehyde for 10 minutes at RT. Co-cultures were washed twice with PBS (Thermo Fisher Scientific). After α-BTX staining, cells were washed twice with PBS and permeabilized with 1X perm/wash buffer (BD, Franklin Lakes, NJ, USA) for 30 minutes at RT. Cells were washed twice with PBS and then blocked with PBS containing 1% BSA and 10% goat serum (Thermo Fisher Scientific) for 1 hour at RT. The following antibodies were used: mouse anti-βIII-tubulin (MAB1195, clone #TuJ1, 1/400; R&D Systems), rabbit anti-ryanodine receptor (anti-RyR; AB9078, 1/200; Millipore), goat anti-choline acetyltransferase (anti-ChAT; ABN100, 1/200; Millipore), goat anti-ChAT (AB144, 1/200; Millipore), and mouse anti-dihydropyridine receptor (anti-DHPR; Ab2864, 1/400; Abcam). These antibodies were incubated overnight at 4°C in 1X perm/wash buffer. Co-cultures were washed with PBS and stained with the corresponding secondary antibodies supplemented with DAPI (1/10,000; Sigma-Aldrich) for 1 hour at RT. Stained co-culture cells were visualized using a Leica SP5 confocal microscope sourced by Leica Microsystems for fluorescent microscopy.
Results
A functional human motor unit platform
The present work describes a functional human motor unit platform established using immortalized skeletal myoblasts and hESCs-derived NPCs that develop into motor neurons in muscle differentiation media (co-culture media) devoid of any complex neural growth factors or serum.
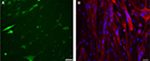
To validate the platform, two monoculture controls were included, one of NPCs and the other of immortalized human myoblasts27 with co-culture media for 7 days. Cultured GFP-transfected NPCs did not show any morphological changes or motor neuron differentiation, but instead they deteriorated and died (Figure 1A). The human myoblasts stained with phalloidin-DAPI showed normal morphology and differentiation features with centrally and peripherally located nuclei, but the myotubes did not spontaneously contract (Figure 1B).
Derivation of NPCs from hESCs and establishment of the NMJs model
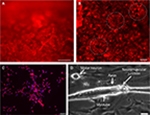
The NPCs were derived from hESCs, as described previously.28 Neural differentiation of hESCs progressed through three stages of differentiation to NECs (Figure 2A) and NRPCs (Figure 2B) which were stained with Nestin red color. To confirm that differentiated NPCs were homogeneous committed neural lineage, cells were stained with DAPI, anti-GFAP (a specific marker for glial cells) and anti-Nestin. Double-positive staining (DAPI blue and Nestin red) confirmed the formation of NPCs while single DAPI staining was not observed, which confirmed that the differentiated cells were NPCs (Figure 2C). Staining of GFAP was not present, confirming again that all of the cells were NPCs (Figure 2C).
The NPCs were co-cultured with human immortalized myoblasts for 7 days in co-culture media. After myogenic differentiation was initiated, the characteristics of functional motor units began to develop. This included myotube formation and axonal sprouting from the NPCs which subsequently formed NMJs along the myotubes (Figure 2D). The myotubes showed spontaneous muscle contractions from approximately day 7 onwards in the absence of any exogenous electrical and chemical stimuli (Video S1). Due to the force of contractions, some myotubes were detached from the culture plate, causing large spaces within the co-culture. Since the myotubes cultured without NPCs did not contract, they did not detach from the culture plate and did not open up large spaces (Figure 1B).
Characterization of co-cultures
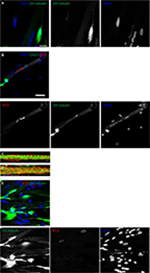
On day 7, the motor neuron formation was assessed using the specific marker for motor neuron differentiation, βIII-tubulin. Figure 3A shows a typical shape of mature motor neurons with axons terminating on myotubes. To further confirm the formation of cholinergic motor neurons, acetyltransferase antibodies (ChAT, a key enzyme for acetylcholine biosynthesis) were used and are shown in green in Figure 3B.
Neurally and aneurally cultured myotubes were characterized by antibody staining against DHPRs and RyRs voltage-gated channels which are located at muscle fiber T-tubules and the sarcoplasmic reticulum, respectively. The DHPR- and RYR-stained images showed transversal triad structures (Figure 3C). These images illustrated mature differentiated myotubes within the co-culture platform with peripherally located nuclei (Figure 3A and B). The aneurally cultured myotubes (control image) differentiated as expected, but did not exhibit appropriate transversal triad structures (Figure 3D).
NMJs display acetylcholine receptor (AChR) clusters along myotubes, and these were assessed by staining for α-BTX, as shown in Figure 3B and E where AChRs are marked by red clusters. As shown in Figure 3A and E, motor neuron axons extend to innervate myotubes and AChR clusters at this same location, marked with α-BTX (red), indicating NMJ formation. Spontaneous myotube contractile activity was determined on day 7. The myotubes co-cultured with motor neurons showed high levels of spontaneous contractile activity (Video S1). Muscle contractions were absent from aneural myoblast cultures.
Discussion
Implications and future uses
This report describes a novel functional human motor unit platform engineered from immortalized skeletal myoblasts and NPCs derived from hESCs. This unique platform was established for investigation of human NMJs and motor unit formation, maintenance, and disease, as well as for drug discovery and toxicity research.
The motor neuron and skeletal muscle co-cultures matured without adding complex cocktails of serum or neural growth factors. NMJ formation was observed and spontaneous myotube contractions were recorded (Video S1) over a period as short as 6–7 days, which is very early compared with the 14 days needed in previous studies16 or where NMJs formed after 20–25 days.29 These are crucial advances for studying the basic aspects of development and for recognizing pathophysiological systems of NMJ disorders associated with disease or aging. It is important that this type of work is carried out with relevant human cultures to increase the possibility for translation to clinical practice. Most previous cell culture model systems used cross-species cell types15,19 and required cocktails of serum30,31 and growth factors.10,17,19,29 These were absent from the present model without any adverse effects, which suggests that nerve and muscle cells release all of the necessary factors needed to stimulate nerve axonal sprouting and formation of NMJs with myotubes. Moreover, eliminating serum, which contains unknown factors that may affect assay reproducibility, simplifies the interpretation of pharmacology and toxicity studies.32 Functional NMJ formation is strongly supported by bidirectional communication1 between nerve and muscle as well as by neural growth factors (such as brain-derived neurotrophic factor, glial cell line-derived neurotrophic factor and neurotrophin-3/4) secreted by muscle to support NMJ formation, maturation and maintenance.33,34 Future investigations using this model will identify key factors released from nerve and muscle to orchestrate axonal sprouting, localization, and NMJ maintenance.
Although animal models can represent essential parts of physiological changes in a human disease,35 in vitro human cell cultures offer many advantages because they are formed of relevant cell types, they can be produced quickly for high-throughput screening, and they are more cost-effective in comparison to animal models.10,17
Conclusion
In summary, human immortalized myoblasts were co-cultured with hESCs-derived NPCs. Over the course of 7 days, myoblasts differentiated into myotubes and NPCs sprouted axons that branched to form multiple NMJ innervation sites along myotubes, and myotubes showed extensive, spontaneous contractile activity. This cell culture platform may be used to study human NMJ growth and disease and may reduce the use of animal models in future related research.
Acknowledgment
This work was sponsored by the School of Healthcare Science, Faculty of Science and Engineering, Manchester Metropolitan University (Manchester, UK).
Author contributions
MAS, JSM, and NAS developed the study concept. All the authors contributed to methodology. MAS, JSM, and NAS carried out the investigation. JSM, TRM, and NAS supervised the study. MAS, JSM, and NAS wrote the original draft of the manuscript. MAS, JSM, VM, and NAS reviewed and edited the draft manuscript. All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Zahavi EE, Ionescu A, Gluska S, Gradus T, Ben-Yaakov K, Perlson E. A compartmentalized microfluidic neuromuscular co-culture system reveals spatial aspects of GDNF functions. J Cell Sci. 2015;128(6):1241–1252. | ||
Gonzalez-Freire M, de Cabo R, Studenski SA, Ferrucci L. The Neuromuscular Junction: Aging at the Crossroad between Nerves and Muscle. Front Aging Neurosci. 2014;6:208. | ||
Zhou C, Wu L, Ni F, Ji W, Wu J, Zhang H. Critical illness polyneuropathy and myopathy: a systematic review. Neural Regen Res. 2014;9(1):101–110. | ||
Wu H, Xiong WC, Mei L. To build a synapse: signaling pathways in neuromuscular junction assembly. Development. 2010;137(7):1017–1033. | ||
van der Worp HB, Howells DW, Sena ES, et al. Can animal models of disease reliably inform human studies? PLoS Med. 2010;7(3):e1000245. | ||
Thomson SR, Wishart TM, Patani R, Chandran S, Gillingwater TH. Using induced pluripotent stem cells (iPSC) to model human neuromuscular connectivity: promise or reality? J Anat. 2012;220(2):122–130. | ||
Berry BJ, Akanda N, Smith AS, et al. Morphological and functional characterization of human induced pluripotent stem cell-derived neurons (iCell Neurons) in defined culture systems. Biotechnol Prog. 2015;31(6):1613–1622. | ||
Umbach JA, Adams KL, Gundersen CB, Novitch BG. Functional neuromuscular junctions formed by embryonic stem cell-derived motor neurons. PLoS One. 2012;7(5):e36049. | ||
Smith AS, Long CJ, Pirozzi K, Hickman JJ. A functional system for high-content screening of neuromuscular junctions in vitro. Technology. 2013;1(1):37–48. | ||
Demestre M, Orth M, Föhr KJ, et al. Formation and characterisation of neuromuscular junctions between hiPSC derived motoneurons and myotubes. Stem Cell Res. 2015;15(2):328–336. | ||
Guo X, Colon A, Akanda N, et al. Tissue engineering the mechanosensory circuit of the stretch reflex arc with human stem cells: Sensory neuron innervation of intrafusal muscle fibers. Biomaterials. 2017;122:179–187. | ||
Guo X, Greene K, Akanda N, et al. In vitro Differentiation of Functional Human Skeletal Myotubes in a Defined System. Biomater Sci. 2014;2(1):131–138. | ||
Santhanam N, Kumanchik L, Guo X, et al. Stem cell derived phenotypic human neuromuscular junction model for dose response evaluation of therapeutics. Biomaterials. 2018;166:64–78. | ||
Happe CL, Tenerelli KP, Gromova AK, Kolb F, Engler AJ. Mechanically patterned neuromuscular junctions-in-a-dish have improved functional maturation. Mol Biol Cell. 2017;28(14):1950–1958. | ||
Arnold AS, Christe M, Handschin C. A functional motor unit in the culture dish: co-culture of spinal cord explants and muscle cells. J Vis Exp. 2012 (62):3616. | ||
Vilmont V, Cadot B, Ouanounou G, Gomes ER. A system for studying mechanisms of neuromuscular junction development and maintenance. Development. 2016;143(13):2464–2477. | ||
Guo X, Gonzalez M, Stancescu M, Vandenburgh HH, Hickman JJ. Neuromuscular junction formation between human stem cell-derived motoneurons and human skeletal muscle in a defined system. Biomaterials. 2011;32(36):9602–9611. | ||
Miles GB, Yohn DC, Wichterle H, Jessell TM, Rafuse VF, Brownstone RM. Functional properties of motoneurons derived from mouse embryonic stem cells. J Neurosci. 2004;24(36):7848–7858. | ||
Guo X, das M, Rumsey J, Gonzalez M, Stancescu M, Hickman J. Neuromuscular junction formation between human stem-cell-derived motoneurons and rat skeletal muscle in a defined system. Tissue Eng Part C Methods. 2010;16(6):1347–1355. | ||
Chin MH, Mason MJ, Xie W, et al. Induced pluripotent stem cells and embryonic stem cells are distinguished by gene expression signatures. Cell Stem Cell. 2009;5(1):111–123. | ||
Chung CY, Khurana V, Auluck PK, et al. Identification and rescue of α-synuclein toxicity in Parkinson patient-derived neurons. Science. 2013;342(6161):983–987. | ||
Kondo T, Asai M, Tsukita K, et al. Modeling Alzheimer’s disease with iPSCs reveals stress phenotypes associated with intracellular Aβ and differential drug responsiveness. Cell Stem Cell. 2013;12(4):487–496. | ||
Sareen D, O’Rourke JG, Meera P, et al. Targeting RNA foci in iPSC-derived motor neurons from ALS patients with a C9ORF72 repeat expansion. Sci Transl Med. 2013;5(208):ra149. | ||
Mouly V, Aamiri A, Périé S, et al. Myoblast transfer therapy: is there any light at the end of the tunnel? Acta Myol. 2005;24(2):128–133. | ||
Webster C, Blau HM. Accelerated age-related decline in replicative life-span of Duchenne muscular dystrophy myoblasts: implications for cell and gene therapy. Somat Cell Mol Genet. 1990;16(6):557–565. | ||
Kaur G, Dufour JM. Cell lines: Valuable tools or useless artifacts. Spermatogenesis. 2012;2(1):1–5. | ||
Mamchaoui K, Trollet C, Bigot A, et al. Immortalized pathological human myoblasts: towards a universal tool for the study of neuromuscular disorders. Skelet Muscle. 2011;1:34. | ||
Fitzpatrick LM, Hawkins KE, Delhove J, et al. NF-κB Activity Initiates Human ESC-Derived Neural Progenitor Cell Differentiation by Inducing a Metabolic Maturation Program. Stem Cell Reports. 2018;10(6):1766–1781. | ||
das M, Rumsey JW, Bhargava N, Stancescu M, Hickman JJ. A defined long-term in vitro tissue engineered model of neuromuscular junctions. Biomaterials. 2010;31(18):4880–4888. | ||
Zhou FM, Hablitz JJ. Layer I neurons of rat neocortex. I. Action potential and repetitive firing properties. J Neurophysiol. 1996;76(2):651–667. | ||
Walsh K, Megyesi J, Hammond R. Human central nervous system tissue culture: a historical review and examination of recent advances. Neurobiol Dis. 2005;18(1):2–18. | ||
Rumsey JW, das M, Stancescu M, Bott M, Fernandez-Valle C, Hickman JJ. Node of Ranvier formation on motoneurons in vitro. Biomaterials. 2009;30(21):3567–3572. | ||
Funakoshi H, Belluardo N, Arenas E, et al. Muscle-derived neurotrophin-4 as an activity-dependent trophic signal for adult motor neurons. Science. 1995;268(5216):1495–1499. | ||
Henderson CE, Camu W, Mettling C, et al. Neurotrophins promote motor neuron survival and are present in embryonic limb bud. Nature. 1993;363(6426):266–270. | ||
Jensen J, Hyllner J, Björquist P. Human embryonic stem cell technologies and drug discovery. J Cell Physiol. 2009;219(3):513–519. |
Supplementary material
Video S1 Phase contrast video micrograph of innervated human myotube contractions at Day 7.
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.